Hypertension and the Fat-Soluble Vitamins A, D and E
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
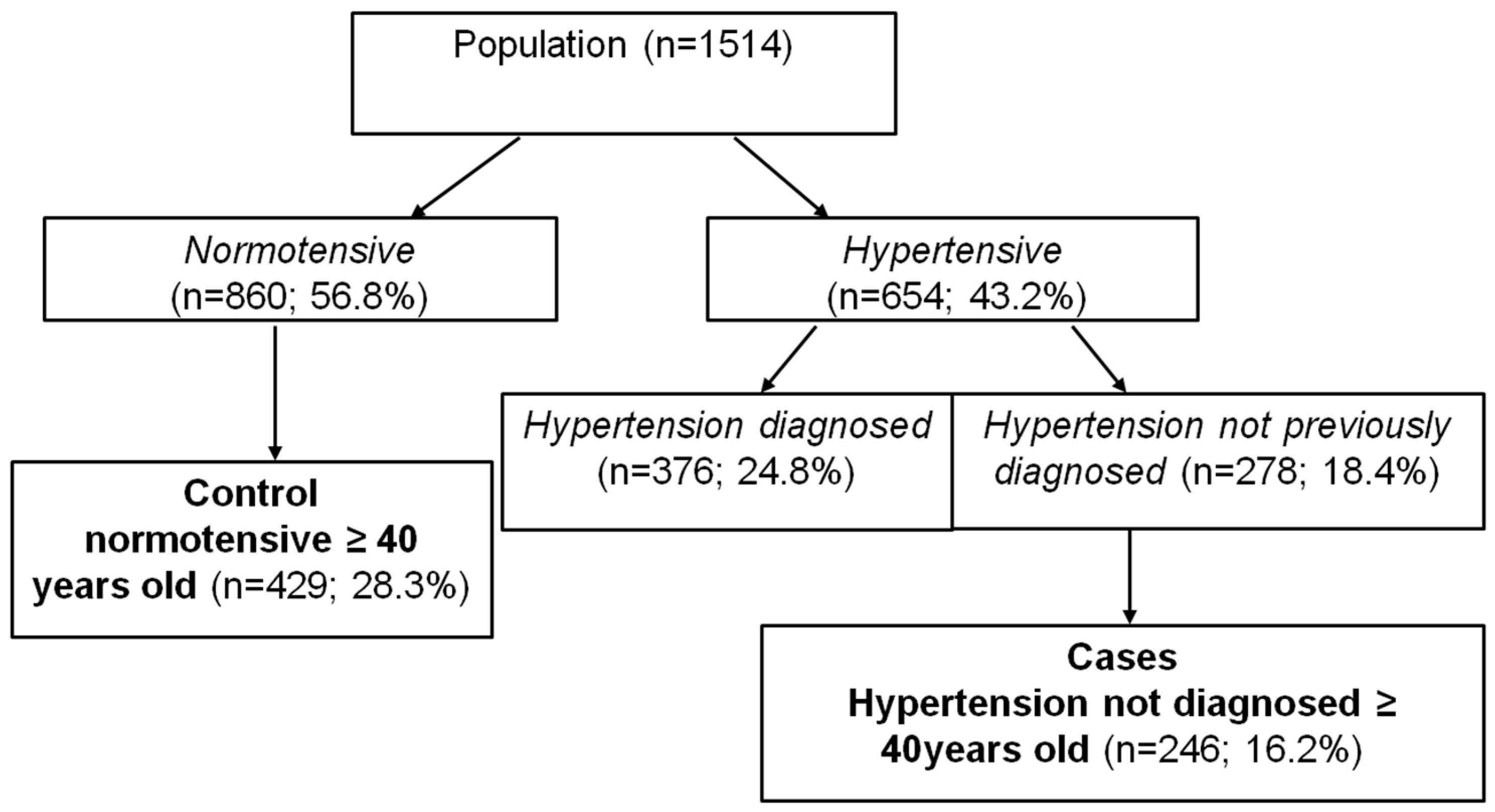
2.3. Patient Data
2.4. Epidemiological Analysis
| Gender | Age (years) | Vitamin A (µg) | Vitamin D (µg) | Vitamin E (µg) |
|---|---|---|---|---|
| Women | 40–49 | 800 | 5 | 8 |
| 50–59 | 800 | 5 | 8 | |
| 60–69 | 800 | 10 | 8 | |
| ≥70 | 700 | 15 | 10 | |
| Men | 40–49 | 1000 | 5 | 10 |
| 50–59 | 1000 | 5 | 10 | |
| 60–69 | 1000 | 10 | 10 | |
| ≥70 | 900 | 15 | 12 |
3. Results
3.1. General Characteristics and Anthropometrical Variables
| Variable | Non-AHT (n = 429; 63.6%) | AHT (n = 246; 36.4%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Women (n = 221; 51.5%) | Men (n = 208; 48.5%) | Women (n = 113; 45.9%) | Men (n = 133; 54.1%) | ||||||
| N | %/Mean ± SD | N | %/Mean ± SD | N | %/Mean ± SD | N | %/Mean ± SD | ||
| Age | 40–59 | 149 | 67.42 * | 128 | 61.54 * | 27 | 23.89 * | 47 | 35.34 * |
| 60–69 | 22 | 9.95 | 21 | 10.10 | 19 | 16.81 | 13 | 9.77 | |
| >70 | 50 | 22.62 * | 59 | 28.37 * | 67 | 59.29 * | 73 | 54.89* | |
| Weight (kg) | 62.21 ± 8.84 * | 201 | 76.44 ± 10.78 | 111 | 66.82 ± 12.96 * | 129 | 77.09 ± 12.01 | ||
| BMI | 25.36 ± 3.58 * | 208 | 26.75 ± 3.22 | 112 | 28.66 ± 4.99 * | 133 | 27.38 ± 3.57 | ||
| Waist/hip | 0.81 ± 0.09 | 206 | 0.94 ± 0.07 * | 110 | 0.84 ± 0.10 | 130 | 0.96 ± 0.06 * | ||
| Medication | |||||||||
| Anti-HTA | |||||||||
| No medication | 99.5 | 207 | 99.5 | 108 | 95.6 | 128 | 96.2 | ||
| On medication | 0.5 | 1 | 0.5 | 5 | 4.4 | 5 | 3.8 | ||
| IECAs | 0 | 0 | 0 | 4 | 80 | 4 | 80 | ||
| Diuretics | 100 | 1 | 100 | 1 | 20 | 1 | 20 | ||
| Anti-DM | |||||||||
| No medication | 99.1 | 199 | 95.7 | 103 | 91.2 | 126 | 94.7 | ||
| On medication | 0.9 | 9 | 0.3 | 10 | 8.8 | 7 | 5.3 | ||
| Oral antidiabetics | 50 | 4 | 44.4 | 4 | 40 | 3 | 42.9 | ||
| Insulin | 50 | 5 | 55.6 | 6 | 60 | 4 | 57.1 | ||
| Anti-hypolipidemics | |||||||||
| No medication | 97.3 | 196 | 94.2 | 104 | 92.0 | 123 | 92.5 | ||
| On medication | 2.7 | 12 | 5.8 | 9 | 8.0 | 10 | 7.5 | ||
3.2. Blood Pressure, Biochemical Parameters and Vitamins Intake
| Variable | Non-AHT (n = 429; 63.6%) | AHT (n = 246; 36.4%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Women (n = 221; 51.5%) | Men (n = 208; 48.5%) | Women (n = 113; 45.9%) | Men (n = 133; 54.1%) | |||||
| N | %/Mean ± SD | N | %/Mean ± SD | N | %/Mean ± SD | N | %/Mean ± SD | |
| Systolic blood pressure (mmHg) | 218 | 118.64 ± 12.11 * | 207 | 123.51 ± 10.44 * | 112 | 152.00 ± 15.42 * | 132 | 150.61 ± 15.90 * |
| Diastolic blood pressure (mmHg) | 218 | 75.70 ± 7.66 * | 207 | 76.41 ± 6.86 * | 112 | 87.30 ± 9.40 * | 132 | 87.13 ± 10.05 * |
| Triglycerides (mg/dL) | 221 | 142.04 ± 67.31 * | 208 | 212.02 ± 107.89 | 113 | 165.64 ± 85.30 * | 133 | 228.25 ± 152.83 |
| HDL cholesterol (mg/dL) | 221 | 59.11 ± 13.58 | 208 | 45.79 ± 11.88 | 113 | 56.25 ± 14.48 | 133 | 47.64 ± 12.24 |
| LDL cholesterol (mg/dL) | 221 | 122.94 ± 32.58 | 208 | 121.79 ± 33.06 | 113 | 128.98 ± 33.79 | 133 | 117.68 ± 35.03 |
| Total cholesterol (mg/dL) | 221 | 210.47 ± 37.35 | 208 | 209.98 ± 34.66 | 113 | 218.36 ± 36.01 | 133 | 210.97 ± 41.73 |
| Fasting glucose (mg/dL) | 221 | 88.77 ± 12.17 * | 208 | 92.30 ± 18.3 3* | 113 | 94.03 ± 14.40 * | 133 | 98.62 ± 24.94 * |
| Diabetes mellitus | 3 | 1.4 * | 11 | 5.3 * | 10 | 8.8 * | 15 | 11.3 * |
| Kidney damage | 5 | 2.3 * | 4 | 1.9 * | 10 | 9.0 * | 13 | 9.8 * |
| Metabolic syndrome | 25 | 11.3 * | 41 | 19.7 * | 40 | 35.4 * | 53 | 39.8 * |
| Hypertriglyceridemia | 108 | 50.5 | 108 | 55.1 | 51 | 49.0 | 58 | 47.2 |
| Hypercholesterolemia | 29 | 13.2 * | 38 | 18.3 | 27 | 23.9 * | 22 | 16.5 |
| Obesity | 35 | 17.0 * | 49 | 24.3 | 46 | 41.4 * | 35 | 26.7 |
| Overweight | 102 | 49.5 * | 151 | 74.8 | 85 | 76.6 * | 99 | 75.6 |
| Total energy (kcal/day) | 221 | 4311.22 ± 1546.59 | 208 | 4566.67 ± 1623.22 | 113 | 4650.59 ± 1470.62 | 133 | 4564.06 ± 1680.60 |
| VITAMINS: Vit. A (µg/day) | 221 | 1650.03 ± 908.85 * | 208 | 1661.76 ± 899.54 | 113 | 1977.00 ± 1016.82 * | 133 | 1533.96 ± 870.43 |
| Vit. D (µg/day) | 221 | 7.70 ± 9.76 | 208 | 8.68 ± 9.29 | 113 | 7.96 ± 9.93 | 133 | 8.27 ± 9.57 |
| Vit. E (µg/day) | 221 | 17.91 ± 8.22 | 208 | 19.27 ± 8.30 | 113 | 18.44 ± 7.48 | 133 | 19.12 ± 8.19 |
| Variable | Non-AHT (n = 429; 63.6%) | AHT (n = 246; 36.4%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Vitamin A | Vitamin D | Vitamin E | Vitamin A | Vitamin D | Vitamin E | |||
| N | Mean (µg) ± SD | Mean (µg) ± SD | Mean (mg) ± SD | N | Mean (µg) ± SD | Mean (µg) ± SD | Mean (mg) ± SD | |
| Diabetes mellitus | 14 | 2688.55 ± 2065.74 * | 16.61 ± 50.11 | 19.73 ± 9.25 | 35 | 1808.94 ± 820.34 * | 32.64 ± 64.98 | 21.80 ± 7.47 |
| Kidney damage | 9 | 868.85 ± 797.70 | 3.45 ± 3.61 | 10.11 ± 7.04 * | 23 | 2830.17 ± 5826.18 | 8.47 ± 12.12 | 17.56 ± 8.68 * |
| Metabolic syndrome | 66 | 2617.44 ± 4422.13 | 12.39 ± 29.83 | 19.40 ± 10.15 | 93 | 2100.90 ± 3068.78 | 13.14 ± 44.83 | 18.72 ± 12.86 |
| Hypertriglyceridemia | 216 | 2327.85 ± 3792.64 | 11.20 ± 27.83 | 18.95 ± 10.61 | 109 | 2149.84 ± 2876.60 | 18.01 ± 52.52 | 19.62 ± 12.32 |
| Hypercholesterolemia | 67 | 2170.14 ± 2712.68 | 10.027 ± 14.22 | 18.58 ± 11.14 | 49 | 2712.68 ± 3997.04 | 15.67 ± 40.59 | 20.41 ± 12.22 |
| Obesity | 84 | 1609.50 ± 2051.38 | 7.09 ± 10.80 * | 16.66 ± 8.34 | 81 | 2260.91 ± 3711.34 | 16.24 ± 40.14 * | 18.17 ± 9.39 |
| Overweight | 253 | 2200.86 ± 3547.93 | 11.64 ± 26.03 | 19.33 ± 10.78 | 184 | 2003.96 ± 2575.37 | 13.74 ± 31.56 | 18.35 ± 9.91 |
3.3. Prevalence of Comorbidities
3.4. Multivariate Analysis
| Variable | RDI | Lower RDI | Higher RDI | |
|---|---|---|---|---|
| Vitamin A | Non-AHT (n (%)) | 36 (60.0) | 34 (63.0) | 250 (60.8) |
| AHT (n (%)) | 24 (40.0) | 20 (37.0) | 161 (39.2) | |
| ORc (95%CI) | 1.00 (ref) | 0.97 (0.57–1.68) | 0.88 (0.41–1.88) | |
| ORa1 (95%CI) | 1.00 (ref) | 1.08 (0.59–1.99) | 0.91 (0.40–2.07) | |
| ORa2 (95%CI) | 1.00 (ref) | 1.17 (0.63–2.15) | 1.80 (0.86–3.74) | |
| ORa3 (95%CI) | 1.00 (ref) | 1.29 (0.65–2.54) | 1.55 (0.73–3.31) | |
| Vitamin D | Non-AHT (n (%)) | 23 (53.5) | 180 (57.9) | 118 (71.1) |
| AHT (n (%)) | 20 (46.5) | 131 (42.1) | 48 (28.9) | |
| ORc (95%CI) | 1.00 (ref) | 0.47 (0.24–0.93) * | 0.84 (0.44–1.59) | |
| ORa1 (95%CI) | 1.00 (ref) | 1.77 (0.83–3.80) | 1.71 (0.83–3.52) | |
| ORa2 (95%CI) | 1.00 (ref) | 1.85 (0.85–4.01) | 1.80 (0.86–3.74) | |
| ORa3 (95%CI) | 1.00 (ref) | 1.91 (0.86–4.24) | 1.55 (0.73–3.31) | |
| Vitamin E | Non-AHT (n (%)) | 19 (65.5) | 17 (60.7) | 240 (66.3) |
| AHT (n (%)) | 10 (34.5) | 11 (39.3) | 122 (33.7) | |
| ORc (95%CI) | 1.00 (ref) | 0.97 (0.44–2.14) | 1.23 (0.42–3.61) | |
| ORa1 (95%CI) | 1.00 (ref) | 0.90 (0.36–2.25) | 0.70 (0.20–2.39) | |
| ORa2 (95%CI) | 1.00 (ref) | 0.91 (0.36–2.26) | 0.73 (0.21–2.52) | |
| ORa3 (95%CI) | 1.00 (ref) | 1.04 (0.38–2.85) | 0.78 (0.22–2.76) | |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Banegas, J.R. El problema del control de la hipertensión en España. Hipertensión 2002, 19, 377–381. [Google Scholar]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet. 2005, 365, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of nutrition and nutraceutical supplements in the treatment of hypertension. World J. Cardiol. 2014, 6, 38–66. [Google Scholar] [CrossRef] [PubMed]
- Kones, R. Primary prevention of coronary heart disease: Integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Des. Dev. Ther. 2011, 5, 325–380. [Google Scholar] [CrossRef]
- Makrakis, J.; Zimanyi, M.A.; Black, M.J. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr. Nephrol. 2007, 22, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Lizuka, Y.; Imai, T.; Namiki, M. Sesame seed and its lignans produce marked enhancement of vitamin E activity in rats fed a low alpha-tocopherol die! Lipids. 1995, 30, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Cieslinski, D.A. Interaction between growth factors and retinoic acid in the induction of kidney tubulogenesis in tissue culture. Exp. Cell. Res. 1992, 201, 8–15. [Google Scholar] [CrossRef] [PubMed]
- European Society of Hypertension-European Society of Cardiology Guidelines Committee. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar]
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.R.; Cook, J.V.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Quadro, L.; Hamberger, L.; Gottesman, M.E.; Wang, F.; Colantuoni, V.; Blaner, W.S.; Mendelsohn, C.L. Pathways of vitamin A delivery to the embryo: Insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005, 146, 4479–4490. [Google Scholar] [CrossRef]
- Moise, A.R.; Noy, N.; Palczewski, K.; Blaner, W.S. Delivery of retinoid-based therapies to target tissues. Biochemistry 2007, 46, 4449–4458. [Google Scholar] [CrossRef] [PubMed]
- Merlet-Benichou, C.; Gilbert, T.; Muffat-Jolly, M.; Lelievre-Pegorier, M.; Leroy, B. Intrauterine growth retardation leads to a permanent nephron deficit in the offspring. Pediatr. Nephrol. 1994, 8, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.; Vehaskari, V.M. Low birth weight-associated adult hypertension in the rat. Pediatr. Nephrol. 2001, 16, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004, 80, 1678–1688. [Google Scholar]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur. J. Clin. Invest. 2005, 35, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhu, J.; Zhou, M.; Cui, L.; Yao, W.; Liu, Y. Meta-analysis of long-term vitamin D supplementation on overall mortality. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kienreich, K.; Grübler, M.; Tomaschitz, A.; Schmid, J.; Verheyen, N.; Rutters, F.; Dekker, J.M.; Pilz, S. Vitamin D, arterial hypertension & cerebrovascular disease. Indian J. Med. Res. 2001, 137, 669–679. [Google Scholar]
- Beveridge, L.A.; Witham, M.D. Vitamin D and the cardiovascular system. Osteoporos Int. 2013, 24, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; Pizot, C.; Mullie, P. Vitamin D status and ill health: A systematic review. Lancet Diabetes Endocrinol. 2014, 2, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Olivieri, O.; Girelli, D.; Guarini, P.; Carletto, A.; Corrocher, R. Anti-oxidant status and lipid peroxidation in patients with essential hypertension. J. Hypertens. 1998, 16, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Arayas, J.; Bachler, J.P. Decreace in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Varizi, N.D.; Wang, X.Q.; Oveisi, F.; Rad, B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension 2000, 36, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Wards, M.; Wilson, C.P.; Strain, J.J.; Horigan, G.; Scott, J.M.; McNulty, H. B-Vitamins, methylenetetrahydrofolate reductase (MTHFR) and hypertension. Int. J. Vitamin Nutr. Res. 2011, 81, 240–244. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Patel, C.J.; Okamura, T.; Chan, Q.; Brown, I.J.; Miura, K.; Ueshima, H.; Zhao, L.; Van Horn, L.; Daviglus, M.L.; et al. A nutrient-wide association study on blood pressure. Circulation. 2012, 126, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Varela, M.M.; Llopis-González, A.; González-Albert, V.; López-Izquierdo, R.; González-Manzano, I.; Cháves, J.; Biosca, V.H.; Martin-Escudero, J.C. Zinc and smoking habits in the setting of hypertension in a Spanish populations. Hypertens. Res. 2015, 38, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hervas, S.; Mansego, M.L.; de Marco, G.; Martinez, F.; Alonso, M.P.; Morcillo, S.; Rojo-Martinez, G.; Real, J.T.; Ascaso, J.F.; Redon, J.; et al. Polymorphisms of the UCP2 gene are associated with body fat distribution and risk of abdominal obesity in Spanish population. Eur. J. Clin. Invt. 2012, 42, 171–178. [Google Scholar] [CrossRef]
- World Health Organization—International Society of Hypertension. Guidelines for the Management of Hypertension. J. Hypertens. 1999, 17, 151–183. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Mañas, M.; Martínez de la Victoria, E. Nutrisalud-Alimentación Y Salud Program (Csg Software); Instute of Nutrition and Food Technology, University of Granada: Granada, Spain, 2007. [Google Scholar]
- Ortega, R.M.; Requejo, A.M.; Navia, B.; Lopez-Sobaler, A.M. Recommended Daily Intakes of Energy and Nutrients for the Spanish Population; Department of Nutrition, Universidad Complutense de Madrid: Madrid, Spain, 2004. [Google Scholar]
- The INTERSALT Cooperative Research Group. Sodium, potassium, body mass, alcohol and blood pressure: The INTERSALT study. J. Hypertens. 1988, 6, S584–S586. [Google Scholar]
- Dupe, V.; Matt, M.; Garnier, J.M.; Chambon, P.; Mark, M.; Ghyselinck, N.B. A new born lethal defect due to inactivation of retinaldehydedeshydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl. Acad. Sci. USA 2003, 100, 14036–14041. [Google Scholar] [CrossRef] [PubMed]
- Bhat, P.V.; Manolescu, D.C. Role of vitamin A on determining nephron mass and possible relationship to hypertension. J. Nutr. 2008, 138, 1407–1410. [Google Scholar] [PubMed]
- Pilz, S.; März, W.; Wellnitz, B.; Seelhorst, U.; Fahrleitner-Pammer, A.; Dimai, H.P.; Boehm, B.O.; Dobnig, H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J. Clin. Endocrinol. Metab. 2008, 93, 3927–3935. [Google Scholar] [CrossRef] [PubMed]
- Yankouskaya, L.; Snezhitskiy, V. Relationship between vascular endothelial function and level of vitamin D and parathyroid hormone in women with arterial hypertension. Pol. Arch. Med. Wewn. 2014, 124, 532–539. [Google Scholar] [PubMed]
- Pittas, A.G.; Chung, M.; Trikalinos, T.; Mitri, J.; Brendel, M.; Petel, K.; Lichtenstein, A.H.; Lau, J.; Balk, E.M. Systematic review: Vitamin D and cardiometabolic outcomes. Ann. Intern. Med. 2010, 152, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., III; Poster-Barriuso, R.; Dalal, D.; Reimersma, R.A.; Appel, L.J. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.T.; Mello, A.P.; Sanches, L.B.; Abdalla, D.S.; Damasceno, N.R. Is plasma alpha-tocopherol associated with electronegative LDL in obese adolescents? J. Nutr. Sci. Vitaminol. 2013, 59, 100–107. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, S.; Neal, B.; Rodgers, A. Hypertension time to move on. Lancet. 2005, 365, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llopis-González, A.; Rubio-López, N.; Pineda-Alonso, M.; Martín-Escudero, J.C.; Chaves, F.J.; Redondo, M.; Morales-Suarez-Varela, M. Hypertension and the Fat-Soluble Vitamins A, D and E. Int. J. Environ. Res. Public Health 2015, 12, 2793-2809. https://doi.org/10.3390/ijerph120302793
Llopis-González A, Rubio-López N, Pineda-Alonso M, Martín-Escudero JC, Chaves FJ, Redondo M, Morales-Suarez-Varela M. Hypertension and the Fat-Soluble Vitamins A, D and E. International Journal of Environmental Research and Public Health. 2015; 12(3):2793-2809. https://doi.org/10.3390/ijerph120302793
Chicago/Turabian StyleLlopis-González, Agustin, Nuria Rubio-López, Monica Pineda-Alonso, Juan Carlos Martín-Escudero, Felipe Javier Chaves, Maximino Redondo, and Maria Morales-Suarez-Varela. 2015. "Hypertension and the Fat-Soluble Vitamins A, D and E" International Journal of Environmental Research and Public Health 12, no. 3: 2793-2809. https://doi.org/10.3390/ijerph120302793






