Abstract
The well-known fatty acids with a Δ5,9 unsaturation system were designated for a long period as demospongic acids, taking into account that they originally occurred in marine Demospongia sponges. However, such acids have also been observed in various marine sources with a large range of chain-lengths (C16–C32) and from some terrestrial plants with short acyl chains (C18–C19). Finally, the Δ5,9 fatty acids appear to be a particular type of non-methylene-interrupted fatty acids (NMA FAs). This article reviews the occurrence of these particular fatty acids in marine and terrestrial organisms and shows the biosynthetic connections between Δ5,9 fatty acids and other NMI FAs.
1. Introduction
The well-known notion of demospongic acid appeared for the first time in 1976 in a historical paper from Litchfield and Morales [1], but at that time only as «demospongiae fatty acids». In another paper published in 1980 [2], Litchfield et al. used the term «demospongic fatty acids» probably for the first time. Since then and up until now [3–5], this term has widely been used. However, about 35 years after Litchfield’s work on sponge lipids, the notion of demospongic acid seems to no longer have significance, mainly due to their controversial definition and to their wide distribution among marine invertebrates and some terrestrial plants. At the time, it seemed to be of interest to precisely identify the function of demospongic acids, in consideration of their biological activities as topoisomerase inhibitors or against cancer cells as recently reviewed [6], whereas the biological interests of terrestrial short-chain Δ5,9 fatty acids (FAs) had already been demonstrated [7,8].
2. What Exactly Is a Demospongic Acid?
The definition of a demospongic acid has never been very clear [1,2]. In their first papers, Litchfield et al. only mentioned very long-chain C24–C30 or C24–C34 acids with the unusual 5,9 unsaturation pattern, but at that time, only fatty acids with an even number of carbons had been found [1,9]. In the 1980s, a lot of work was published on sponge FAs, and it became apparent that “demospongic acids” also contained all odd FAs from C23–C31 [10]. Within this field of research, a consensus was quickly established that agreed that demospongic acids were very long-chain fatty acids, mainly C24–C30, with the atypical 5,9-diunsaturation system, independent of the total number of double bonds. Some years later—and due to many papers being devoted to FAs from sponges—it appeared that:
- the distinction between long-chain fatty acids (LCFAs, C20–C22 ?) and very long-chain FAs (VLCFAs, ≥C23 ?) is not clear and often depends on the authors’ interpretation [10–17].
- the presence of the ever-mentioned 5,9-diunsaturation pattern cannot be considered as characteristic of “demospongic acids” due to the elongation process during their biosynthetic pathways, and diunsaturations such as 5,9-, 7,11-, 9,13-, 11,15-, 17,21-, 19,23- 21,25- and 23,27- can be considered as being similar [18], but other dienoic patterns with short chains such as 6,11-18:2 and 6,11-20:2 have also been considered as “demospongic” acids [19]. Furthermore, several “demospongic acids” display E and Z configurations for Δ5 and Δ9 double bonds [20].
Currently, the best definition for a demospongic acid would be that of Christie [18], stating “bis-methylene-interrupted cis-double bonds, ranging in chain-length from C16 to C34 with a cis, cis-dienoic system, either with the double bonds in position 5 and 9, or derived from 5,9-16:2 by chain elongation”.
At present, the question is whether such acids are not at all specific to demosponges, but have been found in other groups of sponges, especially among hexactinellida, in different phyla of marine invertebrates and in several species of terrestrial plants, especially conifers, and in some species of Apocynaceae, Malvaceae, and Ranunculaceae.
3. Occurrence of “Demospongic” Acids among Other Organisms
Table 1 presents a non-exhaustive list of more than 40 FAs that correspond to Christie’s definition of demospongic acids found in microorganisms, marine invertebrates and terrestrial plants. A particularly interesting point is the presence of 6-Br-5,9-FAs that are very common in demosponges but quite rare in other organisms. To the best of our knowledge only some Cnidaria Hexacorallia were shown to contain these brominated FAs [21–23], which prove the existence of bromoperoxidases in this group of Cnidaria since it has been proved that these brominated demospongic acids are synthesized by the sponge itself in the final stage of biosynthesis [24].

Table 1.
Occurrence of “demospongic”* acids in organisms that are not demosponges.
4. Towards a Classification of Non-Methylene-Interrupted Fatty Acids?
Demospongic acids represent a particular type of non-methylene-interrupted FA and, according to Christie’s definition, it could be interesting to consider at least three classes of non-methylene-interrupted fatty acids (NMI FAs) depending on the number of methylene groups situated between the two first double bonds. Then, group 1 would contain all bis-methylene-interrupted cis-double bonds and would correspond to the series 5,9; 7,11; 9,13… dienoic or polyenoic acids (demospongic acids). Group 2 would be that of tetra-methylene-interrupted cis-double bonds and would contain the series 5,11; 7,13; 9,15… NMI FAs, such as the acids 7,13-20:2 found in the Brittle star (Echinoderm, Ophiuroidea) Ophiura sarsi [37] and in the maritime pine Pinus pisaster [29], or the acid 7,13-22:2 found in the sponge Petrosia ficiformis [38]. Finally, group 3 would contain hexamethylene-interrupted cis-double bonds corresponding to the series 5,13; 7,15; 9,17… NMI FAs, such as the acid 7,15-20:2 found in the sponge Dysidea fragilis [39]. Some other acids of these three groups have been identified in marine invertebrates, especially molluscs and arthropods, and in numerous terrestrial plants, especially gymnosperms, and all of them can be deduced from accepted biosynthetic pathways implying elongases and 5- and 9-desaturases. Figures 1 and 2 give an overview of these putative biosyntheses from palmitic acid (16:0), palmitoleic acid (9-16:1) and linoleic acid (9,12-18:2). These schemes are currently used and have appeared recently in several publications, along with recent reviews on elongases and polyketide synthases [3,9,10,12,40–44].
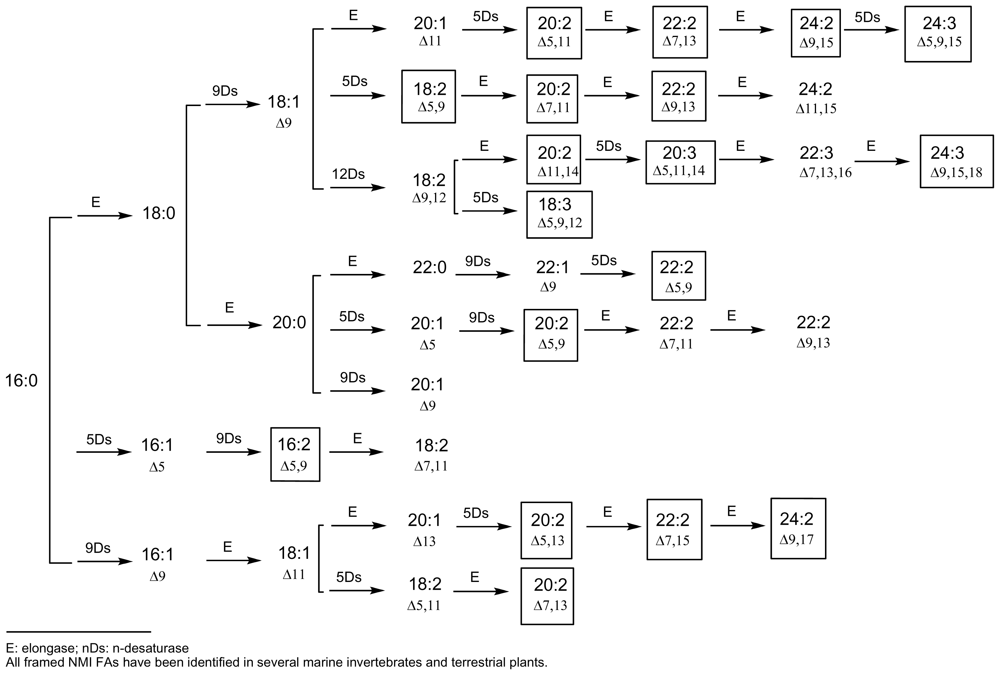
Figure 1.
Currently accepted pathways for the main long-chain NMI FAs (≤C24)E: elongase; nDs: n-desaturaseAll framed NMI FAs have been identified in several marine invertebrates and terrestrial plants.
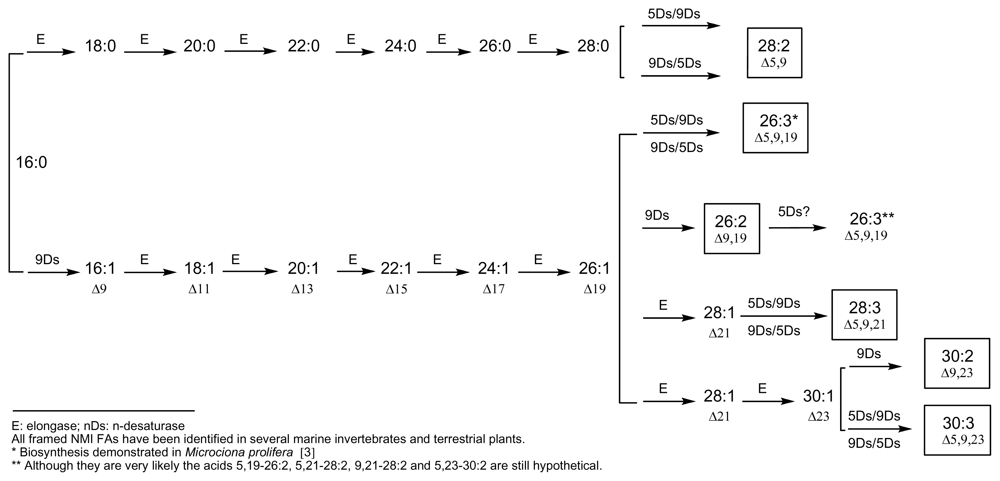
Figure 2.
Currently accepted pathways for the main very long-chain NMI FAs (>C24)E: elongase; nDs: n-desaturaseAll framed NMI FAs have been identified in several marine invertebrates and terrestrial plants.*Biosynthesis demonstrated in Microciona prolifera [3]**Although they are very likely the acids 5,19-26:2, 5,21-28:2, 9,21-28:2 and 5,23-30:2 are still hypothetical.
5. Conclusion
To end this point of view, we think that the former notion of demospongic acid should no longer be used mainly because bis-methylene interrupted 5,9-diunsaturated FAs and related acids are distributed among several phyla of marine organisms and several classes of terrestrial plants. The former “demospongic acids” can be considered as a particular series of NMI FAs produced by different combinations of elongases and Δ5 and Δ9 desaturases on the most common FAs in nature such as palmitic and palmitoleic acids.
References
- Litchfield, C; Morales, RW. Harrison, FW, Cowden, RR, Eds.; Are demospongiae membranes unique among living organisms? In Aspects of Sponge Biology; Academic Press: New York, NY, USA, 1976; pp. 183–200. [Google Scholar]
- Litchfield, C; Tyszkiewicz, J; Dato, V. 5,9,23-Triacontatrienoic acid, principal fatty acid of the marine sponge Chondrilla nucula. Lipids 1980, 15, 200–202. [Google Scholar]
- Djerassi, C; Lam, W-K. Sponge phospholipids. Acc. Chem. Res 1991, 24, 69–75. [Google Scholar]
- Barnathan, G; Kornprobst, J-M. Baudimant, G, Guézennec, J, Roy, P, Samain, J-F, Eds.; Fatty acids from marine organisms: recent research developments. In Marine Lipids; Editions Ifremer: Plouzané, France, 2000; pp. 35–43. [Google Scholar]
- Kornprobst, J-M. Encyclopedia of Marine Natural Products; Wiley-VCH: Weinheim, Germany, 2010; Volume 2, pp. 538–555. [Google Scholar]
- Carballeira, NM. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res 2008, 47, 50–61. [Google Scholar]
- Wolff, RL; Christie, WW; Marpeau, AM. Reinvestigation of the polymethylene-interrupted 18:2 and 20:2 acids of Ginkgo biloba seed lipids. J. Am. Oil. Chem. Soc 1999, 76, 273–276. [Google Scholar]
- Wolff, RL; Deluc, LG; Marpeau, AM. Conifer seeds: oil content and fatty acid compositions. J. Am. Oil. Chem. Soc 1996, 73, 765–771. [Google Scholar]
- Ando, Y; Kawabata, Y; Narukawa, K; Ota, T. Demospongic acids of the marine sponge Halichondria panicea from the coast of Hokkaido, Japan. Fish. Sci 1998, 64, 136–139. [Google Scholar]
- Rezanka, T; Sigler, K. Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog. Lipid Res 2009, 48, 206–238. [Google Scholar]
- Bergé, JP; Barnathan, G. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv. Biochem. Eng. Biotechnol 2005, 96, 49–125. [Google Scholar]
- Johnson, DW. Contemporary clinical usage of LC/MS: analysis of biologically important carboxylic acids. Clin. Biochem 2005, 38, 351–361. [Google Scholar]
- Leonard, AE; Pereira, SL; Sprecher, H; Huang, Y-S. Elongation of long-chain fatty acids. Prog. Lipid Res 2004, 43, 36–54. [Google Scholar]
- Tsydendambaev, VD; Christie, WW; Brechany, EY; Vereshchagin, AG. Identification of unusual fatty acids of four alpine plant species from the Pamir. Phytochemistry 2004, 65, 2695–2703. [Google Scholar]
- Moldovan, Z; Jover, E; Bayona, JM. Gas chromatographic and mass spectrometric methods for the characterization of long-chain fatty acids – Application to wool wax extract. Anal. Chim. Acta 2002, 465, 359–378. [Google Scholar]
- Steinberg, SJ; Wang, SJ; Kim, DG; Mihalik, SJ; Watkins, PA. Human very-long-chain acyl-CoA synthetase: cloning, topography, and relevance to branched-chain fatty acid metabolism. Biochem. Biophys. Res. Commun 1999, 257, 615–621. [Google Scholar]
- Rezanka, T. Very-long-chain fatty acids from the animal and plant kingdoms. Prog. Lipid Res 1989, 28, 147–187. [Google Scholar]
- Christie, WW. AOCS Lipid Library. http://lipidlibrary.aocs.org/Lipids/fa_conj+/index.htm (accessed on 6 July 2010).
- Kulkarni, BA; Chattopadhyay, S; Chattopadhyay, A; Mamdapur, VR. Synthesis of the demospongic compounds, (6Z,11Z)-octadecadienoic acid and (6Z,11Z)-eicosadienoic acid. Molecules 1997, 2, 3–6. [Google Scholar]
- Carballeira, NM; Shalabi, F. Identification of naturally occurring trans, trans Δ-5,9 fatty acids from the sponge Plakortis halichondroides. Lipids 1990, 25, 835–840. [Google Scholar]
- Dembitsky, VM; Srebnik, M. Natural halogenated fatty acids: their analogues and derivatives. Prog. Lipid Res 2002, 41, 315–367. [Google Scholar]
- Carballeira, NM; Medina, JR. New Δ5,9 fatty acids in the phospholipids of the sea anemone Stoichactis helianthus. J. Nat. Prod 1994, 57, 1688–1695. [Google Scholar]
- Carballeira, NM; Reyes, M. Identification of a new 6-bromo-5,9-eicosadienoic acid from the anemone Condylactis gigantea and the Zoanthid Palythoa caribaeorum. J. Nat. Prod 1995, 58, 1689–1694. [Google Scholar]
- Lam, WK; Hahn, S; Ayanoglu, E; Djerassi, C. Phospholipid studies of marine organisms. 22. Structure and biosynthesis of a novel brominated fatty acid from a Hymeniacidonid sponge. J. Org. Chem 1989, 54, 3428–3432. [Google Scholar]
- Schlenk, H. Odd numbered polyunsaturated fatty acids. Prog. Chem. Fats Other Lipids 1970, IX(Part 5), 589–605. [Google Scholar]
- Kawashima, H; Ohnishi, M. Occurrence of novel nonmethylene-interrupted C24 polyenoic fatty acids in female gonad lipids of the limpet Cellana grata. Biosci. Biotechnol. Biochem 2006, 70, 2575–2578. [Google Scholar]
- Kawashima, H. Unusual minor nonmethylene-interrupted di-, tri- and tetraenoic fatty acids in limpet gonads. Lipids 2005, 40, 627–630. [Google Scholar]
- Joseph, JD. Ackman, RG, Ed.; Distribution and composition of lipids in marine invertebrates. In Marine Biogenic Lipids, Fats and Oils; CRC Press: Boca Raton, FL, USA, 1989; Volume II, pp. 49–143. [Google Scholar]
- Asset, G; Leroy, A; Bauge, E; Wolff, RL; Fruchant, J-C; Dallongeville, J. Effects of dietary maritime pine (Pinus pinaster)-seed oil on high-density lipoprotein levels and in vitro cholesterol efflux in mice expressing human apolipoprotein A-I. British J. Nutrition 2000, 84, 353–360. [Google Scholar]
- Sayanova, O; Haslam, R; Venegas Caleron, M; Napier, JA. Cloning and characterization of unusual fatty acid desaturases from Anemone leveillei: Identification of an acyl-coenzyme A C20 Δ5-desaturase responsible for the synthesis of sciadonic acid. Plant Physiol 2007, 144, 455–467. [Google Scholar]
- Treschow, AP; Hodges, LD; Wright, PFA; Wynne, PM; Kalafatis, N; Macrides, T. Novel anti-inflammatory⌉-3 PUFAs from the New Zealand green-lipped mussel, Perna canaliculus. Comp. Biochem. Physiol 2007, 147B, 645–656. [Google Scholar]
- Carballeira, NM; Cruz, C. 5,9-Nonadecadienoic acids in Malvaviscus arboreus and Allamanda cathartica. Phytochemistry 1998, 49, 1253–1256. [Google Scholar]
- Pearce, RE; Stillway, LW. Non-methylene-interrupted and ω4 dienoic fatty acids of the white shrimp Penaeus setiferus. Lipids 1977, 12, 544–549. [Google Scholar]
- Zhukova, NV. Lipid classes and fatty acid composition of the tropical nudibranch mollusks Chromodoris sp. and Phyllidia coelestis. Lipids 2007, 42, 1169–1175. [Google Scholar]
- Imbs, AB; Demidkova, DA; Dautova, TN; Latyshev, NA. Fatty acid biomarkers of symbionts and unusual inhibition of tetracosapolyenoic acid biosynthesis in corals (Octocorallia). Lipids 2009, 44, 325–335. [Google Scholar]
- Thiel, V; Blumenberg, M; Hefter, J; Pape, T; Pomponi, S; Reed, J; Reitner, J; Wörheide, G; Michaelis, W. A chemical view of the most ancient metazoa: biomarker chemotaxonomy of hexactinellid sponges. Naturwissenschaften 2002, 89, 60–66, Erratum: Naturwissenschaften 2002, 89, 233–234. [Google Scholar]
- Sato, D; Ando, Y. Distribution of novel nonmethylene-interrupted fatty acids over neutral and polar lipids of Ophiuroidea (Brittle Star). J. Oleo Sci 2002, 51, 563–567. [Google Scholar]
- Ayanoglu, E; Walkup, RD; Sica, D; Djerassi, C. Phospholipid studies of marine organisms: III. New phospholipid fatty acids from Petrosia ficiformis. Lipids 1982, 17, 617–625. [Google Scholar]
- Christie, WW; Brechany, EY; Stefanov, K; Popov, S. The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids 1992, 27, 640–644. [Google Scholar]
- Hahn, S; Lam, WK; Wu, I; Silva, CJ; Djerassi, C. Unusual pattern of fatty acid biosynthesis. J. Biol. Chem 1989, 264, 21043–21046. [Google Scholar]
- Wallis, JG; Watts, JL; Browse, J. Polyunsaturated fatty acid synthesis: what will they think of next? Trends Biochem. Sci 2002, 27, 467–473. [Google Scholar]
- Buist, PH. Fatty acid desaturases: selecting the dehydrogenation channel. Nat. Prod. Rep 2004, 21, 249–262. [Google Scholar]
- Barnathan, G. Non-methylene-interrupted fatty acids from marine invertebrates: Occurrence, characterization and biological properties. Biochimie 2009, 91, 671–678. [Google Scholar]
- Hochmuth, T; Piel, J. Polyketide synthases of bacterial symbionts in sponges – Evolution-based applications in natural products research. Phytochemistry 2009, 70, 1841–1849. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).