Land-Use Change and Emerging Infectious Disease on an Island Continent
Abstract
:1. Introduction
2. Methods
2.1. Definitions and Boundaries
2.2. Sources of Data on EIDs, Study Procedures and Exclusions

3. Results and Discussion
3.1. Results
3.1.1. Results of Systematic Literature Review

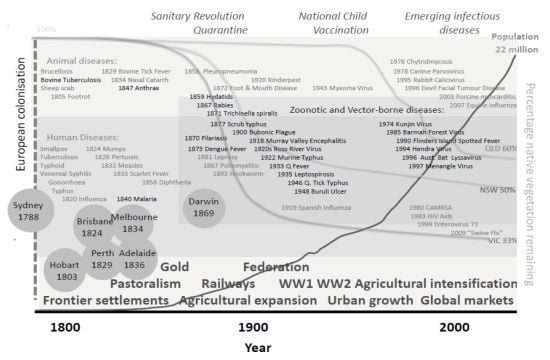
3.1.2. Historical Perspective

| Pathogen | Host | Location | LUCC associated with emergence described in literature |
|---|---|---|---|
| Origin: environment | |||
| Meliodosis Burkholderia pseudomallei * | H D W | NT (endemic) other foci e.g., s.w. WA | (4): Increased soil disturbance through gardening, farming soil cultivation, livestock, irrigation [36,41] |
| Buruli Ulcer * Mycobacterium ulcerans | H D W | Coastal Vic & Q | (5): Nutrient enrichment in coastal developments (e.g., golf courses, storm water drainage) [37,38] |
| Cryptococcosis Cryptococcus gattii * | H D W | Regional mainland Australia | (4): Plantations, residence adjacent to plantations or naturally occurring host trees (River Red Gums Eucalyptus camaldulensis) [42] |
| Photorabdus asymbotica | H | East coast towns, city Q, Vic, NSW | (4): Agricultural intensification: bacteria used in agricultural pest control [43] |
| Origin: human | |||
| Dengue virus Serotypes 1–3 * | H | Q (north coast and hinterland) | (5): Increased residential & urban development increases artificial habitat for vector (including in dry hinterland towns with irrigated gardens) [44] |
| Origin: Wildlife | |||
| Ross River Virus * | H D W | Expanded range Tas; urbanising Q, NSW, WA | (5): Creation of wetlands, reclamation or incorporation of coastal wetlands into residential developments, dryland salinity. Disease is becoming urban and involving urban adapted wildlife hosts [39,45,46,47] |
| Barmah Forest Virus * | H | NE to all states except Tas, SA. | (5): Believed similar to RRV including developments of artificial wetlands [44] |
| Murray Valley Encephalitis virus * | H | Endemic N WA, epidemic SE and SW | (5): Completion of the Argyle Dam in 1971, in Kimberleys, WA provided the opportunity for year round persistence and breeding of mosquitoes and water bird hosts, also increase in human population [48] |
| Flinders Island Spotted Fever Rickettsia honei * | H | Tas, SA, Q (Torres Straits Islands) | (5): Increased human-bush exposure through residential development [49,50] |
| Scrub typhus, Orientia tsuttsugamushi new strains * | H | Northern NT, WA | (1): Increased recreation access to remote (rainforest) locations [51,52] |
| Hendra virus (flying fox to horse to human) | H D | Q (coastal) | (4): Horse farms in traditionally fruit bat habitat areas. Changed virus ecology following urbanisation of fruit bats as natural habitat lost and degraded [53,54,55] |
| Australian Bat Lyssavirus (microbat to human) | H W | Q (coastal) | (5): Changed virus ecology following urbanisation of fruit bats as natural habitat lost and degraded [56,57] |
| Menangle virus (flying fox to pig to human) | H D | NSW (nr Sydney) | (4): intensive piggery in traditional fruit bat habitat areas. Urbanisation of fruit bats, deforestation may play some role [58] |
| Devil Facial Tumour Disease (Tasmanian devil) | W | Tas | (4): habitat converted to agricultural land, persecution leading to loss of genetic variability [59] |
| Amphibian Chytrid Fungus (frogs) | W | All states Tas (roads) | (5): Associated with dirt road construction/maintenance in Tas spreading infected amphibians, soil, and/or water [60] |
| Origin: Domestic animal | |||
| Hydatids E.granulosus (dog to kangaroo to human) | H W | WA Perth | (4); Residential and water catchment area confluence creating new predator-prey cycles [61] |
| H7 Highly Pathogenic Avian Influenza (poultry) | D | Vic, NSW, Qld | (4): Intensification of poultry farms [62] |
| Newcastle Disease virulent strains (poultry) | D | NSW, Qld | (4): Intensification of poultry farms. Contact with wild (water) birds suspected [63] |
Locations: Queensland (Q); New South Wales (NSW); Victoria (Vic); South Australia (SA); Tasmania (Tas); Northern Territory (NT); Western Australia (WA). Hosts: Human (H), Domestic (D), Wildlife (W). Vector-borne disease (*) Land use and cover change categorised as follows (after Lesslie et al., 2010):
| |||
3.2. Discussion
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Smolinski, M.S.; Hamburg, M.A.; Lederberg, J. Microbial Threats to Health: Emergence, Detection and Response; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Patz, J.; Daszak, P.; Tabor, G.; Aguirre, A.; Pearl, M.; Epstein, J.; Wolfe, N.; Kilpatrick, A.; Foufopoulos, J.; Molyneux, D.; Bradley, D.; Working Group on Land Use Change and Disease Emergence. Unhealthy landscapes: Policy recommendations on land use change and infectious disease emergence. Environ. Health Perspect. 2004, 112, 1092–1098. [Google Scholar] [CrossRef]
- Keesing, F.; Belden, L.; Daszak, P.; Dobson, A.; Harvell, C.D.; Holt, R.; Hudson, P.; Jolles, A.; Jones, K.; Mitchell, C.; Myers, S.; Bogich, T.; Ostfeld, R. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 2010, 468, 647–652. [Google Scholar] [CrossRef]
- FAO. The Future of Our Land-Facing the Challenge; Food and Agriculture Organization of the United Nations (FAO) in collaboration with the United Nations Environment Programme (UNEP): Rome, Italy, 1999. Available online: http://www.fao.org/docrep/004/X3810E/ x3810e00.htm (accessed on 1 June 2012).
- Ellis, E.C. Anthropogenic transformation of the terrestrial biosphere. Philos. Transact. R. Soc. London A 2011, 369, 1010–1035. [Google Scholar] [CrossRef]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–994. [Google Scholar] [CrossRef]
- Taylor, L.; Latham, S.; Woolhouse, M. Risk factors for human disease emergence. Philos. Trans. R. Soc. London B 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Myers, S.S.; Patz, J.A. Emerging threats to human health from global environmental change. Annu. Rev. Environ. Resour. 2009, 34, 223–252. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Disease, habitat fragmentation and conservation. Proc. R. Soc. B 2002, 269, 2041–2049. [Google Scholar]
- McFarlane, R.; Becker, N.; Field, H. Investigation of the climatic and environmental context of Hendra virus spillover events 1994–2010. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Bradley, D.J. An exploration of chronotones: A concept for understanding the health process of changing ecosystems. Ecohealth 2004, 1, 165–171. [Google Scholar] [CrossRef]
- Eisenberg, J.N.S.; Desai, M.A.; Levy, K.; Bates, S.J.; Liang, S.; Naumoff, K.; Scott, J.C. Environmental determinants of infectious disease: A framework for tracking causal links and guiding public health research. Environ. Health Perspect. 2007, 115, 1216–1223. [Google Scholar] [CrossRef]
- Forget, G.; Lebel, J. An ecosystem approach to human health. Int. J. Occup. Environ. Health 2001, 7, S3–S38. [Google Scholar]
- Pekkanen, J.; Pearce, N. Environmental epidemiology: Challenges and opportunities. Environ. Health Perspect. 2001, 109, 1–5. [Google Scholar] [CrossRef]
- McMichael, A.J. Prisoners of the proximate: Loosening the constraints on epidemiology in an age of change. Am. J. Epidemiol. 1999, 149, 887–897. [Google Scholar] [CrossRef]
- Susser, M.; Susser, E. Choosing the future for epidemiology II. From Black Box to Chinese Boxes and eco-epidemiology. Am. J. Public Health 1996, 86, 674–677. [Google Scholar]
- Wilcox, B.A.; Colwell, R.R. Emerging and re-emerging infectious diseases: Biocomplexity as an interdisciplinary paradigm. Ecohealth 2005, 2, 244–257. [Google Scholar] [CrossRef]
- Gilbert, M.; Xiao, X.; Chaitaweesub, P.; Kalpravidh, W.; Premashthira, S.; Boles, S.; Slingenbergh, J. Avian influenza, domestic ducks and rice agriculture in Thailand. Agr. Ecosyst. Environ. 2007, 119, 409–415. [Google Scholar] [CrossRef]
- Langlois, J.P.; Fahrig, F.; Merriam, G.; Artsob, H. Landscape structure influences continental distribution of hantavirus in deer mice. Landsc. Ecol. 2001, 16, 255–266. [Google Scholar] [CrossRef]
- Yan, L.; Ren, Y.-H.; Zhang, J.; Li, P.-L.; Wang, X.-L.; Mao, D.-Q.; Wang, S.-W.; Yang, W.-Z. Relationship between the incidence of HFRS and changes of land-use in Big Three Gorges area of Chongqing Municipality, China. Front. Med. China 2010, 4, 199–203. [Google Scholar] [CrossRef]
- Ostfeld, R.; Keesing, F. Biodiversity and disease risk: The case of Lyme disease. Conserv. Biol. 2000, 14, 722–728. [Google Scholar] [CrossRef]
- Eisenberg, J.N.S.; Cevallos, W.; Ponce, K.; Levy, K.; Bates, S.J.; Scott, J.C.; Hubbard, A.; Vieira, N.; Endara, P.; Espinel, M.; Trueba, G.; Riley, L.W.; Trostle, J. Environmental change and infectious disease: How new roads affect the transmission of diarrheal pathogens in rural Ecuador. Proc. Natl. Acad. Sci. 2006, 103, 19460–19465. [Google Scholar] [CrossRef]
- Wilcox, B.A.; Ellis, B. Forests and emerging infectious diseases of humans. (Forests and human health). Unasylva (English ed.) 2006, 57, 11–18. [Google Scholar]
- Sleigh, A.C. Water, Dams and Infections: Asian Challenges. In Population Dynamics and Infectious Diseases in Asia; Sleigh, A.C., Leng, C.H., Yeoh, B.S.A., Hong, P.K., Safman, R., Eds.; World Scientific Publishing Co.: Singapore, 2006. [Google Scholar]
- Hartson, R.; Orlofske, S.; Melin, V.; Dillon, R.; Johnson, P. Land use and wetland spatial position jointly determine amphibian parasite communities. EcoHealth 2011, 8, 485–500. [Google Scholar] [CrossRef]
- Patz, J.; Graczyk, T.; Geller, N.; Vittor, A. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- Bradshaw, C. Little left to lose: Deforestation and forest degradation in Australia since European colonization. Plant Ecol. 2012, 5, 109–120. [Google Scholar]
- IUCN Red List of Threatened Species. Available online: www.iucnredlist.org (accessed on 1 June 2012).
- Australian Bureau of Resource Sciences, Land Use in Australia at a Glance; Government Publishing Service: Canberra, Australia, 2006.
- Mackenzie, J. Emerging Diseases in the Australasian Region. In Emerging Infectious Diseases from the Global to the Local Perspective; Davis, J., Lederberg, J., Eds.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Beaman, M.H. Emerging Infections in Australia. Ann. Acad. Med. Singapore 1997, 26, 609–615. [Google Scholar]
- Leslie, R.; Thackway, R.; Smith, J. A National-Level Vegetation Assets, States and Transitions (VAST) Dataset for Australia (version 2.0); Australian Government Bureau of Rural Sciences: Canberra, Australia, 2010. [Google Scholar]
- Hobbs, R.J. Land Use Changes and Invasions. In Invasive Species in a Changing World; Mooney, H.A., Hobbs, R.J., Eds.; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- DeFries, R.; Foley, J.A.; Asner, G.P. Land use choices: Balancing human needs and ecosystem function. Front. Ecol. Environ. 2004, 2, 249–257. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef]
- Kaestli, M.; Mayo, M.; Harrington, G.; Ward, L.; Watt, F.; Hill, J.V.; Cheng, A.C.; Currie, B.J. Landscape changes influence the occurrence of the Melioidosis Bacterium Burkholderia pseudomallei in soil in Northern Australia. PLoS Negl. Trop. Dis. 2009, 3. [Google Scholar] [CrossRef]
- Veitch, M.; Johnson, P.; Flood, P.; Leslie, D.; Street, A.; Hayman, J. A large localized outbreak of Mycobacterium ulcerans infection on a temperate southern Australian island. Epidemiol. Infect. 1997, 119, 313–318. [Google Scholar] [CrossRef]
- Johnson, P.D.R.; Azuolas, J.; Lavender, C.J.; Wishart, E.; Stinear, T.P.; Hayman, J.A.; Brown, L.; Jenkin, G.A.; Fyfe, J.A.M. Mycobacterium ulcerans in mosquitoes captured during outbreak of buruli ulcer, Southeastern Australia. Emerg. Infect. Dis. 2007, 13, 1653–1660. [Google Scholar] [CrossRef]
- Carver, S.; Spafford, H.; Storey, A.; Weinstein, P. Dryland salinity and the ecology of Ross River Virus: The ecological underpinnings of the potential for transmission. Vect. Borne Zoonot. Dis. 2009, 9, 611–622. [Google Scholar]
- Australian Bureau of Resource Science, Land Use Change, Productivity and Diversification. Final Report of Theme 5.1 to the National Land & Water Resources Audit; Australian Government Printing Service: Canberra, Australia, 2001.
- Currie, B.J.; Fisher, D.A.; Howard, D.M.; Burrow, J.N.C.; Selvanayagam, S.; Snelling, P.L.; Anstey, N.M.; Mayo, M.J. The epidemiology of melioidosis in Australia and Papua New Guinea. Acta Tropica 2000, 74, 121–127. [Google Scholar]
- Sorrell, T.C. Cryptococcus neoformans variety gattii. Med. Mycol. 2001, 39, 155–168. [Google Scholar]
- Gerrard, J.; Waterfield, N.; Vohra, R.; Ffrench-Constant, R. Human infection with Photorhabdus asymbiotica: An emerging bacterial pathogen. Microb. Infect. 2004, 6, 229–237. [Google Scholar] [CrossRef]
- Russell, R.; Dwyer, D. Arboviruses associated with human disease in Australia. Microb. Infect. 2000, 2, 1693–1704. [Google Scholar] [CrossRef]
- Boyd, A.; Hall, R.A.; Gemmell, R.T.; Kay, B. Experimental infection of Australian brushtail possums, Trichosurus vulpecula (Phalangeridae: Marsupialia), with Ross River and Barmah Forest viruses by use of a natural mosquito vector system. Am. J. Trop. Med. Hyg. 2001, 65, 777–782. [Google Scholar]
- Lindsay, M. An outbreak of Ross River Disease in southwestern Australia. Emer. Infect. Dis. 1996, 2, 117–120. [Google Scholar]
- Brokenshire, T.; Symmonds, D.; Reynolds, R.; Doggett, S.; Geary, M.; Russell, R. A cluster of locally-acquired Ross River virus infection in outer Western Sydney. New South Wales Public Health Bulletin 2000, 11, 132–134. [Google Scholar]
- Marshall, I.D. Murray Valley Encephalitis. In Encyclopedia of Arthropod-Transmitted Infections of Man and Domesticated Animals; Service, M.W., Ashford, R.W., Eds.; CABI: London, UK, 2001; pp. 346–355. [Google Scholar]
- Unsworth, N.B.; Stenos, J.; Graves, S.R.; Faa, A.G.; Cox, G.E.; Dyer, J.R.; Boutlis, C.S.; Lane, A.M.; Shaw, M.D.; Robson, J.; Nissen, M.D. Flinders island spotted fever rickettsioses caused by “marmionii” strain of Rickettsia honei, Eastern Australia. Emer. Infect. Dis. 2007, 13, 566–573. [Google Scholar]
- Raoult, D.; Roux, V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 1997, 10, 694–719. [Google Scholar]
- Ralph, A.; Raines, M.; Whelan, P.; Currie, B. Scrub typhus in the Northern Territory: Exceeding the boundaries of Litchfield National Park. Commun. Dis. Intell. 2004, 28, 267–269. [Google Scholar]
- Odorico, D.; Graves, S.; Currie, B.; Catmull, J.; Nack, Z.; Ellis, S.; Wang, L.; Miller, D. New Orientia tsutsugamushi strain from scrub typhus in Australia. Emer. Infect. Dis. 1998, 4, 641–644. [Google Scholar] [CrossRef]
- Plowright, R.; Sokolow, S.; Gorman, M.; Daszak, P.; Foley, J.E. Causal inference in disease ecology: Investigating ecological drivers of disease emergence. Front. Ecol. Environ. 2008, 6, 420–429. [Google Scholar]
- Field, H.; Young, P.; Yob, J.M.; Mills, J.; Hall, L.; Mackenzie, J. The natural history of Hendra and Nipah Viruses. Microb. Infect. 2001, 3, 307–314. [Google Scholar] [CrossRef]
- Field, H. Ecology of Hendra Virus and Australian Bat Lyssavirus. Doctoral Thesis, University of Queensland, Brisbane, Australia, 2005. [Google Scholar]
- McCall, B.; Epstein, J.H.; Neill, A.S.; Heel, K.; Field, H.; Barrett, J.; Smith, G.A.; Selvey, L.A.; Rodwell, B.; Lunt, R. Potential human exposure to Australian bat Lyssa virus, Queensland, 1996–1999. Emer. Infect. Dis. 2000, 6, 259–264. [Google Scholar] [CrossRef]
- Hooper, P.T.; Lunt, R.A.; Gould, A.R.; Samaratunga, H.; Hyatt, A.D.; Gleeson, L.J.; Rodwell, B.J.; Rupprecht, C.E.; Smith, J.S.; Murray, P.K. A new lyssavirus—The first endemic rabies-related virus recognized in Australia. Bulletin de l’Institut Pasteur 1997, 95, 209–218. [Google Scholar] [CrossRef]
- Philbey, A.W.; Kirkland, P.D.; Ross, A.D.; Field, H.E.; Srivastava, M.; Davis, R.J.; Love, R. Infection with Menangle virus in flying foxes (Pteropus spp.) in Australia. Aust. Veter. J. 2008, 86, 449–454. [Google Scholar]
- Obendorf, D.; McGlashan, N. Research priorities in the Tasmanian devil facial tumour debate. Eur. J. Oncol. 2008, 13, 229–238. [Google Scholar]
- Pauza, M.; Driessen, M. Distribution and Potential Spread of Amphibian Chytrid Fungus Batrachochytrium Dendrobatidis in the Tasmanian World Heritage Area; Biodiversity Conservation Branch, Department of Primary Industries and Water: Tasmania, Australia, 2008. [Google Scholar]
- Jenkins, D.J.; Romig, T.; Thompson, R.C.A. Emergence/re-emergence of Echinococcus spp.—A global update. Int. J. Parasitol. 2005, 35, 1205–1219. [Google Scholar] [CrossRef]
- Westbury, H. History of Highly Pathogenic Avian Influenza in Australia and the H7N3 Outbreak 1995. In Proceedings of 4th International Symposium on Avian Influenza; U.S. Animal Health Association: Athens, GA, USA, 1998; pp. 22–30. [Google Scholar]
- Westbury, H. Newcastle disease virus: An evolving pathogen? Avian Pathol. 2001, 30, 5–11. [Google Scholar] [CrossRef]
- Gammage, B. The Biggest Estate on Earth: How Aborigines Made Australia; Allen and Unwin: Sydney, Australia, 2011. [Google Scholar]
- Henzell, T. Australian Agriculture:Its History and Challenges; CSIRO: Melbourne, Australia, 2007. [Google Scholar]
- Webb, S. Palaeopathology of Aboriginal Australians: Health and Disease across a Hunter-Gatherer Continent. In Infectious Disease; Cambridge University Press: Cambridge, UK, 2009; Chapter 6; pp. 125–161. [Google Scholar]
- Dowling, P.J. A Great Deal of Sickness—Introduced Diseases among the Aboriginal People of Colonial Southeast Australia 1788–1900. Doctoral Thesis, Australian National University, Canberra, Australia, 1997. [Google Scholar]
- Turner, A.J. Endemic disease control and regulation in Australia 1901–2010. Aust. Veter. J. 2011, 89, 413–421. [Google Scholar] [CrossRef]
- Turner, A.J. Disease control during the colonial period in Australia. Aust. Veter. J. 2011, 89, 239–242. [Google Scholar] [CrossRef]
- Cumpston, J.H.L.; Lewis, M.J. Health and Disease in Australia: A History; Australian Government Printing Service: Canberra, Australia, 1989; p. 450. [Google Scholar]
- Gandevia, B. The pattern of Australian medical history. Proc. R. Soc. Melbourne 1957, 50, 591–598. [Google Scholar]
- Lewis, M.J. The Peoples Health: Public Health in Australia 1788–1950; Praeger: Westport Co, Mayo, Ireland, 2003; p. 311. [Google Scholar]
- Fenner, F.; Rountree, P. The Early Days of Microbiology in Australia. In History of Microbiology in Australia; Fenner, F., Ed.; Australian Society for Microbiology, Brolga Press: Canberra, Australia, 1990; pp. 1–16. [Google Scholar]
- Heaslip, W. Tsutsugamushi fever in north Queensland, Australia. Med. J. Aust. 1941, 1, 380–392. [Google Scholar]
- Skerratt, L.; Campbell, N.J.; Murrell, A.; Walton, S.; Kemp, D.; Barker, S. The mitochondrial 12S gene is a suitable marker of populations of Sarcoptes scabiei from wombats, dogs and humans in Australia. Parasitol. Res. 2002, 88, 376–379. [Google Scholar] [CrossRef]
- Sherwin, W.; Timms, P.; Wicken, J.; Houlden, B. Analysis and conservation: Implications of Koala Genetics. Conserv. Biol. 2000, 14, 639–649. [Google Scholar]
- Thompson, R.C.A.; Kutz, S.J.; Smith, A. Parasite zoonoses and wildlife: Emerging issues. Int. J. Environ. Res. Public Health 2009, 6, 678–693. [Google Scholar] [CrossRef]
- Harley, D.; Sleigh, A.; Ritchie, S. Ross River virus transmission, infection and disease: A cross-disciplinary review. Clin. Microbiol. Infect. 2001, 14, 909–932. [Google Scholar]
- Graves, S.; Stenos, J. Rickettsioses in Australia. Ann. NY Acad. Sci. 2009, 1166, 151–155. [Google Scholar] [CrossRef]
- Emanuel, M.L.I.; Mackerras, I.M.; Smith, D.J.W. The epidemiology of leptospirosis in North Queensland: I. General survey of animal hosts. J. Hyg. 1964, 62, 451–484. [Google Scholar] [CrossRef]
- MacCallum, P.; Tolhurst, J.C. A new mycobacterial infection in man. I. Clinical aspects. II. Experimental investigations in laboratory animals. III. Pathology of the experimental lesions in the rat. IV. Cultivation of the new mycobacterium. J. Pathol. Biol. 1948, 60, 93–122. [Google Scholar] [CrossRef]
- Doherty, R.L. Arboviruses in Australia. Aust. Veter. J. 1972, 48, 172–180. [Google Scholar] [CrossRef]
- Russell, R.C.; Currie, B.J.; Lindsay, M.D.; Mackenzie, J.S.; Ritchie, S.A.; Whelan, P.I. Dengue and climate change in Australia: Predictions for the future should incorporate knowledge from the past. Med. J. Aust. 2009, 190, 265–268. [Google Scholar]
- Dale, P.; Carlson, D.; Easton, C. Four degrees of latitude: Mosquito control on the “Right” coasts of Australia and Florida, USA? J. Am. Mosquito Cont. Assoc. 2008, 24, 1–11. [Google Scholar] [CrossRef]
- McFarlane, R.A. Patterns of Emerging Infectious Disease and Ecological Change in Australia. In From Healthy Workers to a Healthy Planet; Butler, C., Capon, T., Dixon, J., Eds.; ANU ePress: Canberra, Australia, in press.
- Cleaveland, S.; Laurenson, M.K.; Taylor, L.H. Diseases of humans and their domestic mammals: Pathogen characteristics, host range and the risk of emergence. Phil. Transact. R. Soc. London B Biol. Sci. 2001, 356, 991–999. [Google Scholar]
- Guernier, V.; Hochberg, M.; Guégan, J. Ecology drives the worldwide distribution of human diseases. PLoS Biology 2004, 2. [Google Scholar] [CrossRef] [Green Version]
- Noble, I.; Barson, M.; Dumsday, R.; Friedel, M.; Hacker, R.; McKenzie, N.; Smith, G.; Young, M.; Maliel, M.; Zammit, G. Land Resources; Environment Australia: Canberra, Australia, 1996. [Google Scholar]
- Australian Bureau of Statistics. A Picture of the Nation; Australian Government Printing Service: Canberra, Australia, 2006. Available online: http://www.abs.gov.au/AUSSTATS/[email protected]/ DetailsPage/2070.02006?OpenDocument (accessed on 1 June 2012).
- Plowright, R.K.; Foley, P.; Field, H.E.; Dobson, A.P.; Foley, J.E.; Eby, P.; Daszak, P. Urban habituation, ecological connectivity and epidemic dampening: The emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B 2011, 278, 3703–3712. [Google Scholar] [CrossRef]
- Hall, L.; Richards, G. Flying Foxes Fruit and Blossum Bats of Australia; UNSW Press: Sydney, Australia, 2000; p. 135. [Google Scholar]
- Russell, R. Constructed wetlands and mosquitoes: Health hazards and management options—An Australian perspective. Ecol. Engineer. 1999, 12, 107–124. [Google Scholar] [CrossRef]
- McFarlane, R.A.; Sleigh, A.; McMichael, A.J. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian-Australasian Region. EcoHealth 2012, 9, 24–35. [Google Scholar] [CrossRef]
- Calaby, J.H.; Grigg, G.C. Changes in Macropodoid Communities and Populations in the Past 200 Years, and the Future. In Kangaroos, Wallabies and Rat-Kangaroos; Grigg, G., Jarman, P., Hume, I., Eds.; Surrey Beatty and Sons: Sydney, Australia, 1989; Vol. 2, pp. 813–820. [Google Scholar]
- Glass, K. Ecological mechanisms that promote arbovirus survival: A mathematical model of Ross River virus transmission. Transact. R. Soc. Trop. Med. Hyg. 2005, 99, 252–260. [Google Scholar] [CrossRef]
- Bradford-Hill, A. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar]
- Parmesan, C.; Duarte, C.; Poloczanska, E.; Richardson, A.J.; Singer, M.C. Overstretching attribution. Nature Clim. Change 2011, 1, 2–4. [Google Scholar] [CrossRef]
- Arinaminpathy, N.; McLean, A.R.; Godfray, H.C.J. Future UK land use policy and the risk of infectious disease in humans, livestock and wild animals. Land Use Policy 2009, 26, S124–S133. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McFarlane, R.A.; Sleigh, A.C.; McMichael, A.J. Land-Use Change and Emerging Infectious Disease on an Island Continent. Int. J. Environ. Res. Public Health 2013, 10, 2699-2719. https://doi.org/10.3390/ijerph10072699
McFarlane RA, Sleigh AC, McMichael AJ. Land-Use Change and Emerging Infectious Disease on an Island Continent. International Journal of Environmental Research and Public Health. 2013; 10(7):2699-2719. https://doi.org/10.3390/ijerph10072699
Chicago/Turabian StyleMcFarlane, Rosemary A., Adrian C. Sleigh, and Anthony J. McMichael. 2013. "Land-Use Change and Emerging Infectious Disease on an Island Continent" International Journal of Environmental Research and Public Health 10, no. 7: 2699-2719. https://doi.org/10.3390/ijerph10072699