Purification and Characterization of a Bifunctional Alginate Lyase from Pseudoalteromonas sp. SM0524
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Screening and identification of strain SM0524
2.3. Production and purification of the alginate lyase aly-SJ02
2.4. Protein determination and enzyme assay
2.5. Characterization of the alginate lyase aly-SJ02
2.6. Hydrolysis of polyM, polyG and sodium alginate by aly-SJ02
3. Results
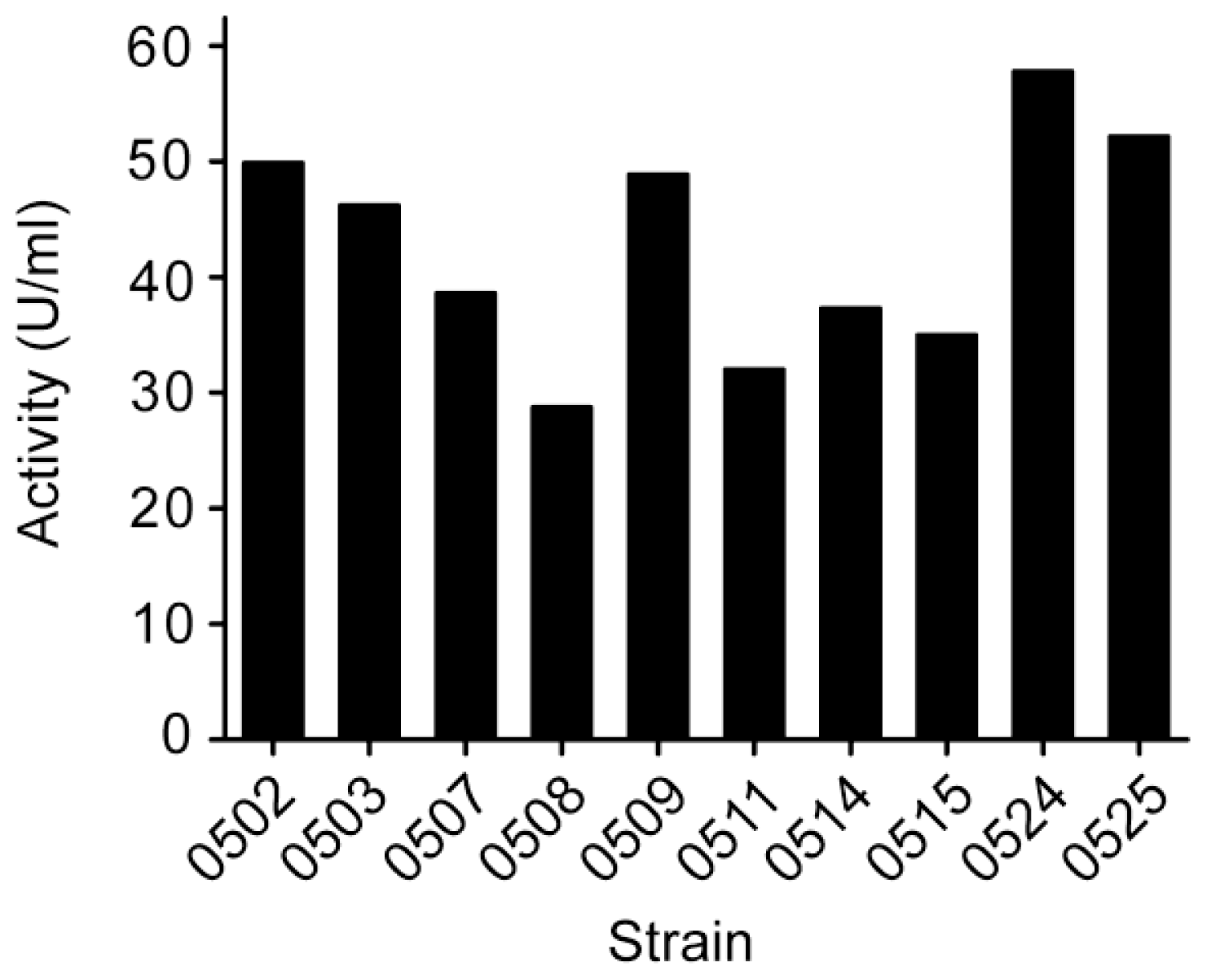
3.1. Screening and identification of strain Pseudoalteromonas sp. SM0524
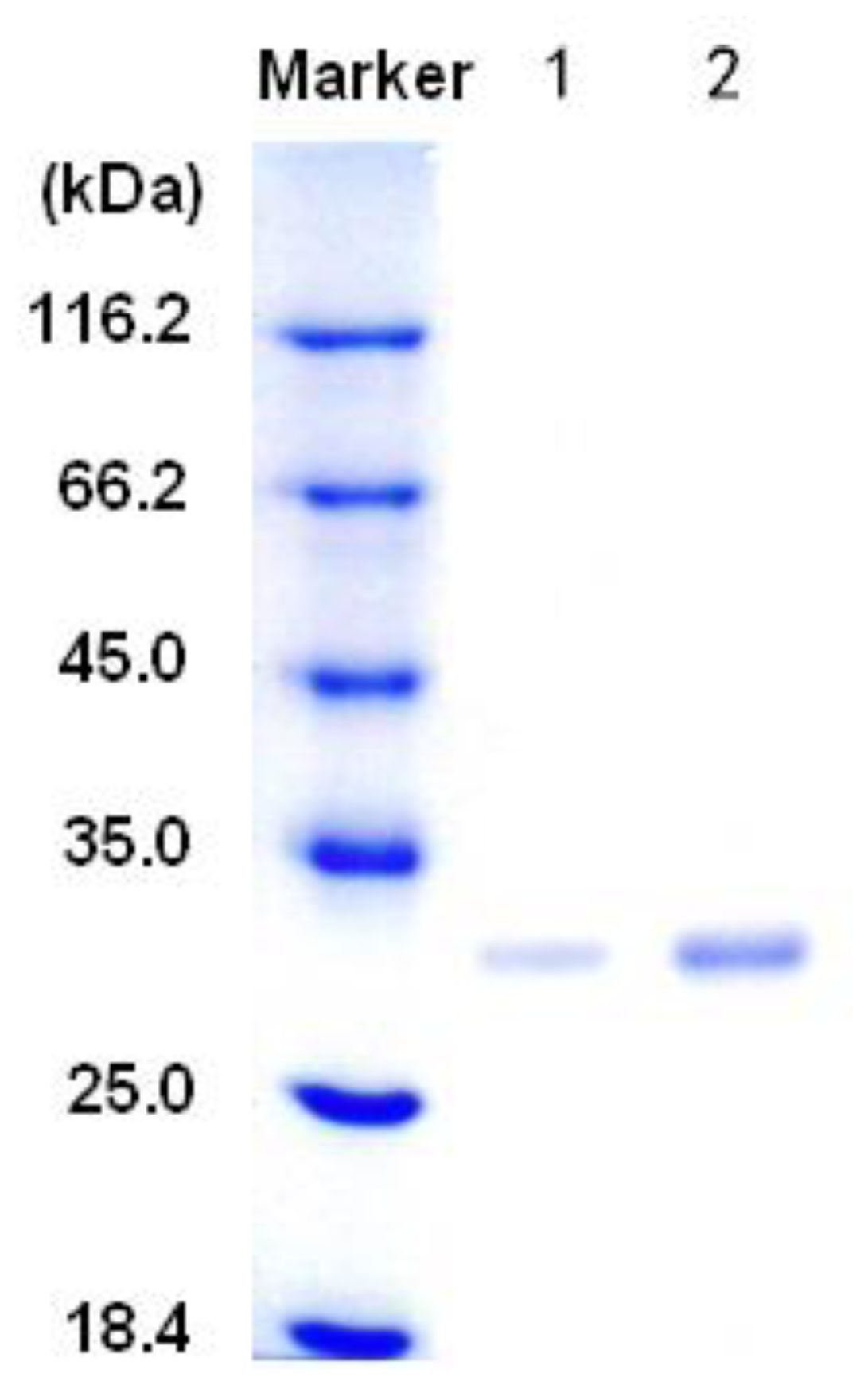
3.2. Purification of the alginate lyase aly-SJ02 from Pseudoalteromonas sp. SM0524
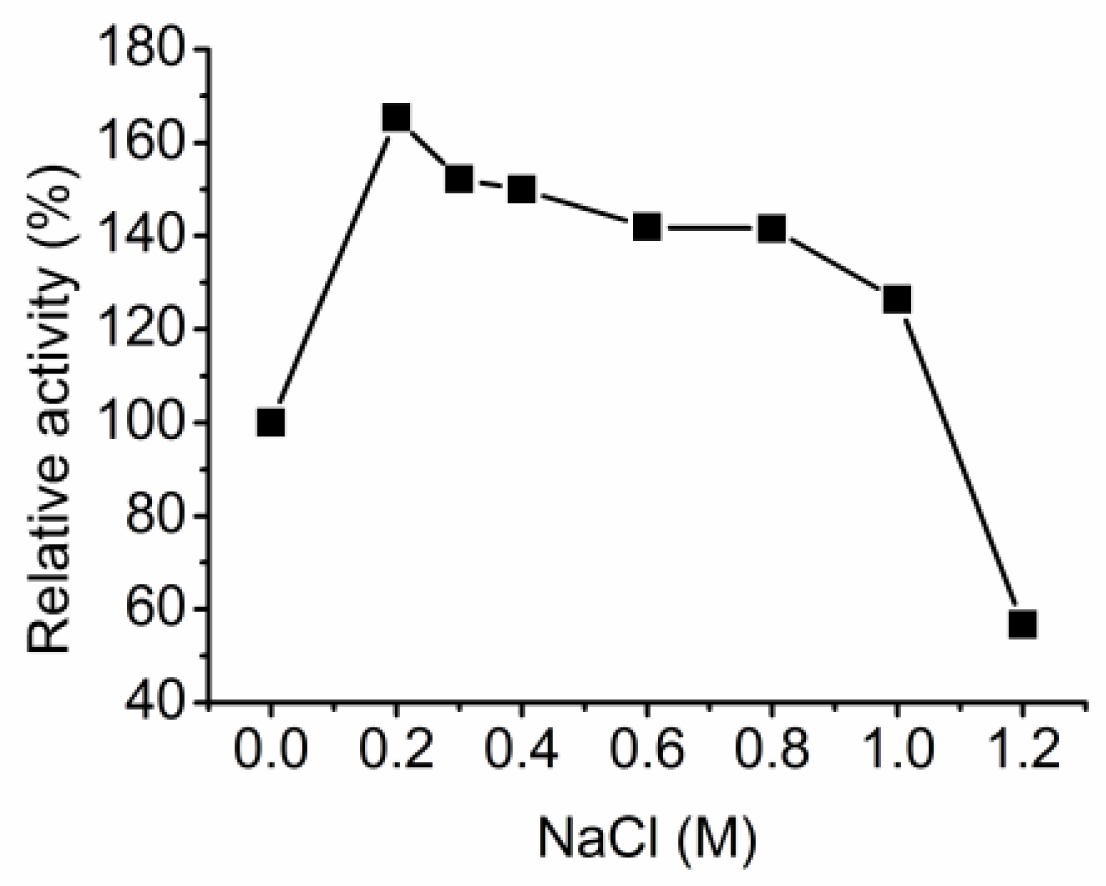
3.3. Characterization of the alginate lyase aly-SJ02 from Pseudoalteromonas sp. SM0524
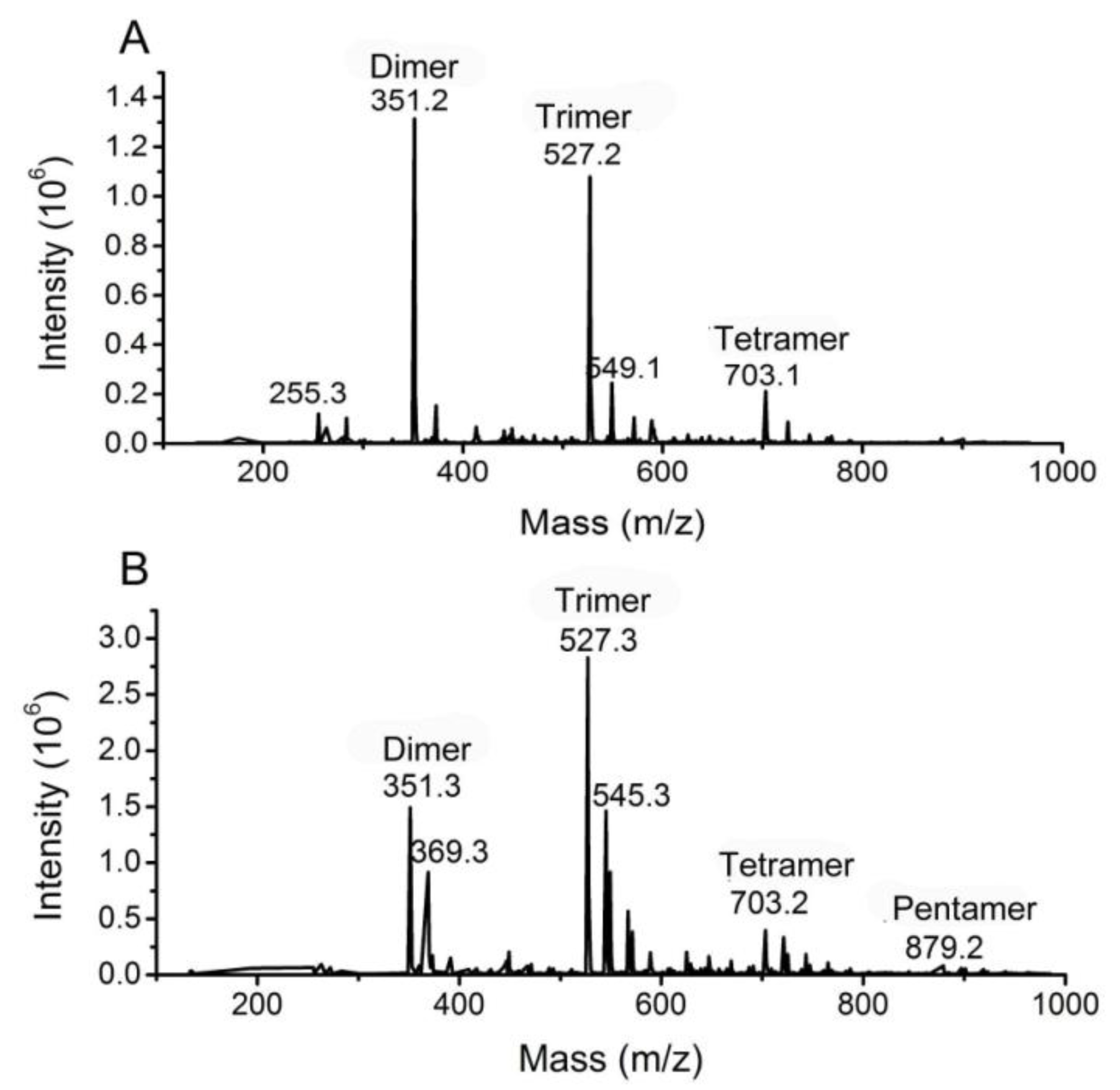
3.4. Analysis of the oligomers released from polyM, polyG and sodium alginate by aly-SJ02
4. Discussion
5. Conclusions
Supplementary Data
Purification of aly-SJ02 by ion-exchange chromatography on a DEAE-Sepharose Fast Flow column.
Purification of aly-SJ02 by gel filtration chromatography on a Sephadex G-100 column.
Figure S4
Secondary mass spectra of the dimers (A) and trimers (B) shown in Figure 6.
Acknowledgements
- Samples Availability: Available from the authors.
References
- Wong, TY; Preston, LA; Schiller, NL. Alginate lyase: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu Rev Microbiol 2000, 54, 289–340. [Google Scholar]
- Gacesa, P. Alginates. Carbohydr Polym 1988, 8, 161–182. [Google Scholar]
- Iwamoto, Y; Iriyama, K; Osatomi, K; Oda, T; Muramatsu, T. Primary structure and chemical modification of some amino acid residues of bifunctional alginate lyase from a marine bacterium Pseudoalteromonas sp. strain No. 272. J Prot Chem 2002, 21, 455–463. [Google Scholar]
- Osawa, T; Matsubara, Y; Muramatsu, T; Kimura, M; Kakuta, Y. Crystal structure of the alginate (poly-α-L-guluronate) lyase from Corynebacterium sp. at 1.2 Å resolution. J Mol Biol 2005, 345, 1111–1118. [Google Scholar]
- Schlesner, H; Bartels, C; Sittig, M; Dorsch, M; Stackebrandt, E. Taxonomic and phylogenetic studies on a new taxon of budding, hyphal Proteobacteria, Hirschia baltica gen. nov., sp. nov. Int J Syst Bacteriol 1990, 40, 443–451. [Google Scholar]
- Hu, ZY; Li, Y. Pseudidiomarina sediminum sp. nov., a marine bacterium isolated from coastal sediments of Luoyuan Bay in China. Int J Syst Evol Microbiol 2007, 57, 2572–2577. [Google Scholar]
- Laemmli, UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J Biol Chem 1952, 195, 19–23. [Google Scholar]
- Gacesa, P. Enzymic degradation of alginates. Int J Biochem 1992, 24, 545–552. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- Chen, XL; Zhang, YZ; Gao, PJ; Luan, XW. Two different proteases produced by a deep-sea psychrotrophic strain Pseudoaltermonas sp. SM9913. Mar. Biol 2003, 143, 989–993. [Google Scholar]
- Thompson, JD; Gibson, TJ; Plewniak, F; Jeanmougin, F; Higgins, DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997, 25, 4876–4882. [Google Scholar]
- Sawabe, T; Ohtsuka, M; Yoshio, E. Novel alginate lyases from marine bacterium Alteromonas sp. strain H-4. Carbohydr Res 1997. [Google Scholar]
- Iwamoto, Y; Araki, R; Iriyama, K; Oda, T; Fukuda, H; Hayashida, S; Muramatsu, T. purification and characterization of bifunctional alginate lyase from Pseudoalteromonas sp. strain No. 272 and its action on saturated oligomeric substrates. Biosci Biotechnol Biochem 2001, 65, 133–142. [Google Scholar]
- Matsushima, R; Danno, H; Uchida, M; Ishihara, K; Suzuki, T; Kaneniwa, M; Ohtsubo, Y; Nagata, Y; Tsuda, M. Analysis of extracellular alginate lyase and its gene from a marine bacterial strain, Pseudoalteromonas atlantica AR06. Appl Microbiol Biotechnol 2010, 86, 567–576. [Google Scholar]
- Natsumc, M; Kamo, Y; Hirayama, M; Adachi, T. Isolation and characterization of alginate-derived oligosaccharides with root growth-promoting activities. Carbohydr Res 1994, 258, 187–197. [Google Scholar]
- Tomoda, Y; Umemura, K; Adachi, T. Promotion of barley root elongation under hypoxic conditions by alginate lyase-lysate. Biosci Biotechnol Biochem 1994, 58, 202–203. [Google Scholar]
- Yonemoto, Y; Tanaka, H; Yamashita, T; Kitabatake, N; Ishida, Y. Promotion of germination and shoot elongation of some plants by alginate oligomers prepared with bacterial alginate lyase. J Ferment Bioeng 1993, 75, 68–70. [Google Scholar]
- Akiyama, H; Endo, T; Nakakita, R; Murata, K; Yonemoto, Y; Okayama, K. Effect of depolymerized alginates on the growth of bifidobacteria. Biosci Biotechnol Biochem 1992, 56, 355–56. [Google Scholar]
- Murata, K; Inose, T; Hisano, T; Abe, S; Yonemoto, Y. Bacterial alginate lyase: enzymology, genetics and application. J Ferment Bioeng 1993, 76, 427–37. [Google Scholar]
- Kawada, A; Hiura, N; Shiraiwa, M; Tajima, S; Hiruma, M. Stimulation of human keratinocyte growth by alginate oligosaccharides, a possible co-factor for epidermal growth factor in cell culture. FEBS Lett 1997, 408, 43–46. [Google Scholar]
- Fujihara, M; Nagumo, T. The effect of the content of D-mannuronic acid and L-guluronic acid blocks in alginates on antitumor activity. Carbohydr Res 1992, 224, 343–347. [Google Scholar]
- Fujihara, M; Nagumo, T. An influence of the structure of alginate on the chemotactic activity of macrophages and the antitumor activity. Carbohydr Res 1993, 243, 211–216. [Google Scholar]
- Otterlei, M; Østgaard, K; Sjåk-Bræk, G; Smidsrød, O; Soon-Shoing, ET. Induction of cytokine production from human monocytes stimulated with alginate. J Immunother 1991, 10, 286–291. [Google Scholar]
Abbreviations
| G | α-l-guluronic acid |
| M | β-d-mannuronic acid |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PL | polysaccharide lyase family |
| TLC | thin layer chromatography |


| Purification step | Total volume (mL) | Total protein (mg) | Total activity (U) | Specific activity (U/mg) | Purification fold | Yield (%) |
|---|---|---|---|---|---|---|
| Culture supernatant | 130 | 124.5 | 8139.3 | 65.4 | 1 | 100 |
| Ion exchange | 36 | 2.916 | 6536.5 | 2241.6 | 34.3 | 80.3 |
| Gel filtration | 15 | 0.765 | 3674.1 | 4802.7 | 73.4 | 45.1 |
| Metal ion (mM) | Relative activity a (%) | Metal ion/EDTA (mM) | Relative activity (%) |
|---|---|---|---|
| control | 100 | K+ (10) | 117.0 |
| Ba2+ (1) | 143.6 | Mn2+ (1) | 115.6 |
| Na+ (10) | 143.4 | Ni2+ (1) | 110.5 |
| Ca2+ (1) | 136.0 | Zn2+ (1) | 100.6 |
| Mg2+ (10) | 125.7 | Cu2+ (1) | 98.9 |
| Sr2+ (1) | 124.1 | Sn2+ (1) | 95.3 |
| Co2+ (1) | 122.7 | EDTA (1) | 48.3 |
| Substrate | sodium alginate | polyG | polyM |
|---|---|---|---|
| Specific activitya (U/mg) | 4802.7 | 3073.7 | 4153.8 |
| Kmb (mg/mL) | 1.086 | 0.465 | 2.751 |
| Vmaxb (OD235/h) | 8.074 | 5.318 | 7.131 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, J.-W.; Dong, S.; Song, J.; Li, C.-B.; Chen, X.-L.; Xie, B.-B.; Zhang, Y.-Z. Purification and Characterization of a Bifunctional Alginate Lyase from Pseudoalteromonas sp. SM0524. Mar. Drugs 2011, 9, 109-123. https://doi.org/10.3390/md9010109
Li J-W, Dong S, Song J, Li C-B, Chen X-L, Xie B-B, Zhang Y-Z. Purification and Characterization of a Bifunctional Alginate Lyase from Pseudoalteromonas sp. SM0524. Marine Drugs. 2011; 9(1):109-123. https://doi.org/10.3390/md9010109
Chicago/Turabian StyleLi, Jian-Wei, Sheng Dong, Jie Song, Chun-Bo Li, Xiu-Lan Chen, Bin-Bin Xie, and Yu-Zhong Zhang. 2011. "Purification and Characterization of a Bifunctional Alginate Lyase from Pseudoalteromonas sp. SM0524" Marine Drugs 9, no. 1: 109-123. https://doi.org/10.3390/md9010109




