Correlation of Zinc with Oxidative Stress Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
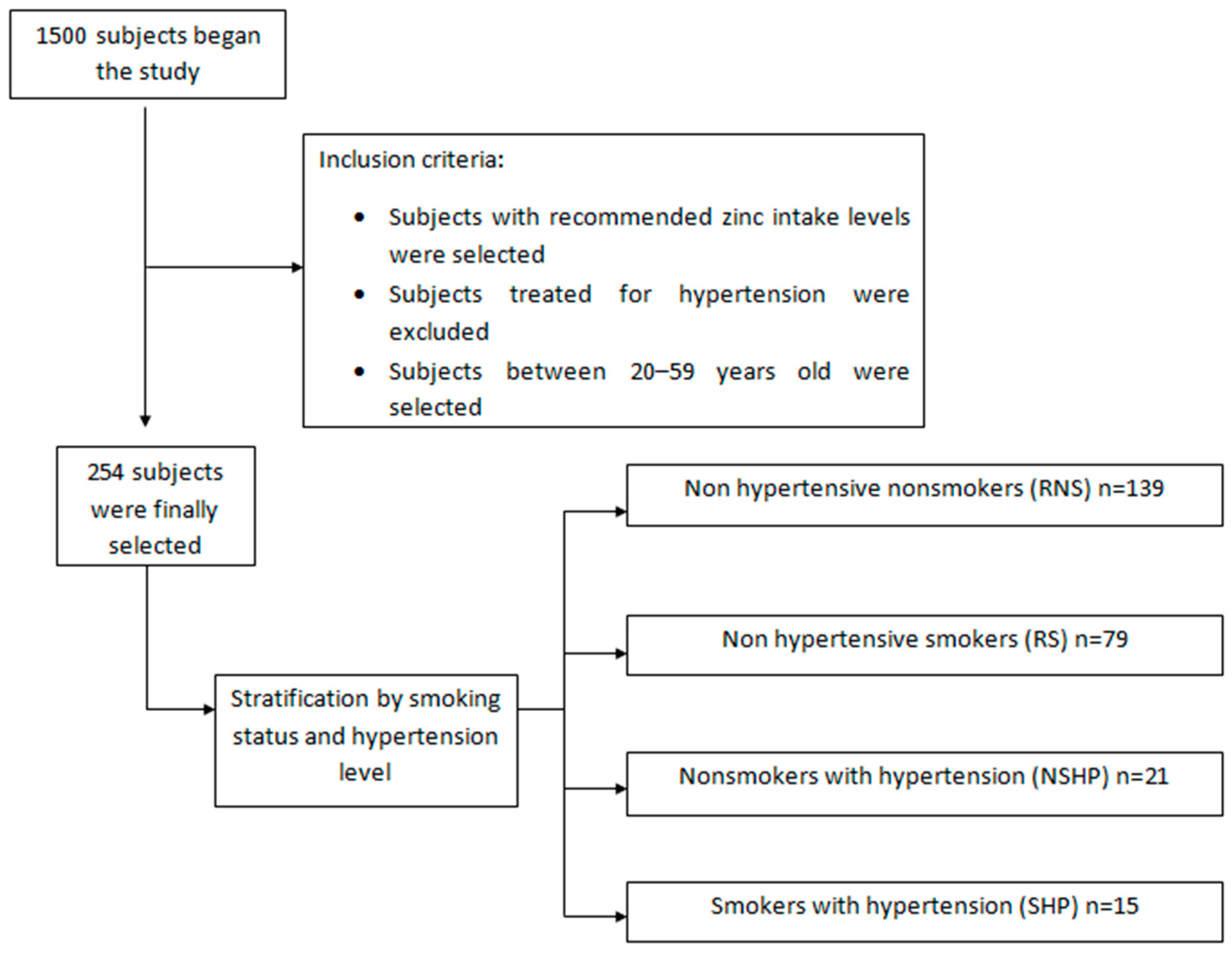
2.1. Study Population
2.2. Determining Zinc in Biological Samples
2.3. Measuring Markers of Oxidative Stress
2.4. Study Procedure
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
| Parameters | RNS (n = 139) | RS (n = 79) | NSHP (n = 21) | SHP (n = 15) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | Mean ± SD | 95%CI | Mean ± SD | 95%CI | |
| Height (cm) | 159.10 ± 32.05 | 155.51–162.69 | 164.61 ± 8.24 | 163.1–166.1 | 165.67 ± 10.68 | 161.31–170.09 | 169.40 ± 9.28 | 164.83–173.97 |
| Weight (kg) | 64.59 ± 17.59 | 62.63–66.57 | 66.80 ± 12.20 | 64.56–69.04 | 75.81 ± 16.35 | 69.07–82.53 | 78.53 ± 11.49 | 72.84–84.16 |
| BMI (kg/m2) | 23.59 ± 5.60 | 22.97–24.23 | 24.60 ± 3.72 | 23.92–25.28 | 27.50 ± 4.68 | 25.57–29.43 | 27.56 ± 4.82 | 25.24–29.96 |
| SBP (mmHg) | 114.69 ± 15.74 | 112.94–116.46 | 113.22 ± 17.39 | 110.01–116.39 | 143.49 ± 11.57 | 138.74–148.26 | 133.51 ± 13.78 | 126.71–140.29 |
| DBP (mmHg) | 73.81 ± 10.19 | 72.66–74.94 | 73.67 ± 11.54 | 71.59–75.81 | 91.16 ± 5.73 | 88.86–93.54 | 86.58 ± 9.69 | 81.83–91.37 |
| PP (mmHg) | 40.94 ± 10.22 | 39.76–42.04 | 39.60 ± 10.53 | 37.67–41.53 | 52.31 ± 12.76 | 47.05–57.55 | 47.27 ± 14.78 | 40.02–54.58 |
| Waist (cm) | 81.73 ± 12.20 | 80.33–83.07 | 81.50 ± 10.48 | 79.57–83.43 | 89.72 ± 12.24 | 84.69–94.71 | 91.43 ± 8.01 | 87.47–95.33 |
| Hip (cm) | 99.60 ± 6.11 | 98.92–101.28 | 101.51 ± 14.93 | 98.77–104.23 | 104.06 ± 10.17 | 99.91–108.29 | 104.79 ± 8.96 | 100.37–109.23 |
| Waist/hip ratio | 0.83 ± 0.09 | 0.79–0.81 | 0.81 ± 0.09 | 0.78–0.82 | 0.86 ± 0.09 | 0.86–0.94 | 0.88 ± 0.09 | 0.85–0.95 |
| Number of cigarettes smoked per day | 14.73 ± 9.49 | 12.96–16.44 | 18.47 ± 13.90 | 11.66–25.34 | ||||
| Years of non-smoking | 6.18 ± 6.41 | 5.48–6.92 | 14.10 ± 9.91 | 10.04–18.16 | ||||
| Parameters | RNS | RS | NSHP | SHP | Total | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | ||
| Diabetes | No | 139 | 54.7 | 78 | 30.7 | 21 | 8.3 | 15 | 5.9 | 253 | 99.6 | 254 | 100 |
| Yes | 0 | 1 | 1.3 | 0 | 0 | 1 | 0.4 | ||||||
| No | 139 | 54.7 | 79 | 31.1 | 21 | 8.3 | 15 | 5.9 | 254 | 100 | 254 | 100 | |
| yes | 0 | 0 | 0 | 0 | 5.9 | 0 | |||||||
| No | 77 | 30.3 | 32 | 12.6 | 11 | 4.3 | 6 | 2.4 | 126 | 49.6 | 253 | 99.6 | |
| Yes | 62 | 24.4 | 47 | 18.5 | 9 | 3.5 | 9 | 3.5 | 127 | 50.0 | |||
| No | 120 | 47.2 | 69 | 27.2 | 16 | 6.3 | 12 | 4.7 | 217 | 85.4 | 254 | 100 | |
| Yes | 19 | 7.5 | 10 | 3.9 | 5 | 2.0 | 3 | 1.2 | 37 | 14.6 | |||
| No | 139 | 54.7 | 79 | 31.1 | 20 | 7.9 | 15 | 5.9 | 253 | 99.6 | 254 | 100 | |
| Yes | 0 | 0 | 1 | 0.4 | 0 | 1 | 0.4 | ||||||
| No | 127 | 50.0 | 67 | 26.4 | 14 | 5.5 | 10 | 3.9 | 218 | 85.8 | 254 | 100 | |
| Yes | 12 | 4.7 | 12 | 4.7 | 7 | 2.8 | 5 | 2.0 | 36 | 14.2 | |||
| 0 | 54 | 21.3 | 26 | 10.2 | 0 | 1 | 0.4 | 81 | 31.9 | 254 | 100 | ||
| 1 | 45 | 17.7 | 25 | 9.8 | 7 | 2.8 | 5 | 2.0 | 82 | 32.3 | |||
| 2 | 28 | 11.0 | 17 | 6.7 | 7 | 2.8 | 4 | 1.6 | 56 | 22.0 | |||
| 3 | 8 | 3.1 | 8 | 3.1 | 3 | 1.2 | 4 | 1.6 | 23 | 9.1 | |||
| 4 | 4 | 1.6 | 3 | 1.2 | 4 | 1.6 | 1 | 0.4 | 12 | 4.7 | |||
| No | 139 | 54.7 | 79 | 31.1 | 21 | 8.3 | 15 | 5.9 | 254 | 100 | 254 | 100 | |
| Yes | 0 | 0 | 0 | 0 | 0 | ||||||||
| Biomarkers (1) | Non-hypertensive (n = 218) | Hypertensive (n = 36) | p-value (2) | p-value (3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-smoker (n = 139) | Smoker (n = 79) | p value | Non-smoker (n = 21) | Smoker (n = 15) | p value | |||||||
| Mean ± SD | 95%CI | Mean ± SD | 95%CI | Mean ± SD | 95%CI | Mean ± SD | 95%CI | |||||
| Zn serum (μmol/L) | 13.39 ± 4.35 | 12.92–13.88 | 12.80 ± 3.77 | 12.10–13.50 | 0.314 | 12.32 ± 3.19 | 10.99–13.61 | 12.23 ± 4.30 | 10.08–14.32 | 0.942 | 0.281 | 0.601 |
| Zn urine (μmol/L) | 2.55 ± 2.91 | 2.27–2.93 | 2.59 ± 2.74 | 2.10–3.10 | 0.921 | 2.78 ± 2.13 | 1.94–3.66 | 2.69 ± 3.57 | 0.93–4.47 | 0.771 | 0.728 | 0.902 |
| Zn serum/urine | 43.74 ± 67.19 | 36.17–51.23 | 43.13 ± 69.24 | 30.40–55.79 | 0.949 | 28.79 ± 60.36 | 4.01–53.59 | 15.40 ± 17.36 | 6.84–23.96 | 0.336 | 0.337 | 0.082 |
| 8-OHdG | 3.24 ± 1.72 | 3.05–3.43 | 3.34 ± 1.41 | 3.08–3.60 | 0.660 | 2.66 ± 0.79 | 2.34–2.98 | 2.03 ± 0.90 | 1.59–2.47 | 0.032 | 0.115 | 0.0008 |
| MDA | 0.63 ± 0.66 | 0.56–0.70 | 0.59 ± 0.70 | 0.46–0.72 | 0.674 | 0.62 ± 0.58 | 0.38–0.86 | 0.49 ± 0.22 | 0.38–0.60 | 0.342 | 0.948 | 0.314 |
| GSH | 16.30 ± 7.89 | 15.42–17.18 | 17.68 ± 7.52 | 16.30–19.06 | 0.208 | 16.28 ± 7.14 | 13.35–19.21 | 16.45 ± 6.18 | 13.41–19.49 | 0.941 | 0.991 | 0.553 |
| GSSG | 0.77 ± 0.71 | 0.69–0.85 | 0.75 ± 0.51 | 0.66–0.84 | 0.826 | 0.97 ± 0.79 | 0.65–1.29 | 0.80 ± 0.31 | 0.65–0.95 | 0.353 | 0.237 | 0.482 |
| GSSG/GSH (4) | 15.67 ± 35.56 | 11.68–19.66 | 10.60 ± 24.49 | 6.11–15.09 | 0.262 | 21.44 ± 56.73 | −1.85–44.73 | 10.81 ± 21.92 | 0.03–21.59 | 0.385 | 0.508 | 0.975 |
| G Total | 17.85 ± 8.20 | 16.93–18.77 | 19.18 ± 7.65 | 17.78–20.58 | 0.239 | 18.22 ± 7.22 | 15.26–21.18 | 18.05 ± 6.25 | 14.98–21.12 | 0.942 | 0.845 | 0.592 |
3.2. Association of OS Markers with Zn Serum/Urine Quotient and Cotinine Levels
3.3. OS Risk Factors
| Biomarkers | Zn Serum/Urine | |||||||
| RNS | RS | NSHP | SHP | |||||
| C. Pearson | p-value | C. Pearson | p-value | C. Pearson | p-value | C. Pearson | p-value | |
| 8-hydroxydeoxyguanosine (8-OHdG) | 0.123 | 0.150 | 0.178 | 0.118 | 0.417 | 0.060 | −0.217 | 0.436 |
| Malondialdehyde (MDA) | 0.049 | 0.565 | −0.038 | 0.739 | −0.184 | 0.424 | −0.097 | 0.731 |
| Reduced glutathione (GSH) | −0.067 | 0.434 | 0.166 | 0.146 | 0.156 | 0.500 | −0.571 | 0.026 |
| Oxidized glutathione (GSSG) | −0.087 | 0.310 | −0.106 | 0.356 | −0.245 | 0.285 | −0.264 | 0.341 |
| Percentage oxidized glut. (GSSG/GSH) | 0.062 | 0.467 | −0.145 | 0.205 | −0.146 | 0.526 | 0.639 | 0.010 |
| Total glutathione | −0.079 | 0.353 | 0.149 | 0.192 | 0.100 | 0.665 | −0.591 | 0.020 |
| Biomarkers | Cotinine | |||||||
| RNS | RS | NSHP | SHP | |||||
| C. Pearson | p-value | C. Pearson | p-value | C. Pearson | p-value | C. Pearson | p-value | |
| 8-hydroxydeoxyguanosine (8-OHdG) | --- | --- | 0.019 | 0.869 | --- | --- | 0.087 | 0.759 |
| Malondialdehyde (MDA) | --- | --- | 0.166 | 0.143 | --- | --- | 0.008 | 0.978 |
| Reduced glutathione (GSH) | --- | --- | −0.045 | 0.696 | --- | --- | −0.154 | 0.584 |
| Oxidized glutathione (GSSG) | --- | --- | −0.084 | 0.461 | --- | --- | 0.000 | 1.0 |
| Percentage oxidized glut. GSSG/GSH | --- | --- | 0.044 | 0.700 | --- | --- | −0.097 | 0.732 |
| Total glutathione | --- | --- | −0.055 | 0.630 | --- | --- | −0.152 | 0.589 |
| Biomarkers | P 75 (nmol/mmol creat.) | Non-hypertensive and Non-smoker/Non-hypertensive and Smoker | Non-hypertensive and Non-smoker/Hypertensive and Non-smoker | Non-hypertensive and Non-smoker/Hypertensive and Smoker | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | AOR (1) | 95%CI | OR | 95%CI | AOR (1) | 95%CI | OR | 95%CI | AOR (1) | 95%CI | ||
| 8-OHdG | P < 4.10 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 4.10 | 1.27 | 0.68–2.36 | 1.41 | 0.73–2.73 | 0.15 | 0.02–1.19 | 0.16 | 0.02–1.34 | --- | --- | --- | --- | |
| MDA | P < 0.75 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 0.75 | 1.12 | 0.59–2.10 | 1.05 | 0.54–2.04 | 0.72 | 0.23–2.31 | 0.49 | 0.12–1.96 | 0.22 | 0.03–1.74 | 0.17 | 0.02–1.68 | |
| GSH | P < 21.34 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 21.34 | 0.81 | 0.43–1.51 | 0.86 | 0.44–1.68 | 0.78 | 0.28–2.16 | 0.77 | 0.24–2.49 | 0.62 | 0.19–1.95 | 0.47 | 0.13–1.69 | |
| GSSG | P < 0.87 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 0.87 | 0.98 | 0.51–1.86 | 0.98 | 0.50–1.91 | 2.80 | 1.09–7.18 | 3.06 | 0.96–9.71 | 1.12 | 0.34–3.76 | 1.73 | 0.45–6.67 | |
| GSSG/GSH | P < 6.7 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 6.7 | 0.72 | 0.36–1.43 | 0.64 | 0.32–1.30 | 1.54 | 0.58–4.14 | 1.92 | 0.59–6.27 | 0.77 | 0.20–2.89 | 1.25 | 0.29–5.38 | |
| Total glutathione | P < 23.14 | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- | 1 | --- |
| P > 23.14 | 1.09 | 0.57–2.06 | 1.02 | 0.51–2.02 | 1.60 | 0.60–4.31 | 1.57 | 0.49–4.99 | 1.17 | 0.35–3.91 | 1.58 | 0.41–6.19 | |
4. Discussion
4.1. Hypertension and the Zinc Serum/Urine Quotient
4.2. Hypertension, Smoking, Zinc Alterations and OS
4.3. Study Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fagard, R.H. Resistant hypertension. Heart 2012, 98, 254–261. [Google Scholar] [CrossRef]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; Atkinson, C.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2013; WHO: Geneva, Switzerland, 2013; pp. 66–67. [Google Scholar]
- Boyd, A.C.; Eshoo, S.; Richards, D.A.; Thomas, L. Hypertension accelerates the “normal” aging process with a premature increase in left atrial volume. J. Am. Soc. Hypertens. 2013, 7, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I. Zinc and the modulation of redox homeostasis. Free Radic. Biol. Med. 2012, 53, 1748–1759. [Google Scholar] [CrossRef] [PubMed]
- Shazia, Q.; Mohammad, Z.H.; Rahman, T.; Shekhar, H.U. Correlation of oxidative stress with serum trace element levels and antioxidant enzyme status in Beta thalassemia major patients: A review of the literature. Anemia 2012, 2012. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Zinc; ATSDR: Atlanta, GA, USA, 2005; pp. 75–77. [Google Scholar]
- Talukder, M.A.; Johnson, W.M.; Varadharaj, S.; Lian, J.; Kearns, P.N.; El-Mahdy, M.A.; Liu, X.; Zweier, J.L. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, 388–396. [Google Scholar] [CrossRef]
- Afridi, H.I.; Kazi, T.G.; Brabazon, D.; Naher, S. Interactions between zinc, cadmium and lead in scalp hair samples of Pakistani and Irish smokers rheumatoid arthritis subjects in relation to controls. Biol. Trace Elem. Res. 2012, 48, 139–147. [Google Scholar] [CrossRef]
- Suárez-Varela, M.M.; Llopis-González, A.; González-Albert, V.; López-Izquierdo, R.; González-Manzano, I.; Cháves, J.; Huerta-Biosca, V.; Martín-Escudero, J.C. Zinc and smoking habits in the setting of hypertension in a Spanish populations. Hypertens. Res. 2014, 38, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Hoshide, S.; Shimada, K.; Kario, K. The impact of cigarette smoking on 24-hour blood pressure, inflammatory and hemostatic activity, and cardiovascular risk in Japanese hypertensive patients. J. Clin. Hypertens. 2013, 15, 234–240. [Google Scholar] [CrossRef]
- Singh, R.; Devi, S.; Gollen, R. Role of free radical in atherosclerosis, diabetes and dyslipidemia: Larger-than-life. Diabetes Metab. Res. Rev. 2015, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta 2004, 339, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sámano, J.; Torres-Durán, P.V.; Júarez-Oropeza, M.A. El glutatión y su asociación con las enfermedades neurodegenerativas, la esquizofrenia, el envejecimiento y la isquemia cerebral. REB 2011, 30, 56–67. [Google Scholar]
- Pilger, A.; Rüdiger, H.W. 8-Hydroxy-2’-deoxyguanosine as a marker of oxidative DNA damage related to occupational and environmental exposures. Int. Arch. Occup. Environ. Health. 2006, 80, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, E.Y.; Lee, K.H.; Park, J.D.; Kim, Y.D.; Hong, Y.C. Differential oxidative stress response in young children and the elderly following exposure to PM2.5. Environ. Health Prev. Med. 2009, 14, 60–66. [Google Scholar] [CrossRef]
- Sentellas, S.; Morales-Ibáñez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH ratios in cryopreserved rat and human hepatocytes as a biomarker for drug induced oxidative stress. Toxicol. in Vitro. 2014, 28, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Oliveiri, O.; Girelli, D.; Faccini, G.; Zenari, M.L.; Lombardi, S.; Corrocher, R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J. Hypertens. 1998, 16, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Federación Española de sociedades de Nutrición, Alimentación y Dietética (FESNAD). Ingestas dietéticas de referencia (IDR) para la población española, 2010. Actividad. Dietética 2010, 14, 196–197. [Google Scholar]
- Richter, P.A.; Bishop, E.E.; Wang, J.; Kaufmann, R. Trends in tobacco smoke exposure and blood lead levels among youth and adults in the United States: The National Health and Nutrition Examination Survey, 1999–2008. Prev. Chron. Dis. 2013. [Google Scholar] [CrossRef]
- Medline Plus. Available online: http://www.nlm.nih.gov/medlineplus/highbloodpressure.html. (accessed on 15 January 2015).
- Galán-Chilet, I.; Téllez-Plaza, M.; Guallar, E.; De Marco, G.; López-Izquierdo, R.; González-Manzano, I.; Carmen Tormos, M.; Martín-Núñez, G.M.; Rojo-Martínez, G.; Sáez, G.T.; et al. Plasma selenium levels and oxidative stress biomarkers: A gene-environment interaction population-based study. Free Radic. Biol. Med. 2014, 74, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.G.; Jamali, M.K.; Arain, M.B.; Sirajuddin; Baig, J.A.; Kandhro, G.A.; Wadhwa, S.K.; Shah, A.Q. Evaluation of cadmium, lead, nickel and zinc status in biological samples of smokers and nonsmokers hypertensive patients. J. Hum. Hypertens. 2010, 24, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Guichard, C.; Bachler, J.P. Relationship between oxidative stress and essential hypertension. Hypertens. Res. 2007, 30, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Singhal, U.; Hossain, M.M.; Islam, N.; Rizvi, I. The role of the endogenous antioxidant enzymes and malondialdehyde in essential hypertension. J. Clin. Diagn. Res. 2013, 7, 987–990. [Google Scholar] [PubMed]
- De Tursi, L.; Vázquez, A.; Vázquez, A.; Sáez, G.; Mahmoud, A.; Gumbau, V. Oxidative stress; a comparative study between normal and morbid obesity group population. Nutr. Hosp. 2013, 28, 671–675. [Google Scholar] [PubMed]
- Kowalska, K.; Zalewska, M.; Milnerowicz, H. The application of capillary electrophoresis in the determination of glutathione in healthy women’s blood. J. Chromatogr. Sci. 2014, 53, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Ma, Y.C.; Chen, P.C.; Wu, C.C.; Chen, Y.C. Oxidative stress of office workers relevant to tobacco smoking and inner air quality. Int. J. Environ. Res. Public Health. 2014, 11, 5586–5597. [Google Scholar] [CrossRef] [PubMed]
- Sciskalska, M.; Zalewska, M.; Grzelak, A.; Milnerowicz, H. The influence of the occupational exposure to heavy metals and tobacco smoke on the selected oxidative stress markers in smelters. Biol. Trace Elem. Res. 2014, 159, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chia, T.; Hsu, C.Y.; Chen, H.L. Oxidative damage of workers in secondary metal recovery plants affected by smoking status and joining the smelting work. Ind. Health. 2008, 46, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Fang, W.; Chia, S.L.; Leong, D.T. Nanotoxicology of common metal oxide based nanomaterials: Their ROS-y and non-ROS-y consequences. Asia Pac. J. Chem. Eng. 2013, 8, 205–217. [Google Scholar] [CrossRef]
- Chia, S.L.; Tay, C.Y.; Setyawati, M.I.; Leong, D.T. Biomimicry 3D gastrointestinal spheroid platform for the assessment of toxicity and inflammatory effects of zinc oxide nanoparticles. Small. 2015, 11, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Zhao, X.; Tan, E.C.; Khamis, N.; Assodani, A.; Xiong, S.; Ruedl, C.; Ng, K.W.; Loo, J.S. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch. Toxicol. 2011, 85, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Giovanni, M.; Tay, C.Y.; Setyawati, M.I.; Xie, J.; Ong, C.N.; Fan, R.; Yue, J.; Zhang, L.; Leong, D.T. Toxicity profiling of water contextual zinc oxide, silver, and titanium dioxide nanoparticles in human oral and gastrointestinal cell systems. Environ. Toxicol. 2014. [Google Scholar] [CrossRef]
- Ng, K.W.; Khoo, S.P.; Heng, B.C.; Setyawati, M.I.; Tan, E.C.; Zhao, X.; Xiong, S.; Fang, W.; Leong, D.T.; Loo, J.S. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials 2011, 32, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Effect of zinc oxide nanomaterials-induced oxidative stress on the p53 pathway. Biomaterials 2013, 34, 10133–10142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Zhu, K.; Dong, Y.H.; Sun, C.H. Effects of supplementation with multivitamin and mineral on blood pressure and C-reactive protein in obese Chinese women with increased cardiovascular disease risk. Asia Pac. J. Clin. Nutr. 2009, 18, 121–130. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Suárez-Varela, M.; Llopis-González, A.; González-Albert, V.; López-Izquierdo, R.; González-Manzano, I.; Cháves, J.; Huerta-Biosca, V.; Martin-Escudero, J.C. Correlation of Zinc with Oxidative Stress Biomarkers. Int. J. Environ. Res. Public Health 2015, 12, 3060-3076. https://doi.org/10.3390/ijerph120303060
Morales-Suárez-Varela M, Llopis-González A, González-Albert V, López-Izquierdo R, González-Manzano I, Cháves J, Huerta-Biosca V, Martin-Escudero JC. Correlation of Zinc with Oxidative Stress Biomarkers. International Journal of Environmental Research and Public Health. 2015; 12(3):3060-3076. https://doi.org/10.3390/ijerph120303060
Chicago/Turabian StyleMorales-Suárez-Varela, María, Agustín Llopis-González, Verónica González-Albert, Raúl López-Izquierdo, Isabel González-Manzano, Javier Cháves, Vicente Huerta-Biosca, and Juan C. Martin-Escudero. 2015. "Correlation of Zinc with Oxidative Stress Biomarkers" International Journal of Environmental Research and Public Health 12, no. 3: 3060-3076. https://doi.org/10.3390/ijerph120303060






