Terpenoids from the Soft Coral Sinularia sp. Collected in Yongxing Island
Abstract
:1. Introduction
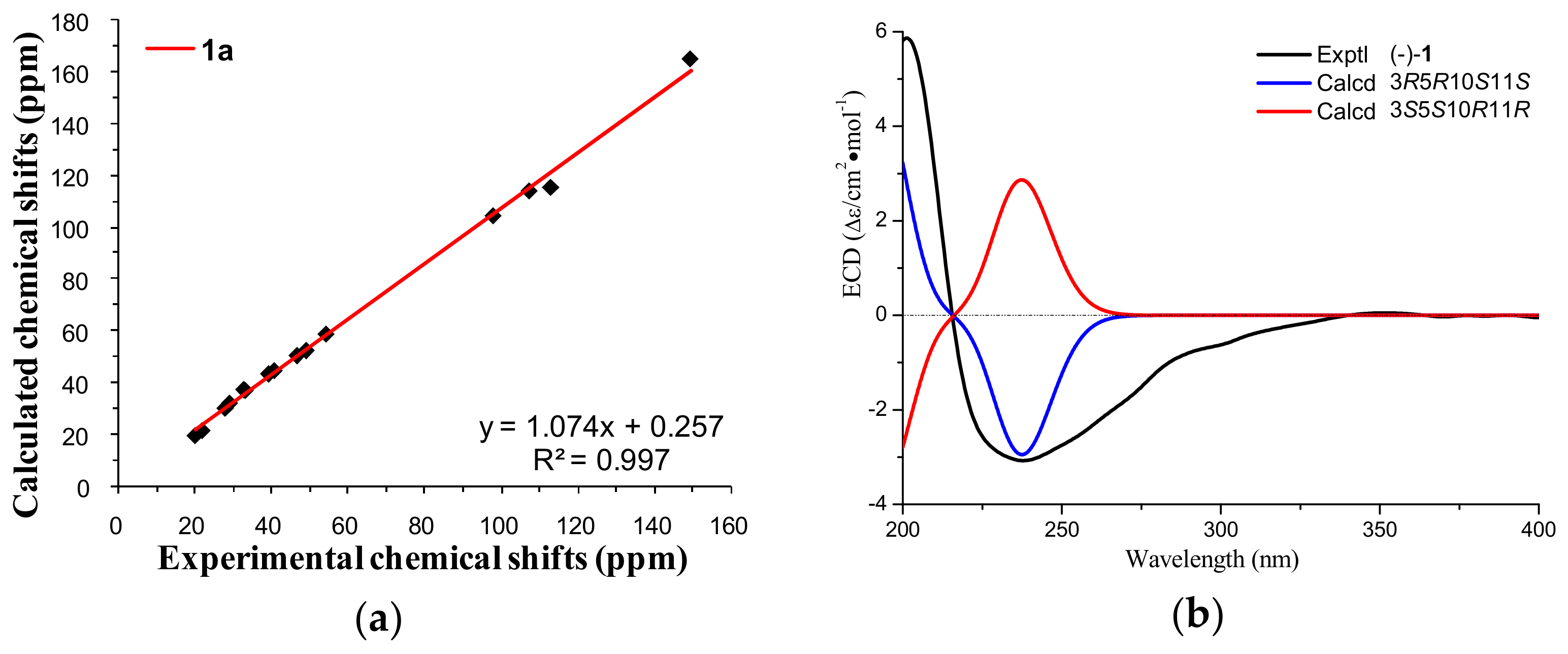
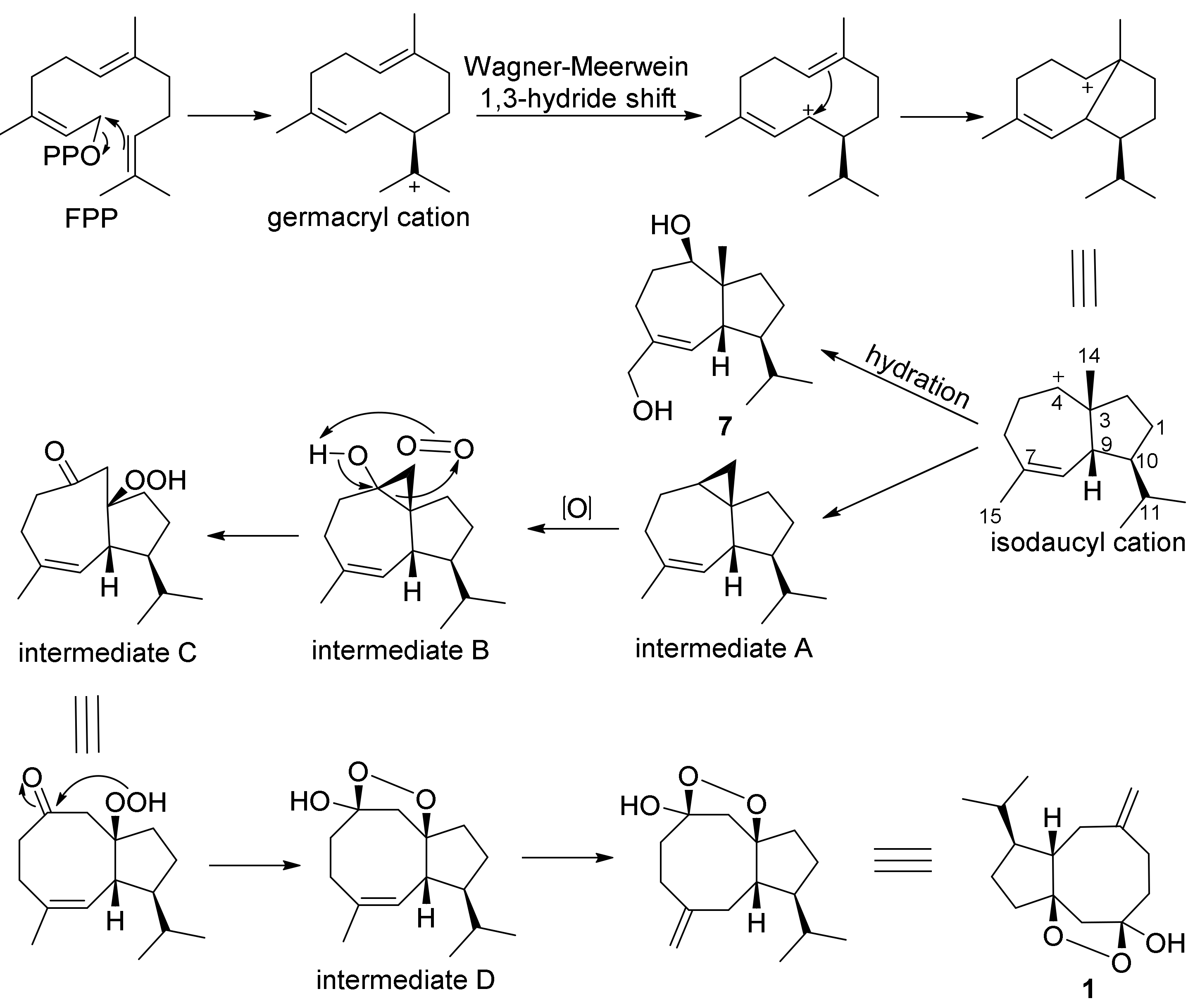
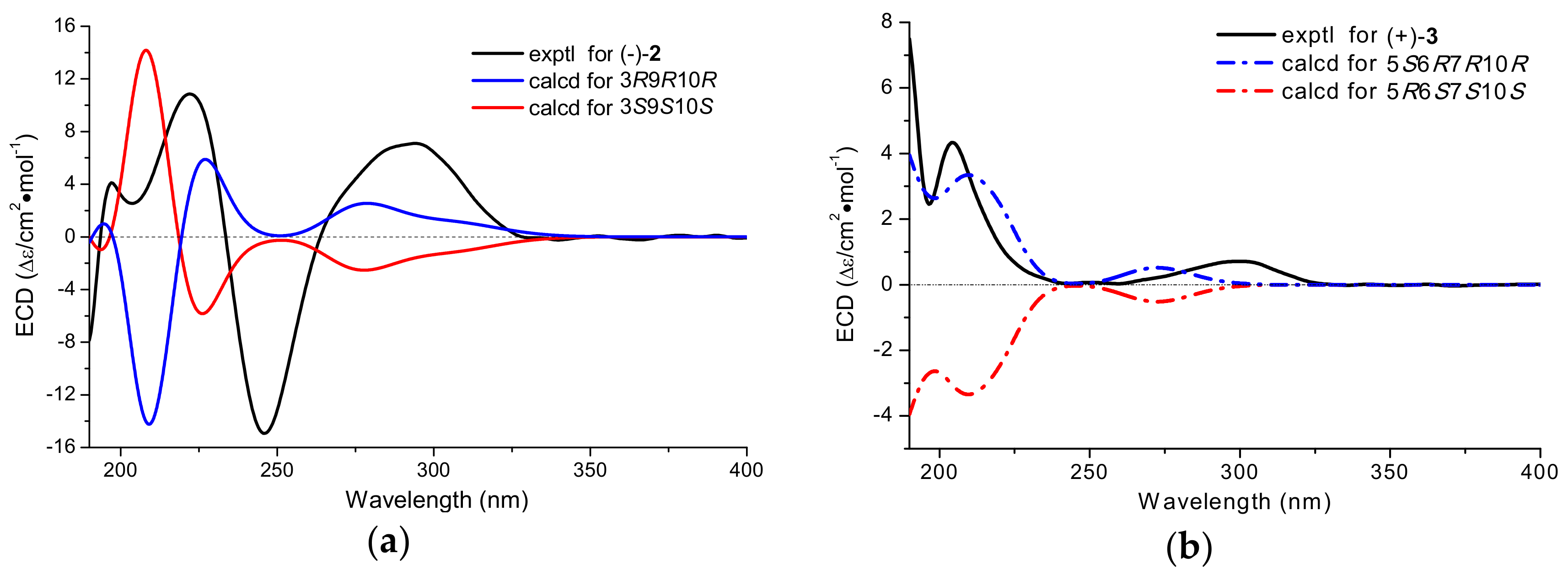
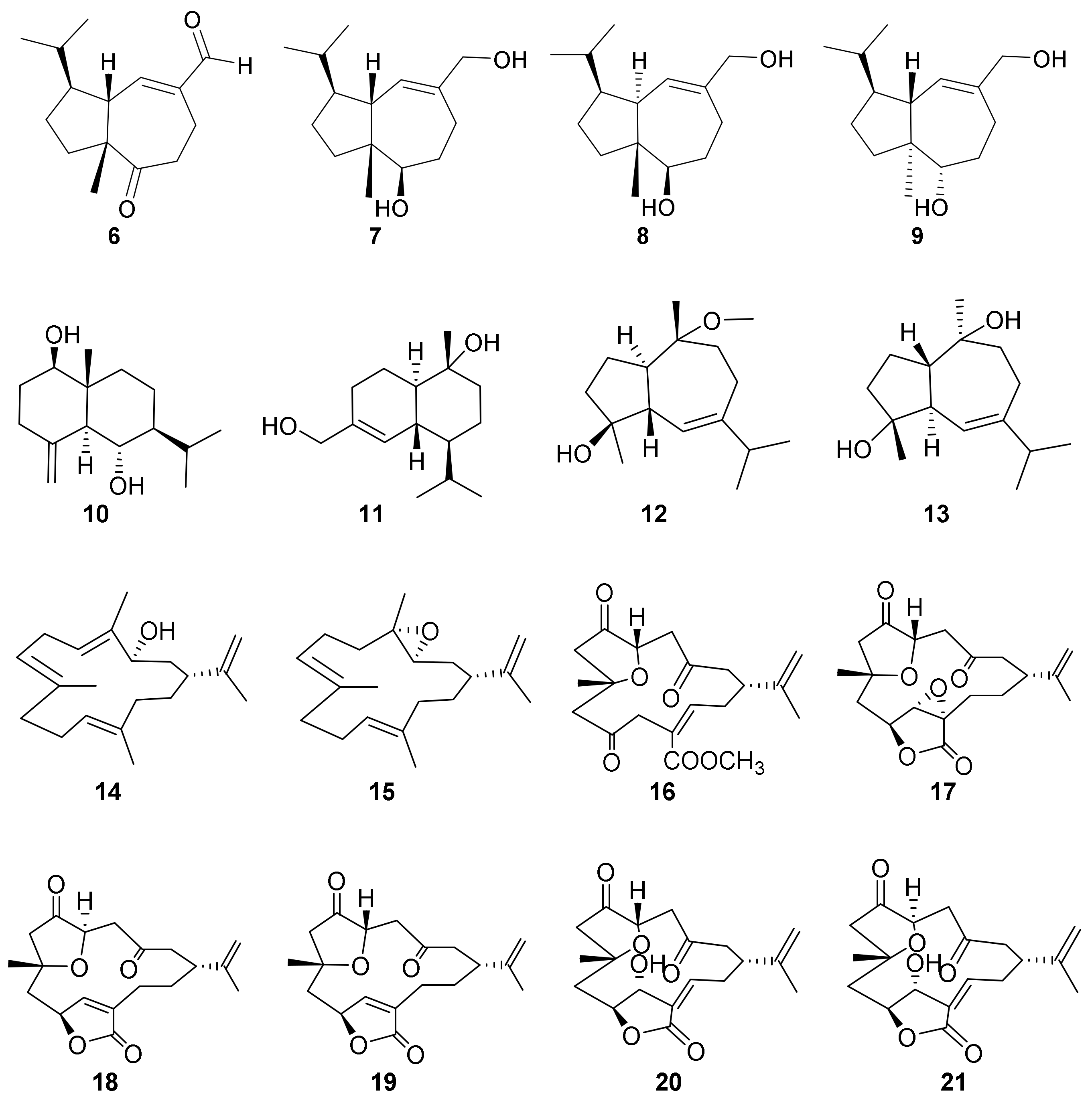
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Computational Section
3.5. Antimalarial Activity
3.6. Immunosuppressive Activity
3.7. Cytotoxic Activities
3.8. Antiviral Activities
3.9. Target Inhibitory Activities
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- World Register of Marine Species (WoRMS). Available online: http://www.marinespecies.org/aphia.php?p=taxlist (accessed on 9 February 2018).
- Chen, W.T.; Li, Y.; Guo, Y.W. Terpenoids of Sinularia soft corals: Chemistry and bioactivity. Acta Pharm. Sin. B 2012, 2, 227–237. [Google Scholar] [CrossRef]
- Lakshmi, V.; Kumar, R. Metabolites from Sinularia species. Nat. Prod. Res. 2009, 23, 801–850. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.Y.; Yu, S.J.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Sinularones A–I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs 2012, 10, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.L.; Li, G.Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.X.; Tang, X.L.; van Ofwegen, L.; Xue, L.; Song, W.J.; Li, P.L.; Li, G.Q. Cyclopentenone derivatives and polyhydroxylated steroids from the soft coral Sinularia acuta. Chem. Biodivers. 2015, 12, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Rustaiyan, A.; Sigari, H.; Jakupovic, J.; Grenz, M. A sesquiterpene lactone from Artemisia diffusa. Phytochemistry 1989, 28, 2723–2725. [Google Scholar] [CrossRef]
- Casteel, D.A. Peroxy natural products. Nat. Prod. Rep. 1999, 16, 55–73. [Google Scholar] [CrossRef]
- Bu, M.; Yang, B.B.; Hu, L.M. Natural endoperoxides as drug lead compounds. Curr. Med. Chem. 2016, 23, 383–405. [Google Scholar] [CrossRef] [PubMed]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Nagashima, H.; Tanaka, N.; Kashiwada, Y.; Kobayashi, J.; Kojoma, M. Hitorins A and B, hexacyclic C25 terpenoids from Chloranthus japonicus. Org. Lett. 2016, 18, 5420–5423. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Z.; Kurtán, T.; Mándi, A.; Tang, H.; Chou, Y.; Soong, K.; Su, L.; Sun, P.; Zhuang, C.L.; Zhang, W. Immunomodulatory polyketides from a Phoma-like fungus isolated from a soft coral. J. Nat. Prod. 2017, 80, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.D.; Li, X.M.; Xu, G.M.; Liu, Y.; Wang, B.G. Wentinoids A–F, six new isopimarane diterpenoids from Aspergillus wentii SD-310, a deep-sea sediment derived fungus. RSC Adv. 2017, 7, 4387–4394. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley and Sons, Publication: West Sussex, UK, 2009; pp. 210–217. ISBN 978-0-470-74168-9. [Google Scholar]
- Bülow, N.; König, W.A. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Nes, W.D. Biosynthesis of cholesterol and other sterols. Chem. Rev. 2011, 111, 6423–6451. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, T.; Larson, T.R.; Catania, T.M.; Harvey, D.; Brown, G.D.; Graham, I.A. Artemisia annua mutant impaired in artemisinin synthesis demonstrates importance of nonenzymatic conversion in terpenoid metabolism. Proc. Natl. Acad. Sci. USA 2016, 113, 15150–15155. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Trullinger, T.K.; Liu, X.J.; Dussault, P.H. Asymmetric synthesis of 1,2-dioxolane-3-acetic acids: Synthesis and configurational assignment of plakinic acid A. J. Org. Chem. 2006, 71, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Moriyasu, M.; Takeuchi, S.; Ichimaru, M.; Nakatani, N.; Nishiyama, Y.; Kato, A.; Mathenge, S.G.; Juma, F.D.; ChaloMutiso, P.B. Pyrenes and pyrendiones from Uvaria lucida. J. Nat. Med. 2012, 66, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, B.R.; Tatipamula, V.B.; Dokuburra, C.B.; Mangamuri, U.K.; Tuniki, V.R.; Kalivendi, S.V.; Bunce, R.A.; Yenamandra, V. Pambanolides A–C from the South Indian soft coral Sinularia inelegans. Tetrahedron 2016, 72, 1933–1940. [Google Scholar] [CrossRef]
- Sato, A.; Fenical, W.; Zheng, Q.T.; Clardy, J. Norcembrene diterpenoids from Pacific soft-corals of the genus Sinularia (Alcyonacea; Octocorallia). Tetrahedron 1985, 41, 4303–4308. [Google Scholar] [CrossRef]
- Nishizawa, M.; Inoue, A.; Hayashi, Y.; Sastrapradja, S.; Kosela, S.; Iwashita, T. Structure of aphanamol I and II. J. Org. Chem. 1984, 49, 3660–3662. [Google Scholar] [CrossRef]
- Liu, H.B.; Zhang, C.R.; Dong, S.H.; Yang, S.P.; Sun, Q.; Geng, M.Y.; Yue, J.M. Sesquiterpenes from Dysoxylum oliganthum and Dysoxylum excelsum. J. Asian Nat. Prod. Res. 2012, 14, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.W.; Cheng, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Capillosananes S–Z, new sesquiterpenoids from the soft coral Sinularia capillosa. Tetrahedron Lett. 2014, 55, 3077–3082. [Google Scholar] [CrossRef]
- Hu, J.F.; Bai, S.P.; Jia, Z.J. Eudesmane sesquiterpenes from Artemisia eriopoda. Phytochemistry 1996, 43, 815–817. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Chen, C.H.; Chien, S.C.; Lin, Y.L. Five new cadinane-type sesquiterpenes from the heartwood of Chamaecyparis obtusa var. formosana. J. Nat. Prod. 2002, 65, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.G.; Jin, Q.; Kim, A.R.; Choi, H.; Lee, J.H.; Kim, Y.S.; Lee, D.G.; Woo, E.R. A new triterpenoid from Alisma orientale and their antibacterial effect. Arch. Pharm. Res. 2012, 35, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Hamann, M.T. A new norcembranoid dimer from the Red Sea soft coral Sinularia gardineri. J. Nat. Prod. 1996, 59, 687–689. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.F.; Coll, J.C.; Mitchell, S.J.; Kazlauskas, R. Studies of Australian soft corals. XXIV. Two cembranoid diterpenes from the soft coral Sinularia facile. Aust. J. Chem. 1981, 34, 1551–1556. [Google Scholar] [CrossRef]
- Saitman, A.; Rulliere, P.; Sullivan, S.D.E.; Theodorakis, E.A. Total synthesis of norcembrenolide B and scabrolide D. Org. Lett. 2011, 13, 5854–5857. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Mohamed, T.A.; Elshamy, A.I.; Al-Hammady, M.A.; Ohta, S.; Paré, P.W. Casbane diterpenes from Red Sea coral Sinularia polydactyla. Molecules 2016, 21, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Shiue, R.T.; Wang, G.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Five novel norcembranoids from Sinularia leptoclados and S. parva. Tetrahedron 2003, 59, 7337–7344. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Ahmed, A.F.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Sinulochmodins A−C, three novel terpenoids from the soft coral Sinularia lochmodes. Org. Lett. 2005, 7, 3813–3816. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.R.; Pan, F.; Parvez, S.; Fleig, A.; Chong, C.R.; Xu, J.; Dang, Y.; Zhang, J.; Jiang, H.; Penner, R.; et al. Clofazimine inhibits human Kv1.3 potassium channel by perturbing calcium oscillation in T lymphocytes. PLoS ONE 2008, 3, e4009. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Wang, W.; Zhang, P.; Yu, G.L.; Li, C.X.; Hao, C.; Qi, X.; Zhang, L.J.; Guan, H.S. Preparation and anti-influenza A virus activity of k-carrageenan oligosaccharide and its sulphated derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Liu, S.B.; Chen, H.Q.; Guo, Z.K.; Dong, W.H.; Wang, J.; Mei, W.L.; Dai, H.F. Phragmalin-type limonoids from the roots of Trichilia sinensis. RSC Adv. 2017, 7, 28994–29003. [Google Scholar] [CrossRef]
- Du, L.; Ai, J.; Li, D.H.; Zhu, T.J.; Wang, Y.; Knauer, M.; Bruhn, T.; Liu, H.B.; Geng, M.Y.; Gu, Q.Q.; et al. Aspergiolides C and D: Spirocyclic aromatic polyketides with potent protein kinase c-Met inhibitory effects. Chem. Eur. J. 2011, 17, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
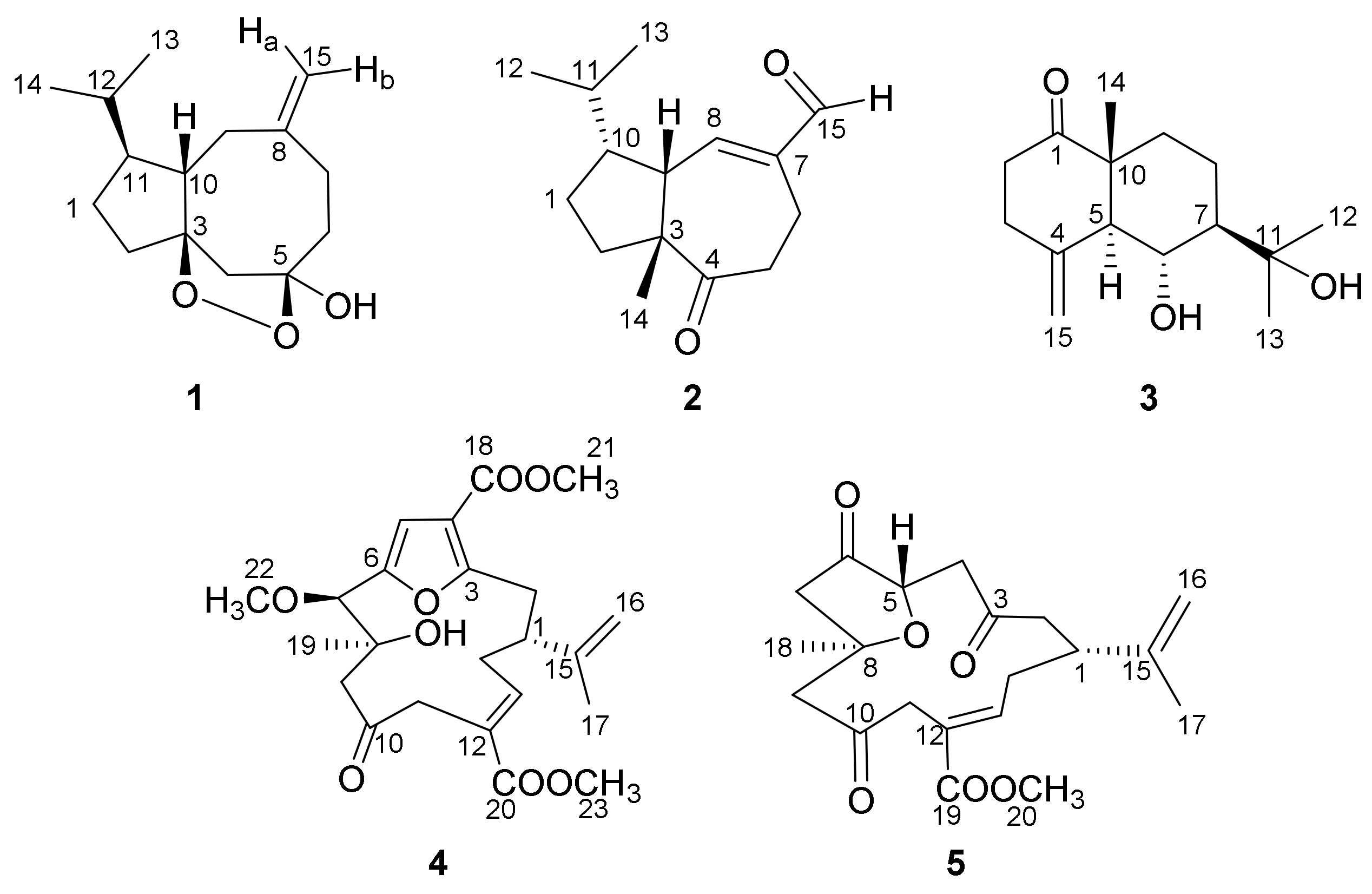
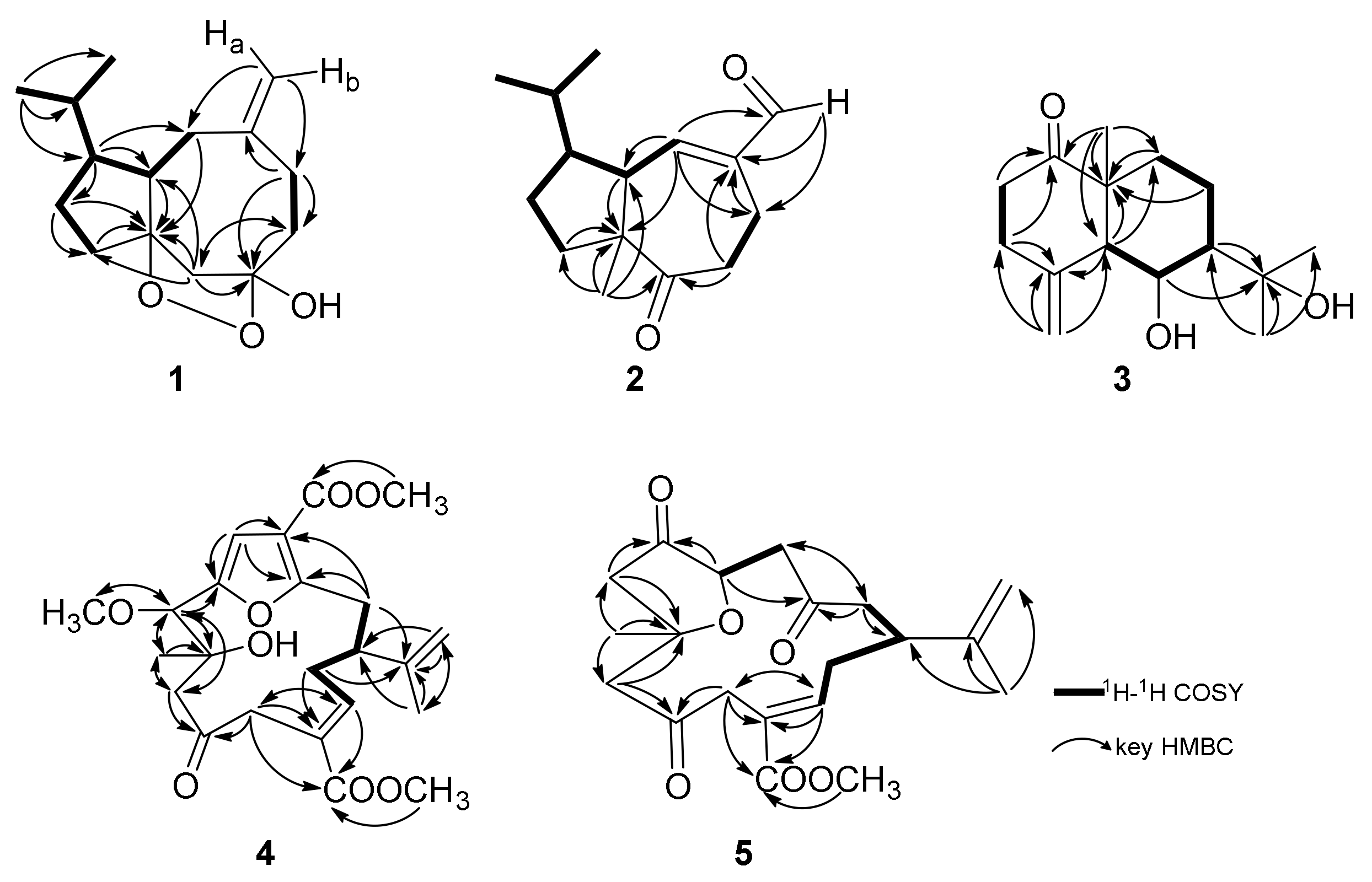
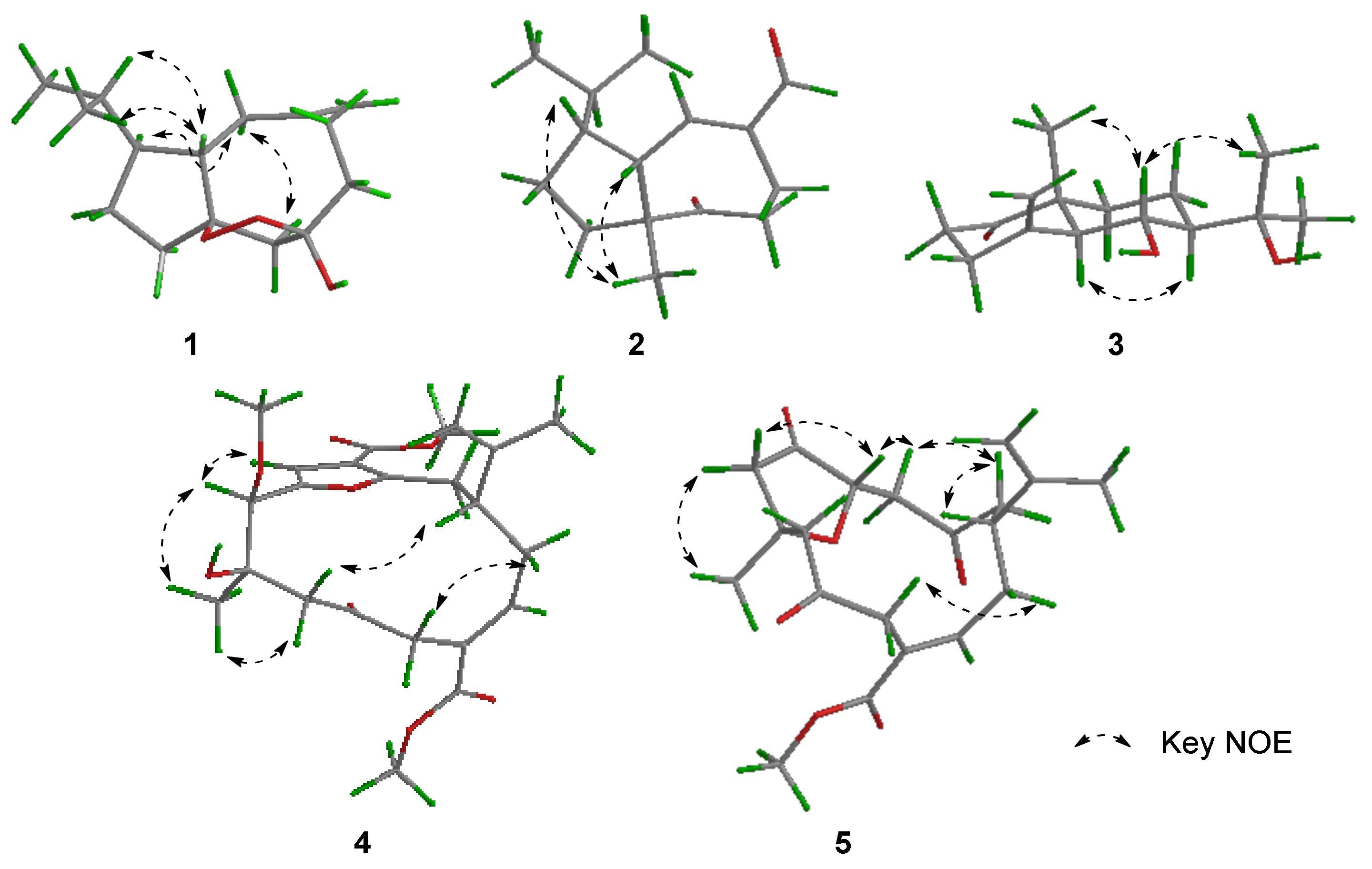
| No. | δH (J in Hz) a | δC (Type) b | 1H-1H COSY | HMBC | NOE |
|---|---|---|---|---|---|
| 1a 1b | 1.72 (1H, m) 1.37 (1H, m) | 28.0 (CH2) | H-1b, 11 H-1a, 2 | C-2, 3, 10 C-2, 11 | - |
| 2 | 1.80 (2H, m) | 33.2 (CH2) | H-1a | C-1, 3, 10, 11 | - |
| 3 | - | 98.0 (C) | - | - | - |
| 4α 4β | 3.14 (1H, d, 13.4) 2.28 (1H, d, 13.4) | 49.2 (CH2) | H-4β H-4α | C-6, 10 C-2, 3, 5, 10 | H-9α - |
| 5 | - | 107.4 (C) | - | - | - |
| 6α 6β | 1.92 (1H, td, 13.2, 4.2) 2.06 (1H, m) | 39.3 (CH2) | H-6β, 7β H-6α,7α | C-5, 7, 8 C-4, 5, 8 | - |
| 7α 7β | 2.29 (1H, m) 2.44 (1H, td, 13.1, 2.8) | 29.1 (CH2) | H-6β, 7β H-6α,7α | C-5, 6, 8, 9, 15 C-5, 6, 8, 15 | - |
| 8 | - | 149.4 (C) | - | - | - |
| 9α 9β | 2.04 (1H, m) 2.55 (1H, brd, 17.1) | 40.8 (CH2) | H-9β, 10 H-9α, 10, 15a, 15b | C-3, 7, 8, 10, 11, 15 - | H-4α, 11 H-10 |
| 10 | 2.39 (1H, m) | 46.8 (CH) | H-9α, 9β, 11 | C-3, 9, 11, 12 | H-9β, 12, 13 |
| 11 | 1.31 (1H, m) | 54.3 (CH) | H-1a, 10, 12 | C-1, 9, 10, 12, 13, 14 | H-9α |
| 12 | 1.65 (1H, m) | 32.8 (CH) | H-11, 13, 14 | C-1, 10, 11, 13, 14 | H-10 |
| 13 | 0.95 (3H, d, 6.7) | 22.0 (CH3) | H-12 | C-11, 12, 14 | H-10 |
| 14 | 0.89 (3H, d, 6.7) | 20.1 (CH3) | H-12 | C-11, 12, 13 | - |
| 15a 15b | 4.90 (1H, brs) 4.93 (1H, brs) | 113.0 (CH2) | H-9β H-9β | C-7, 9 C-7, 9 | - |
| No. | δH (J in Hz) a | δC (TYPE) b | 1H-1H COSY | HMBC | NOE |
|---|---|---|---|---|---|
| 1 | 1.91 (1H, m) 1.53 (1H, m) | 23.9 (CH2) | H-2, 10 | C-2, 9, 10 | - |
| 2 | 2.24 (1H, m) 1.57 (1H, m) | 33.3 (CH2) | H-1 | C-1, 3, 14 | - |
| 3 | - | 60.3 (C) | - | - | - |
| 4 | - | 213.2 (C) | - | - | - |
| 5 | 2.56 (1H, ddd, 13.5, 7.0, 2.5) 2.43 (1H, dd, 13.5, 2.8) | 38.0 (CH2) | H-6 | C-4, 6, 7 | - |
| 6 | 3.00 (1H, ddd, 15.6, 7.1, 3.2) 2.05(1H, m) | 20.1 (CH2) | H-5 | C-4, 5, 7, 8, 15 - | - |
| 7 | - | 143.7 (C) | - | - | - |
| 8 | 6.78(1H, dd, 4.8, 1.8) | 157.4 (CH) | H-9 | C-3, 6, 9, 10, 15 | - |
| 9 | 2.48 (1H, dd, 10.4, 4.3) | 49.0 (CH) | H-8, 10 | C-3, 4, 7, 8, 10, 11 | H-14 |
| 10 | 2.08 (1H, m) | 49.4 (CH) | H-1, 9, 11 | C-1, 8, 9, 11, 12, 13 | H-14 |
| 11 | 1.67 (1H, m) | 31.5 (CH) | H-10, 12, 13 | C-1, 9, 10, 12, 13 | - |
| 12 | 0.88 (3H, d, 6.7) | 18.9 (CH3) | H-11 | C-10, 11, 13 | - |
| 13 | 0.93 (3H, d, 6.7) | 21.5 (CH3) | H-11 | C-10, 11, 12 | - |
| 14 | 1.02 (3H, s) | 19.5 (CH3) | - | C-2, 3, 4, 9 | H-9, 10 |
| 15 | 9.49 (1H, s) | 192.7 (CH) | - | C-6, 7 | - |
| No. | δH (J in Hz) a | δC (Type) b | 1H-1H COSY | HMBC | NOE |
|---|---|---|---|---|---|
| 1 | - | 213.0 (C) | - | - | - |
| 2 | 2.66 (1H, m) 2.41 (1H, m) | 38.3 (CH2) | H-3 | C-1, 3 | - |
| 3 | 2.60 (1H, m) 2.39 (1H, m) | 35.3 (CH2) | H-2 | C-1, 2, 4, 5, 15 | - |
| 4 | - | 142.9 (C) | - | - | - |
| 5 | 2.21 (1H, d, 9.9) | 54.9 (CH) | H-6 | C-1, 3, 4, 6, 9, 10, 14, 15 | H-7 |
| 6 | 4.17 (1H, dd, 9.9, 9.9) | 68.7 (CH) | H-5, 7 | C-4, 5, 7, 11 | H-13, 14 |
| 7 | 1.55 (1H, td, 9.6, 3.5) | 52.7 (CH) | H-6, 8 | C-6, 8, 11 | H-5 |
| 8 | 1.73 (1H, ddd, 10.5, 6.6, 3.3) 1.14 (1H, ddd, 26.7, 13.4, 3.4) | 21.9 (CH2) | H-7, 9 | C-7, 10 | - |
| 9 | 1.78 (1H, dt, 13.7, 3.0) 1.62 (1H, td, 13.8, 3.6) | 31.5 (CH2) | H-8 | C-8, 10 | - |
| 10 | - | 49.8 (C) | - | - | - |
| 11 | - | 74.6 (C) | - | - | - |
| 12 | 1.26 (3H, s) | 30.0 (CH3) | - | C-7, 11, 13 | - |
| 13 | 1.30 (3H, s) | 24.1 (CH3) | - | C-7, 11, 12 | H-6 |
| 14 | 1.01 (3H, s) | 17.9 (CH3) | - | C-1, 5, 9, 10 | H-6 |
| 15 | 5.28 (1H, s) 5.05 (1H, s) | 110.6 (CH2) | H-5 | C-3, 4, 5 | - |
| No. | δH (J in Hz) a | δC (Type) b | 1H-1H COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 2.49 (1H, m) | 44.1 (CH) | H-2, 14 | C-2 | H-9β |
| 2 | 3.12 (1H, dd, 15.1, 3.0) 3.07 (1H, dd, 15.1, 8.6) | 30.6 (CH2) | H-1 | C-1, 3, 4, 14, 15 | - |
| 3 | - | 161.0 (C) | - | - | - |
| 4 | - | 114.6 (C) | - | - | - |
| 5 | 6.63 (1H, s) | 112.2 (CH) | - | C-3, 4, 6 | H-19 |
| 6 | - | 149.0 (C) | - | - | - |
| 7 | 4.07 (1H, s) | 83.7 (CH) | - | C-5, 8, 9, 19, 22 | H-5, 19 |
| 8 | - | 73.1 (C) | - | - | - |
| 9α 9β | 2.47 (1H, d, 17.8) 3.19 (1H, d, 17.8) | 46.0 (CH2) | - | C-7, 8, 10, 19 | H-19 H-1 |
| 10 | - | 208.6 (C) | - | - | - |
| 11a 11b | 3.60 (1H, d, 18.2) 3.55 (1H, d, 18.1) | 42.0 (CH2) | - | C-10, 12, 13, 20 | - H-14 |
| 12 | - | 125.1 (C) | - | - | - |
| 13 | 6.96 (1H, dd, 11.0, 4.0) | 144.1 (CH) | H-14 | C-1, 11, 14, 20 | - |
| 14a 14b | 2.45 (1H, m) 2.28 (1H, m) | 31.4 (CH2) | H-1, 13 | C-1, 2, 12, 13, 15 | H-11 - |
| 15 | - | 145.9 (C) | - | - | - |
| 16 | 4.87 (1H, s) 4.79 (1H, s) | 111.4 (CH2) | H-17 | C-1, 15, 17 | - |
| 17 | 1.81 (3H, s) | 20.8 (CH3) | H-16 | C-1, 15, 16 | - |
| 18 | - | 164.0 (C) | - | - | - |
| 19 | 1.35 (3H, s) | 27.9 (CH3) | - | C-7, 8, 9 | H-7, 9α |
| 20 | - | 167.3 (C) | - | - | - |
| 21 | 3.79 (3H, s) | 51.4 (-OCH3) | - | C-18 | - |
| 22 | 3.26 (3H, s) | 57.8 (-OCH3) | - | C-7 | - |
| 23 | 3.72 (3H, s) | 52.1 (-OCH3) | - | C-20 | - |
| No. | δH (J in Hz) a | δC (Type) b | 1H-1H COSY | HMBC | NOESY |
|---|---|---|---|---|---|
| 1 | 2.90 (1H, m) | 38.9 (CH) | H-2, 14 | C-2 | H-2β |
| 2α β | 2.70 (1H, m) 2.37 (1H, m) | 44.3 (CH2) | H-1 | C-1, 3, 4 | - H-1, 4β |
| 3 | - | 207.1 (C) | - | - | - |
| 4β α | 2.80 (1H, m)2.39 (1H, m) | 46.5 (CH2) | H-5 | C-3, 5 | H-5, 2β - |
| 5 | 4.32 (1H, brd, 11.1) | 76.8 (CH) | H-4 | C-3, 4, 6 | H-4β, 7β |
| 6 | - | 212.1 (C) | - | - | - |
| 7β α | 2.67 (1H, m) 2.43 (1H, m) | 48.8 (CH2) | - | C-6, 8, 9, 18 | H-5 H-18 |
| 8 | - | 79.8 (C) | - | - | - |
| 9 | 2.82 (1H, m) 2.40 (1H, m) | 50.7 (CH2) | - | C-7, 8, 10, 18 | - |
| 10 | - | 205.7 (C) | - | - | - |
| 11a 11b | 3.51 (1H, d, 17.6) 3.44 (1H, d, 17.7) | 43.5 (CH2) | - | C-10, 12, 13, 19 | - H-14 |
| 12 | - | 128.6 (C) | - | - | - |
| 13 | 6.80 (1H, dd, 7.0, 7.0) | 140.9 (CH) | H-14 | C-1, 11, 12, 19 | - |
| 14 | 2.27 (2H, m) | 28.8 (CH2) | H-1, 13 | C-1, 2, 12, 13, 15 | H-11b |
| 15 | - | 144.9 (C) | - | - | - |
| 16a b | 4.95 (1H, s) 4.75 (1H, s) | 112.2 (CH2) | H-17 | C-1, 15, 17 | - |
| 17 | 1.75 (CH3, s) | 21.2 (CH3) | - | C-1, 15, 16 | - |
| 18 | 1.41 (CH3, s) | 27.5 (CH3) | - | C-7, 8, 9 | H-7β |
| 19 | - | 167.3 (C) | - | - | - |
| 20 | 3.71 (CH3, s) | 52.0 (CH3) | - | C-19 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.-F.; Tang, X.-L.; Sun, Y.-T.; Luo, X.-C.; Zhang, J.; Van Ofwegen, L.; Sung, P.-J.; Li, P.-L.; Li, G.-Q. Terpenoids from the Soft Coral Sinularia sp. Collected in Yongxing Island. Mar. Drugs 2018, 16, 127. https://doi.org/10.3390/md16040127
Qin G-F, Tang X-L, Sun Y-T, Luo X-C, Zhang J, Van Ofwegen L, Sung P-J, Li P-L, Li G-Q. Terpenoids from the Soft Coral Sinularia sp. Collected in Yongxing Island. Marine Drugs. 2018; 16(4):127. https://doi.org/10.3390/md16040127
Chicago/Turabian StyleQin, Guo-Fei, Xu-Li Tang, Yan-Ting Sun, Xiang-Chao Luo, Jing Zhang, Leen Van Ofwegen, Ping-Jyun Sung, Ping-Lin Li, and Guo-Qiang Li. 2018. "Terpenoids from the Soft Coral Sinularia sp. Collected in Yongxing Island" Marine Drugs 16, no. 4: 127. https://doi.org/10.3390/md16040127






