1. Introduction
The presence of arsenic in water results from the dissolution of minerals, geogenic activities, industrial effluents and the air. Nevertheless, other causes of arsenic in nature depend on anthropogenic activities such as the leaching of mine residues [
3] and the use of insecticides. The degree of the toxicity of arsenic depends on the way in which it dissolves in the medium in which it is found. Arsenic is present in its four forms of oxidation, as arsenate (As
5+), arsenite (As
3+), arsine (As
3−) and its fundamental state (As
0). Generally, the form in which it is present in water bodies is its trivalent and pentavalent state, with As
3+ being the most toxic [
4].
In Mexico, several cases of groundwater contamination have presented themselves, one of which is located in the Huautla in the municipality of Tlaquiltenango, State of Morelos. Another case of contamination is present in the Comarca Lagoon, with values as high as 0.348 mg·L−1, 14 times greater than the maximum limit permissible by the Mexican Standard NOM-127-SSA1-1994 and the WHO. In both of these locations, the water is frequently used as a source of supply for human consumption.
The consumption of water contaminated with arsenic can produce cancerous diseases in a variety of organs in the body, including the lungs, kidneys, bladder, liver and skin, as well as hydroarsenicism [
5]. The aim of this work was to develop and evaluate the efficiency of two different sorption media, characterized by their iron oxides and hydroxides contents, for the removal of As(V) from water for human consumption.
3. Results and Discussion
Table 3 shows the results of the analysis of the composition of silica sand coated with Fe(III) and goethite. The primary elements present in both media are carbon (C), oxygen (O) and iron (Fe) with the highest content. Aluminum and silica compounds were found as constituents of the silica sand.
Table 3.
Elemental analysis of adsorbents.
Table 3.
Elemental analysis of adsorbents.
| Element | Percentage by Weight (%) |
|---|
| Silica Sand Coated with Fe(III) | Goethite |
|---|
| C | 18.57 | 15.50 |
| O | 38.77 | 33.68 |
| Al | 0.63 | - |
| Si | 9.77 | - |
| Fe | 32.25 | 50.82 |
Table 4 shows the characterization of the adsorbent media used in the batch tests. The surface area of goethite was observed to be 17 times greater than that of silica sand coated with Fe(III); both media were classified as mesoporous. In previous studies, such as that of Garrido (2008) [
7], the greatest amount of As(V) found in water in Huautla was species HASO
42−, according to the species diagram at pH 8.57 [
9], with a radius of 3.97 Å. Therefore, the diameters of both media were porous enough for the As
5+ molecule to enter.
Table 4.
Characterization of the adsorbent media obtained in the laboratory.
Table 4.
Characterization of the adsorbent media obtained in the laboratory.
| Analysis | Units | Silica Sand Coated with Fe(III) | Goethite |
|---|
| Surface area | m2·g−1 | 2.44 | 43.04 |
| Accumulated micropore volume | cm3·g−1 | 0.002 | 0.12 |
| Average pore diameter | A | 53.60 | 119.05 |
| Pore structure | | Mesoporous | Mesoporous |
| Particulate size | mm | 0.3–0.6 | 0.1–0.6 |
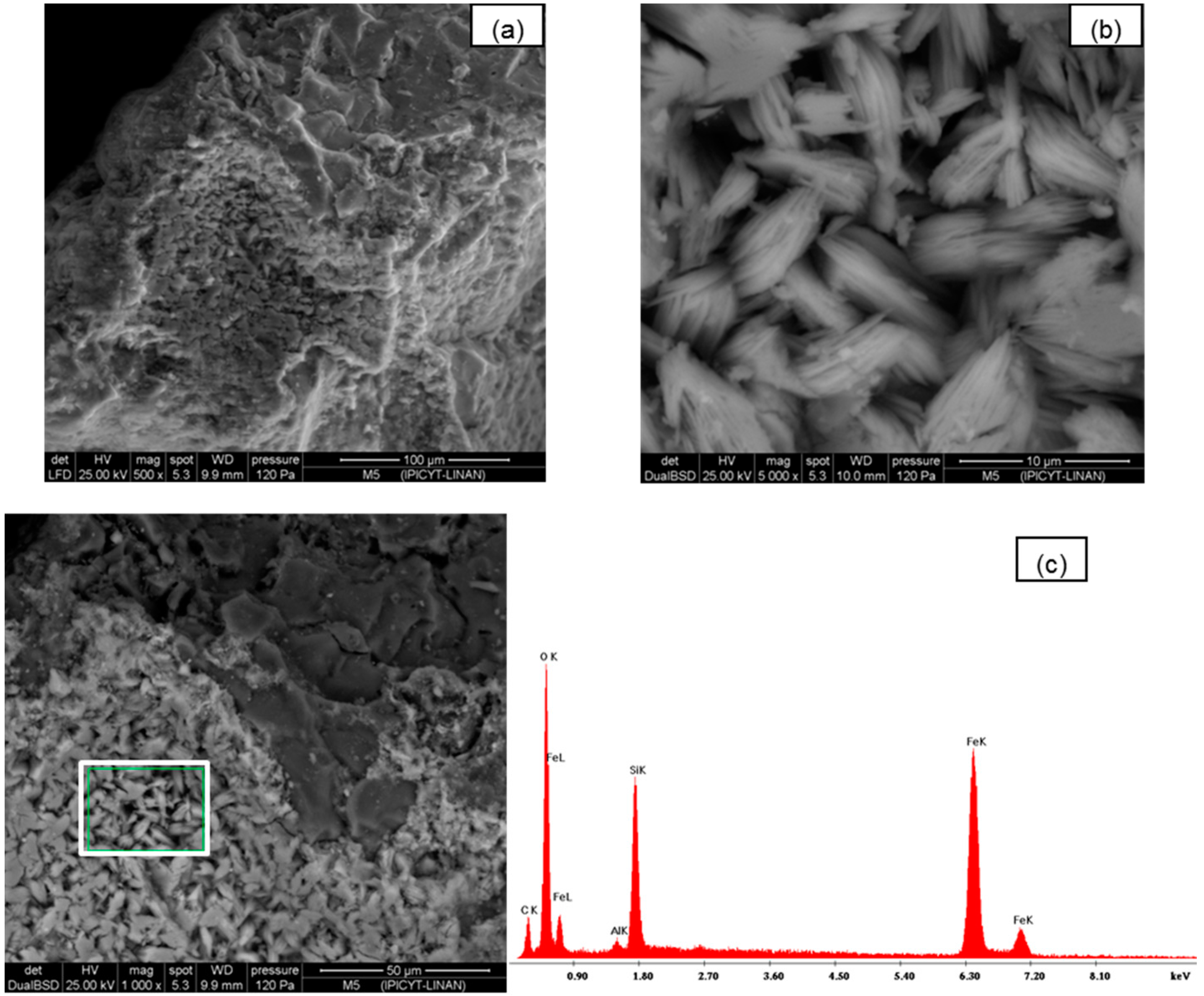
Scanning electron microscopy (SEM) was used to study the morphological structure of the two media. In
Figure 1a–c, the images of silica sand coated with Fe(III) untreated are presented. In these images the surface amorphous structure is shown. In
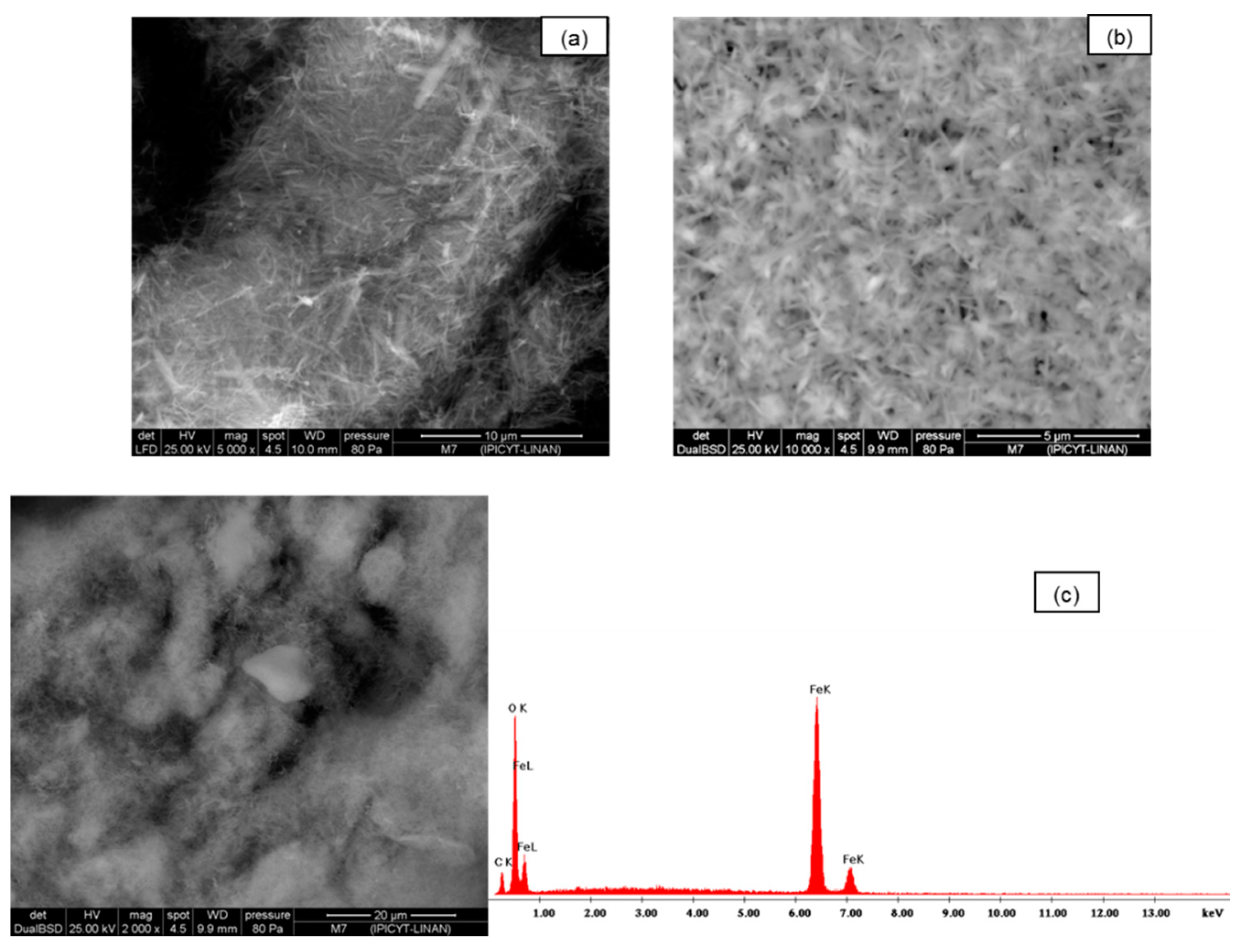
Figure 2a–c, the SEM images of synthetic goethite, which is a material of different sizes, are observed at 2000×; when this is viewed at higher magnifications of 5000× and 1000×, very fine fibers that are less than 5 μm with crystalline materials of spherical structure are shown.
Figure 1.
(a,b) SEM images of silica sand coated with Fe(III) untreated; (c) Fragment where EDS (white box) and chemical spectrograph quantification EDS of adsorbent was performed.
Figure 1.
(a,b) SEM images of silica sand coated with Fe(III) untreated; (c) Fragment where EDS (white box) and chemical spectrograph quantification EDS of adsorbent was performed.
The infrared spectra (IR) from silica sand coated with Fe(III) in the absence and presence of As(V), as shown in
Figure 3, were used to analyze the presence of the main sorption functional groups. It is noted that the two spectra are very similar. The small peak at 1635 cm
−1 indicates the presence of free water molecules and water molecules bonded onto silica. The intense band at 1160 cm
−1 is characteristic of Si-O bonds as is the peak noticeable at 800 cm
−1. The band in the presence of As(V) between 600 and 520 cm
−1 belongs to the stretching mode Fe-O, and Fe-O-As, small shoulder, respectively [
12].
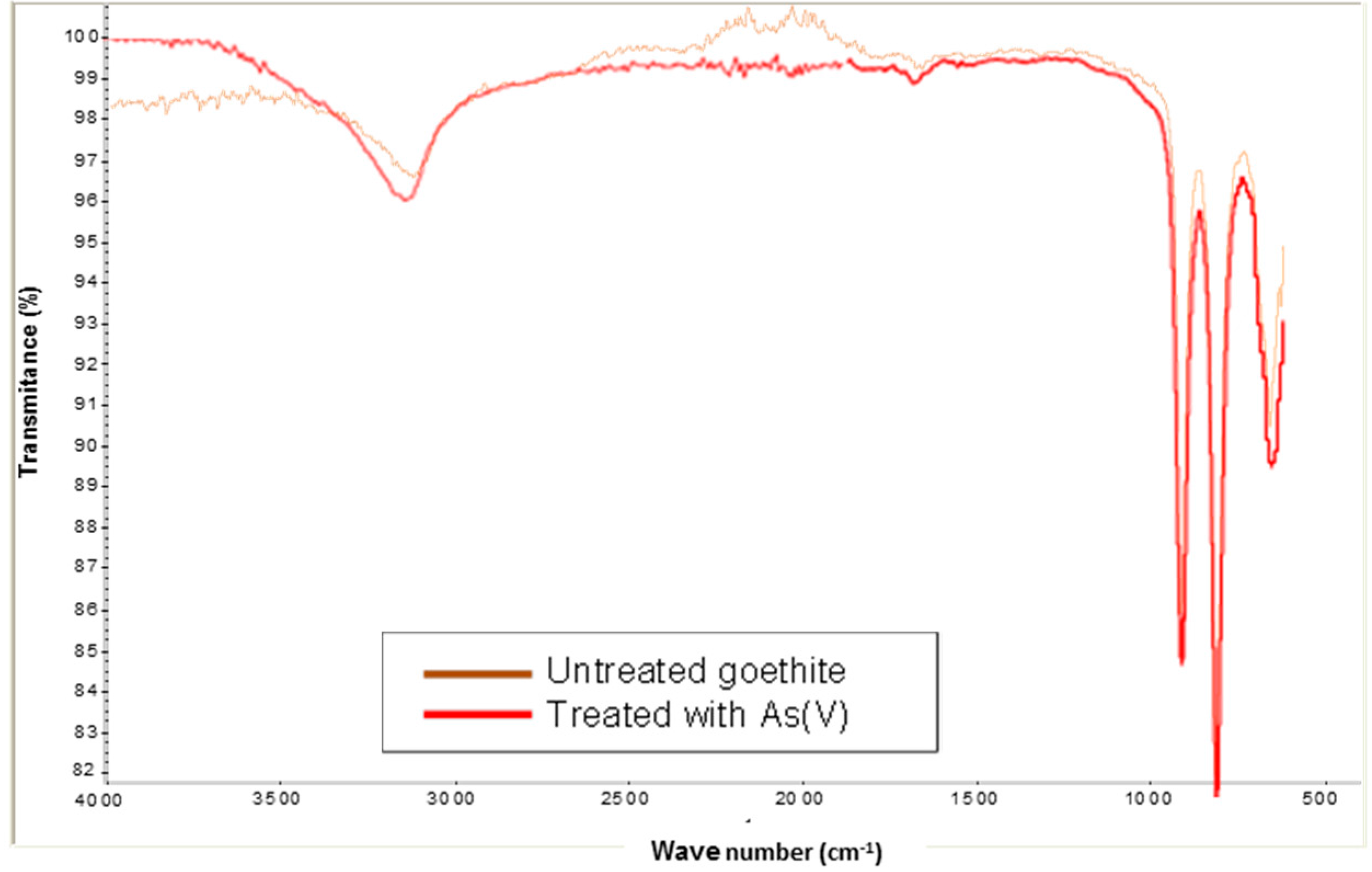
Figure 4 shows the spectrum (IR) from goethite in the absence and presence of As(V). It is noted that the two spectra are very similar. The region of 3500–3000 cm
−1 reveals a very high increase in O-H stretching with for both bands. The band in the absence and presence of As(V) between 600 and 520 cm
−1 belongs to the stretching mode Fe-O [
12]. The band at 780–800 cm
−1 observed in the presence of arsenic can be assigned to the As-OH stretching [
12,
13]. It may be due to the sorption of HAsO4
2− on goethite.
Figure 2.
(a,b) SEM images of goethite untreated; (c) Chemical spectrograph quantification EDS of adsorbent was performed.
Figure 2.
(a,b) SEM images of goethite untreated; (c) Chemical spectrograph quantification EDS of adsorbent was performed.
Figure 3.
FTIR spectrum of silica sand coated with Fe(III) in absence and presence of As(V).
Figure 3.
FTIR spectrum of silica sand coated with Fe(III) in absence and presence of As(V).
Figure 4.
FTIR spectrum of goethite in absence and presence of As(V).
Figure 4.
FTIR spectrum of goethite in absence and presence of As(V).
Based on the design of the experiments, a statistically significant difference (
p < 0.005) was found for the two adsorbent media for the initial concentration of arsenic, while a statistically significant difference (
p < 0.005) for mass was shown only for goethite. The optimal conditions obtained for silica sand coated with Fe(III) were: As concentration of 0.392 mg·L
−1, pH of 8.5 and mass of 4 g·L
−1. Those for goethite were: As concentration of 0.360 mg·L
−1, pH of 7.5 and mass of 2.0 g·L
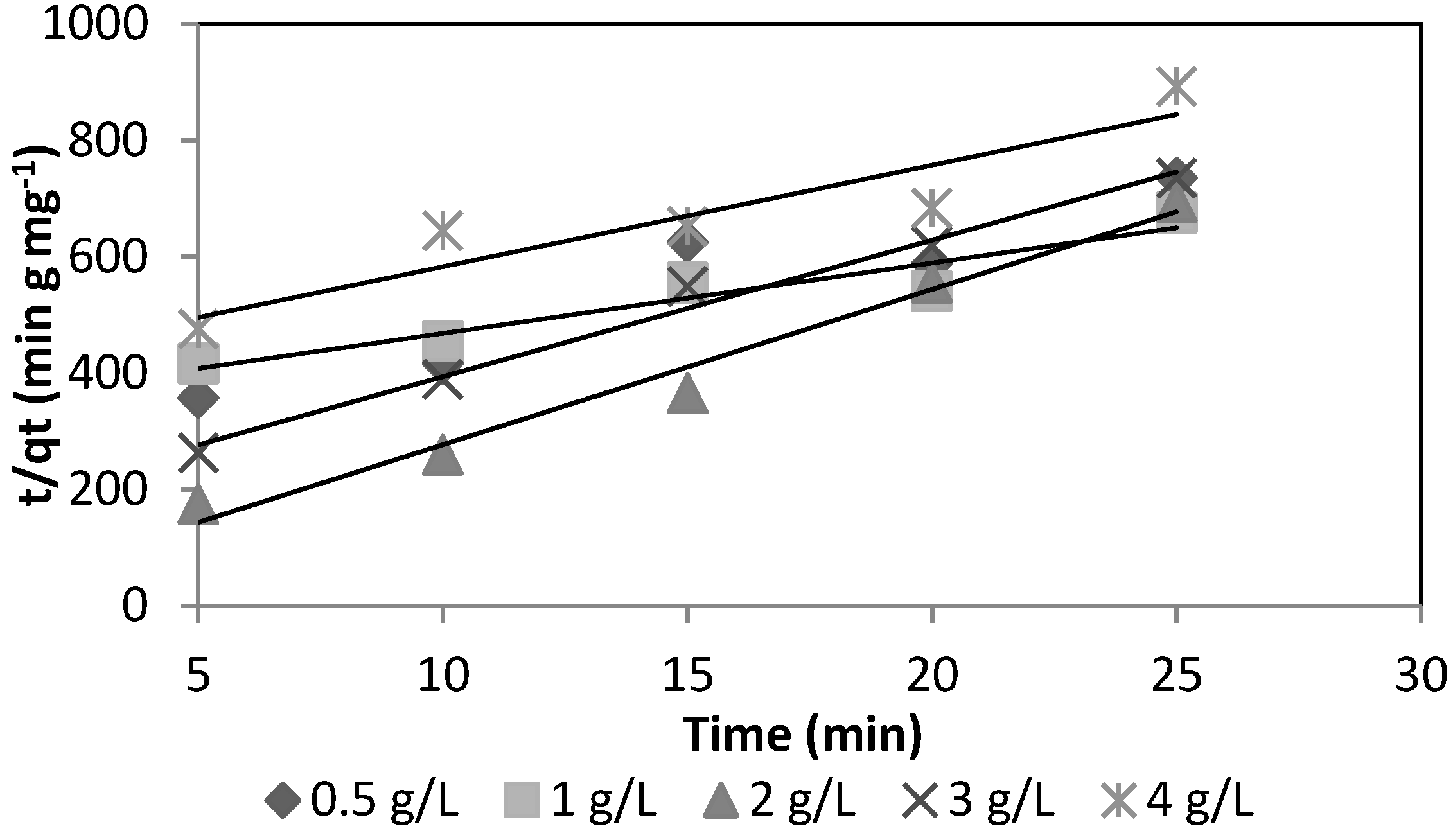
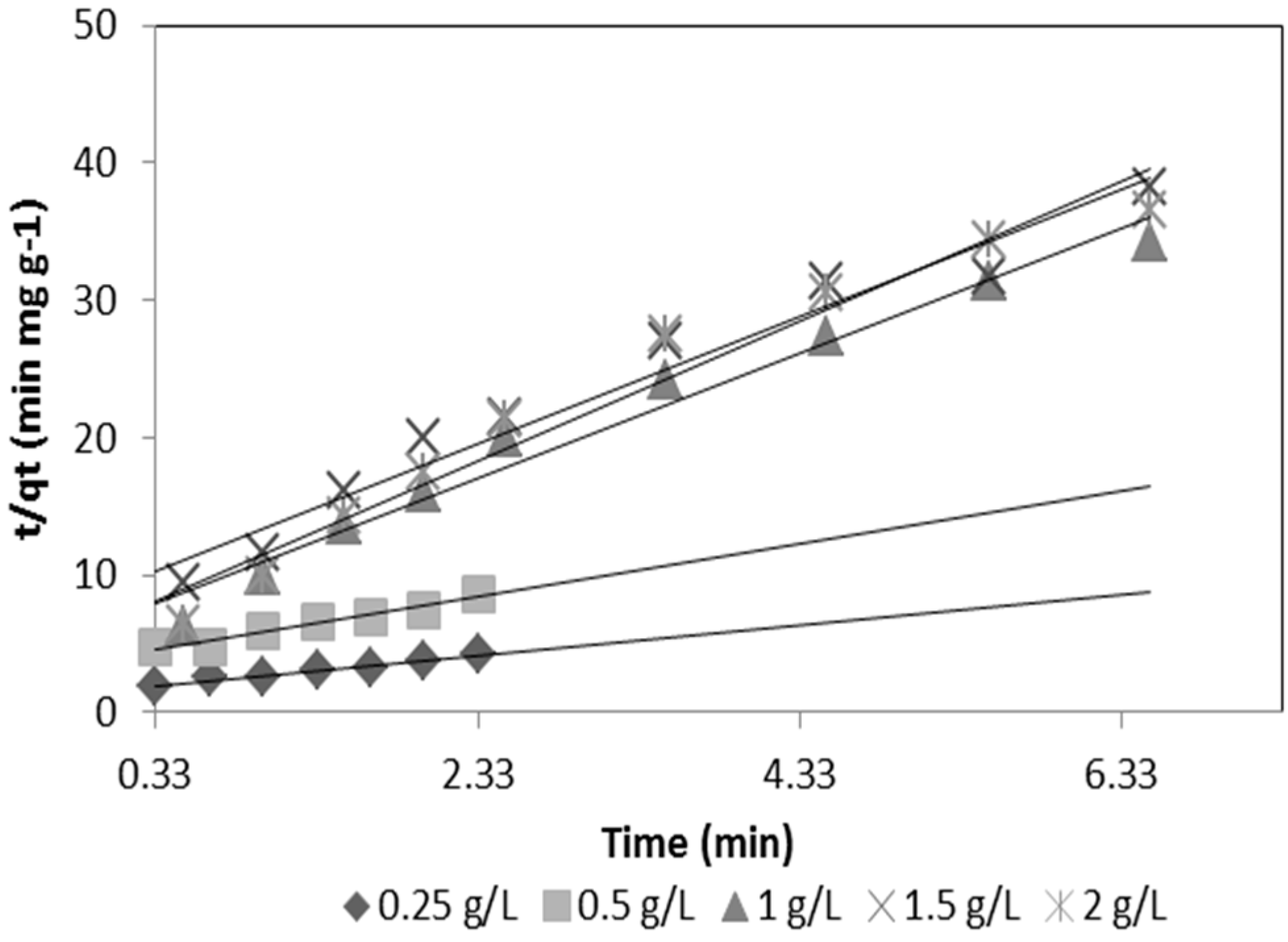
−1. The sorption kinetics for the two media is shown in
Figure 5 and
Figure 6.
To quantify the changes in sorption with respect to the time required by a suitable kinetic model, we use the pseudo-second-order equation:
where
qt is the sorption capacity (mg·g
−1),
qe is the sorption capacity in equilibrium (mg·g
−1),
kad is the rate constant of sorption (g·mg
−1·min
−1) and
h is the initial sorption rate (mg·g
−1·min
−1) at t = 0:
Figure 5 and
Figure 6 show the As(V) kinetics sorption for silica sand coated with Fe(III) and goethite.
Figure 5.
Kinetic sorption of As(V) for silica sand coated with Fe(III).
Figure 5.
Kinetic sorption of As(V) for silica sand coated with Fe(III).
Figure 6.
Kinetic sorption of As(V) for goethite.
Figure 6.
Kinetic sorption of As(V) for goethite.
Table 5 shows that the rate constant of sorption (k
ad) for goethite is 5.39 times greater than the k
ad for silica sand coated with Fe(III) and, therefore, synthesized goethite has a greater sorption capacity due to its larger surface area as compared to silica sand coated with Fe(III). It can be seen that the initial sorption rate (h) of goethite is higher than the silica sand coated with Fe(III). Studies conducted by Thirunavukkarasu
et al. (2001; 2003) [
6,
14] and Paredes (2012) [
9] determined k
ad values of 0.033 and 7.409 g·mg
−1 min
−1 for granular iron hydroxide and goethite, respectively.
Table 5.
Pseudo-second-order kinetic sorption constants.
Table 5.
Pseudo-second-order kinetic sorption constants.
| | Units | Silica Sand Coated with Fe (III) | Goethite |
|---|
| Mass | (g) | 4 | 2 |
| qe | mg·g−1 | 0.058 | 0.196 |
| kad | (g·mg−1·min−1) | 0.745 | 4.019 |
| h | (mg·g−1·min−1) | 0.0024 | 0.155 |
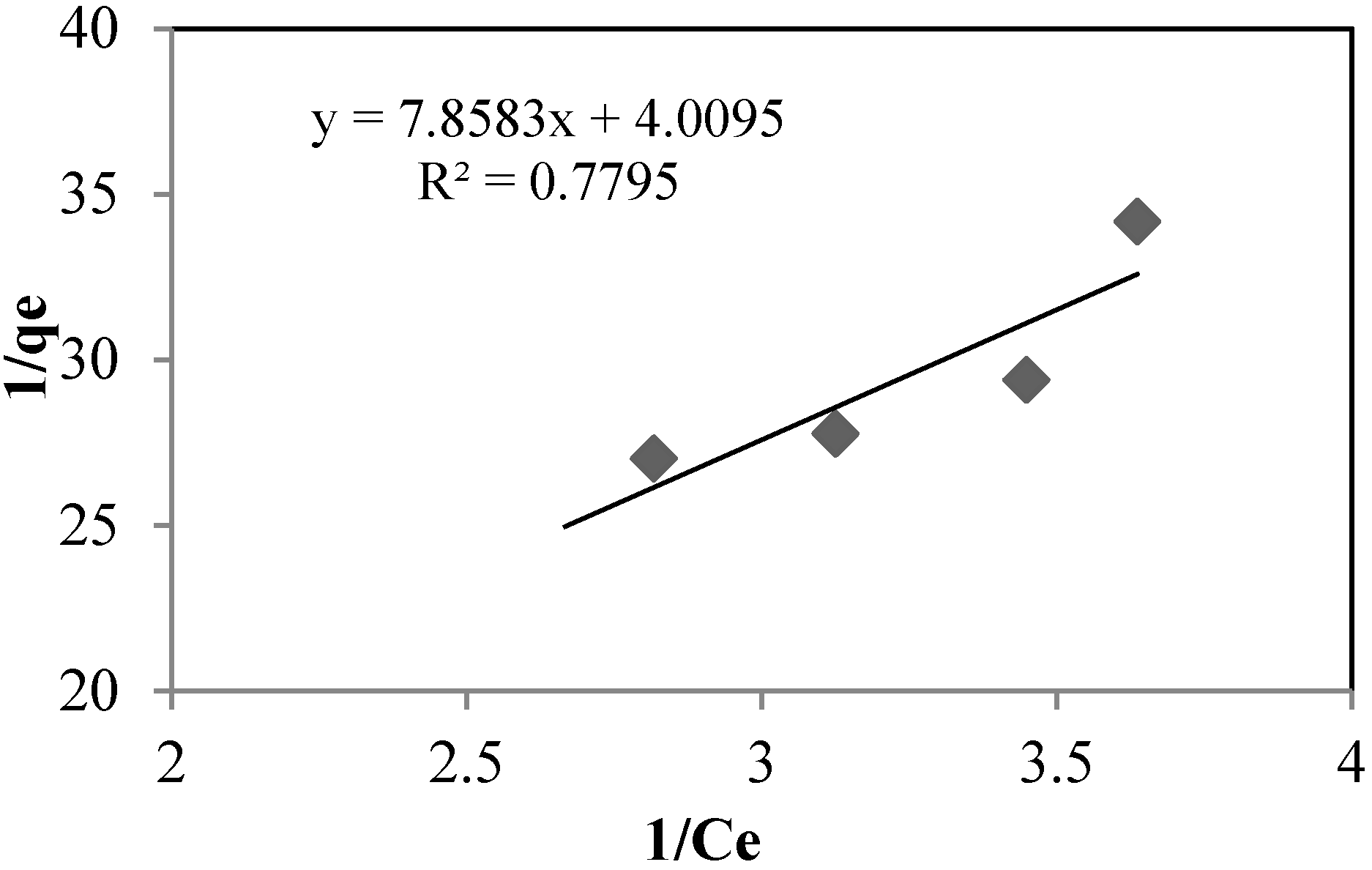
The sorption isotherms of arsenate using silica sand coated with Fe(III) and goethite at pH 8.5 and 7.5, respectively, are shown in
Figure 7 and
Figure 8, and the isotherm constants are shown in
Table 6. For both, adsorption takes place according to the Langmuir model, type (II):
Langmuir (II)
where
qe is the sorption capacity in the equilibrium (mg·g
−1),
qm is the maximum sorption capacity (mg·g
−1),
Ce is the concentration in equilibrium (mg·L
−1) and
b is the constant related with the energy.
Figure 7.
Langmuir (II) isotherm for silica sand coated with Fe(III). Asinitial: 0.392 mg·L−1. T: 22 °C.
Figure 7.
Langmuir (II) isotherm for silica sand coated with Fe(III). Asinitial: 0.392 mg·L−1. T: 22 °C.
Figure 8.
Langmuir (II) isotherm for goethite. Asinitial: 0.360 mg·L−1. T: 22 °C.
Figure 8.
Langmuir (II) isotherm for goethite. Asinitial: 0.360 mg·L−1. T: 22 °C.
Table 6.
Langmuir (II) isotherm constants.
Table 6.
Langmuir (II) isotherm constants.
| | Units | Silica Sand Coated with Fe (III) | Goethite |
|---|
| qm | (mg·g−1) | 0.2494 | 0.4822 |
| b | - | 0.5102 | 5.0841 |
| R2 | - | 0.7795 | 0.9085 |
A comparison of the removal capacities of the selected sorbent materials towards As(V) is given in
Table 7. The As(V) uptake determined in this work was higher than: iron oxide–coated sand (IOCS) ferrihydrite (FH) for goethite and granular ferric hydroxide (GFH). The goethite adsorbent seems to be a good alternative for the removal of As(V) species from an aqueous systems.
Table 7.
A comparison of the produced materials’ As(V) sorption capacity (qm) with data from the literature, and the best fit Langmuir isotherm model.
Table 7.
A comparison of the produced materials’ As(V) sorption capacity (qm) with data from the literature, and the best fit Langmuir isotherm model.
| Adsorbent | Co (mg·L−1) | pH | qm (mg·g−1) | Reference |
|---|
| Silica sand coated with Fe(III) | 0.392 | 8.5 | 0.2494 | This study |
| Goethite synthesized | 0.360 | 7.5 | 0.4822 | This study |
| Natural goethite | 0.220 | 7.5 | 9.30 | [15] |
| Iron oxide coated sand (IOCS) | 0.325 | 7.4 | 0.183 | [6] |
| Ferrihydrite (FH) | - | - | 0.285 | [6] |
| Crystalline hydrous ferric oxide (CHFO) | 50 | - | 25 | [16] |
| Granular ferric hydroxide (GFH) | 0.50 | 7.6 | 0.159 | [14] |
| Sulphuric acid acidified laterite (ALS) | 0.1 | 7.0 | 0.923 | [17] |
| Silica coated with Fe oxides (8%) | 50 | 5.0 | 30.59 | [17] |
| Silica coated with Al oxides (8%) | 50 | 4.0 | 6.52 | [17] |