Ω3 Supplementation and Intermittent Hypobaric Hypoxia Induce Cardioprotection Enhancing Antioxidant Mechanisms in Adult Rats
Abstract
:1. Introduction
2. Results
2.1. Body Weight and Cardiac Parameters
| N | N + Ω3 | IH | IH + Ω3 | |
|---|---|---|---|---|
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | |
| Cardiac/Body Weight | ||||
| mg/g | 0.31 ± 0.09 | 0.33 ± 0.10 | 0.36 ± 0.15 | 0.28 ± 0.07 |
| Heart Rate | ||||
| beats/min | 310 ± 2.2 | 295 ± 1.8 | 308 ± 1.7 | 298 ± 2.3 |
2.2. Left Ventricular Function
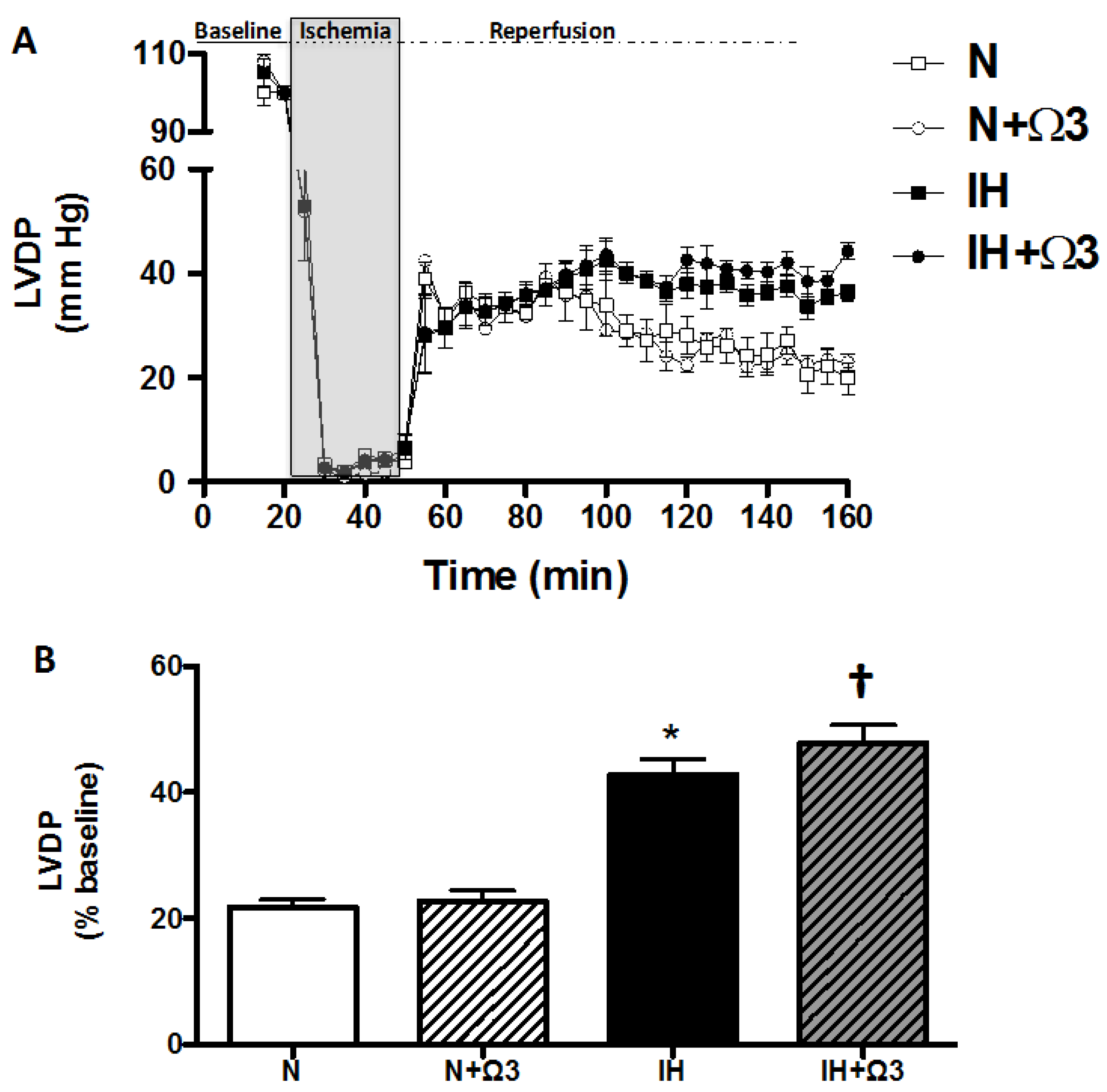
| N | N + Ω3 | IH | IH + Ω3 | |
|---|---|---|---|---|
| (n = 7) | (n = 7) | (n = 7) | (n = 7) | |
| Baseline values LVEDP (mmHg) | 5.7 ± 0.8 | 5.1 ± 0.5 | 6.1 ± 0.9 | 5.5 ± 0.7 |
| After 120 min reperfusion LVEDP (mmHg) | 34.9 ± 1.8 | 33.1 ± 2.3 | 21.7 ± 1.9 * | 20.6 ± 3.7 * |
2.3. LDH Levels
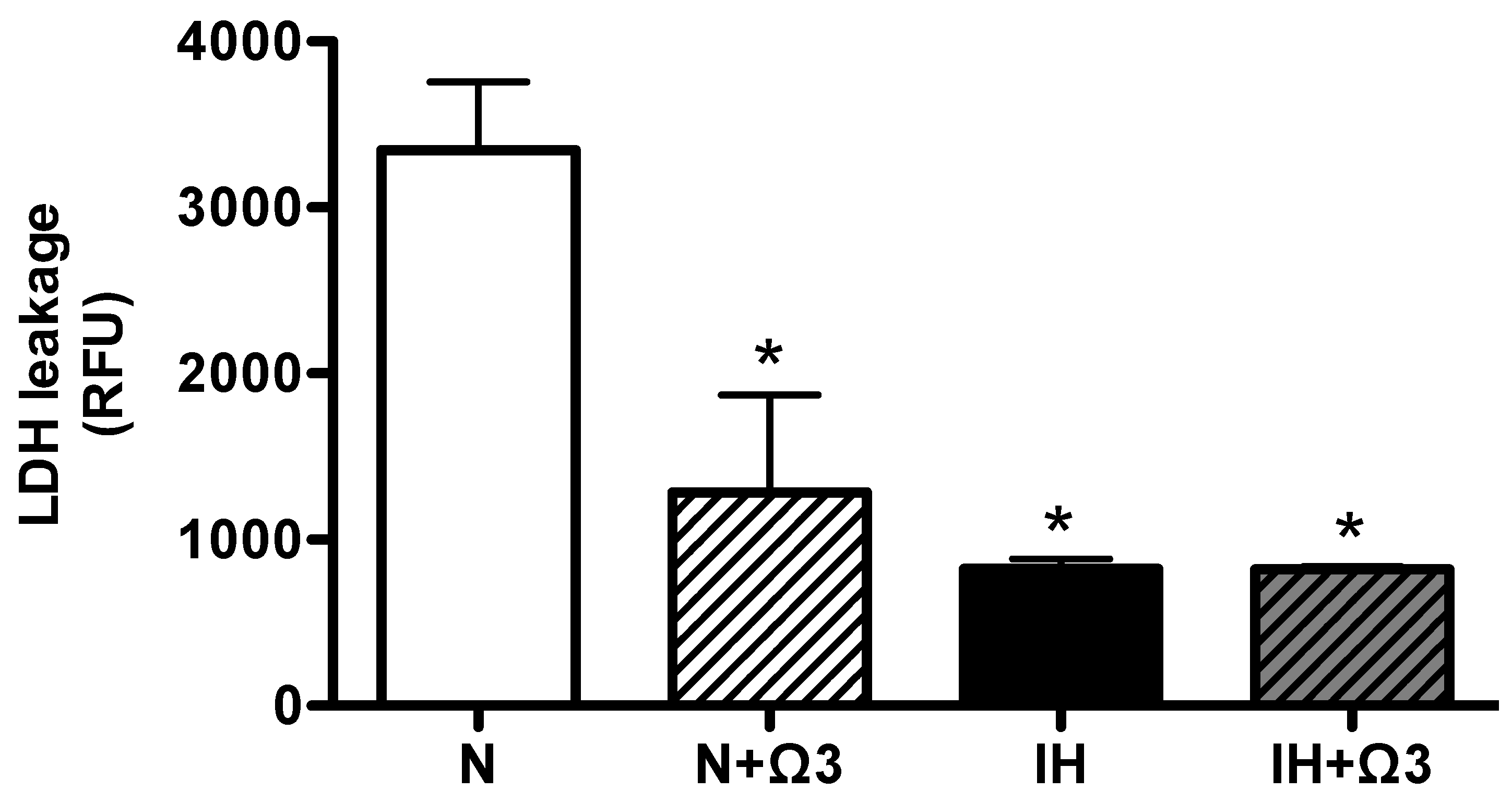
2.4. HIF-1α and ATP Levels
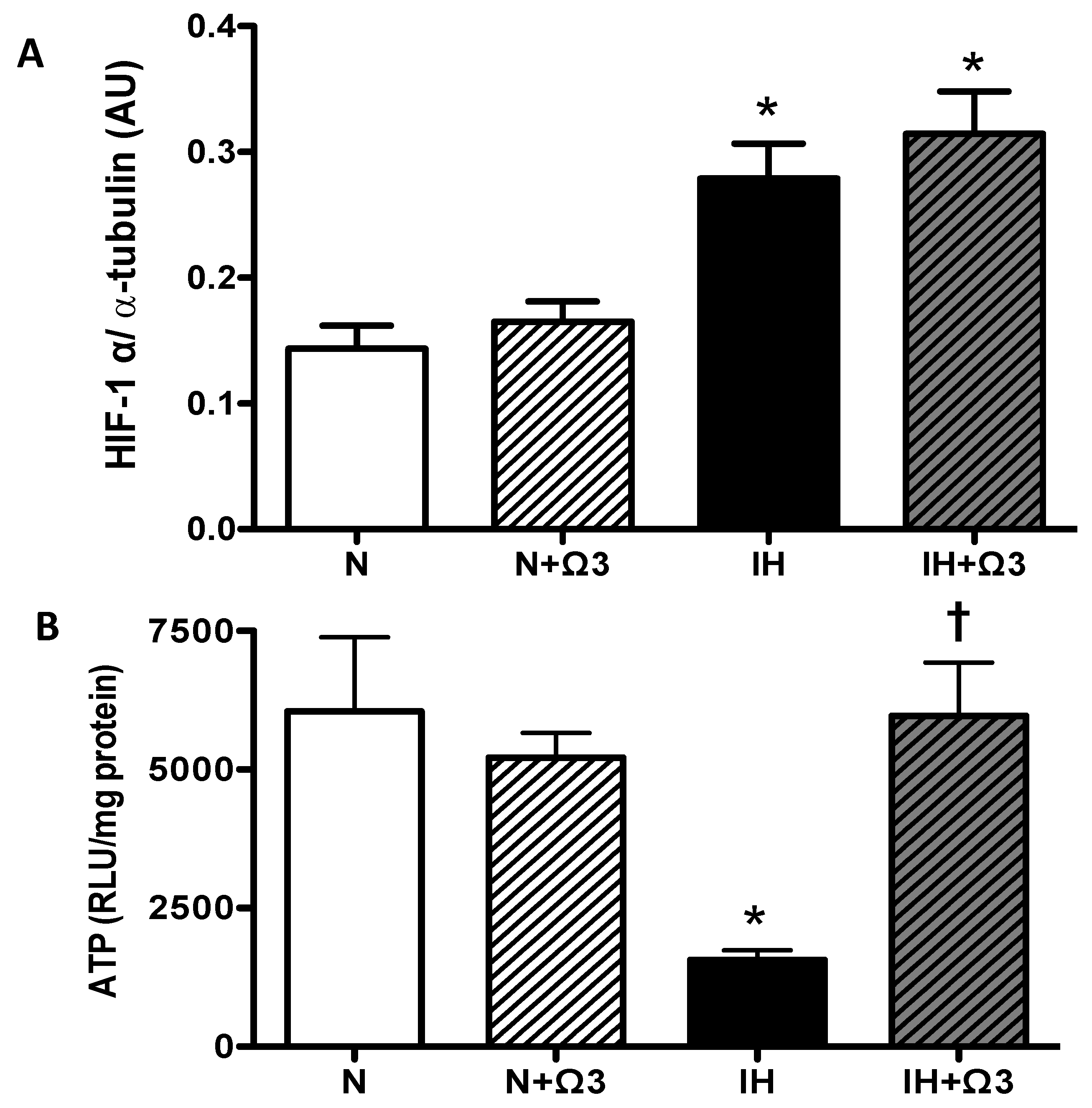
2.5. Oxidative Stress Markers
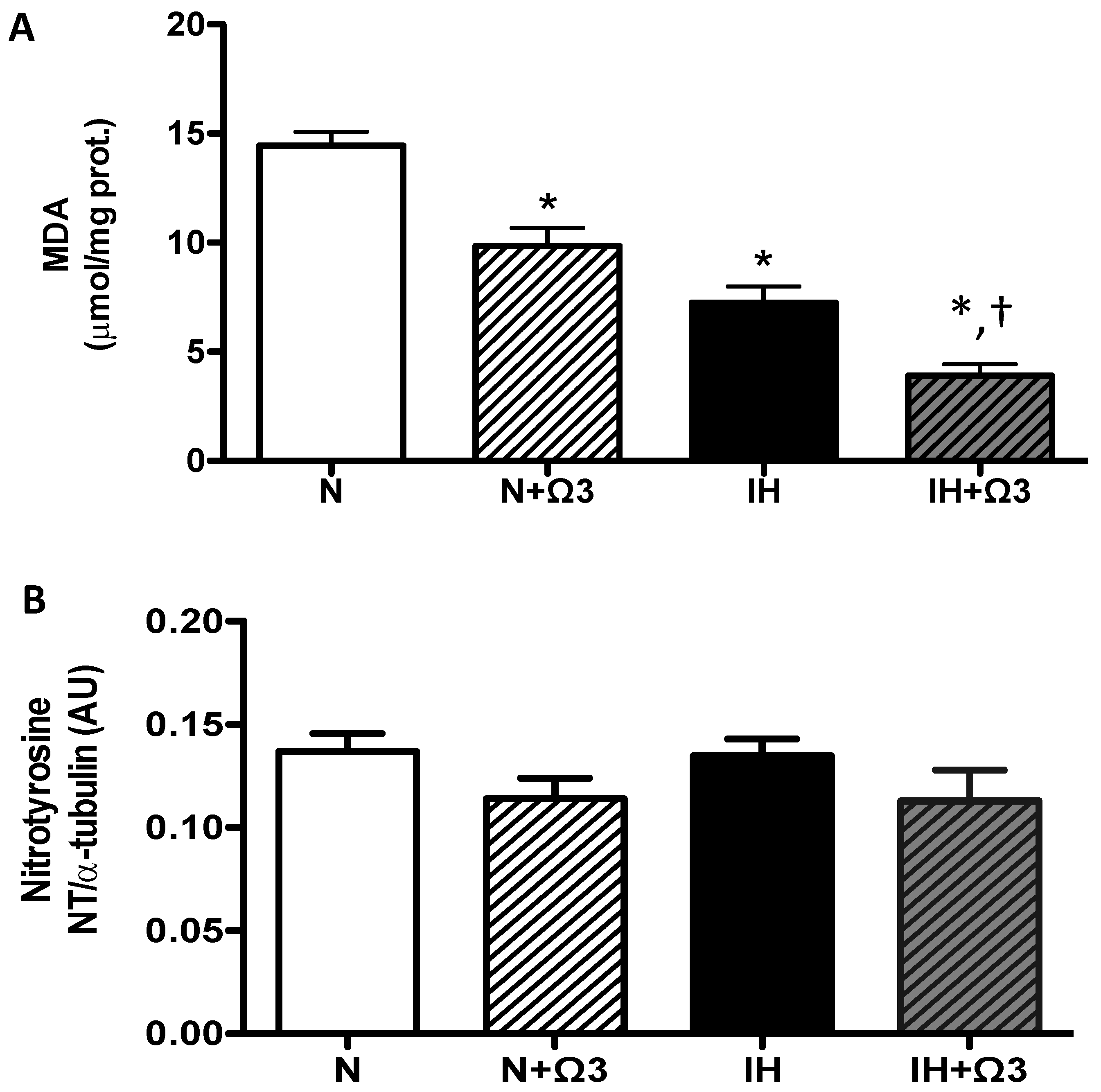
2.6. Antioxidant Enzymes Levels
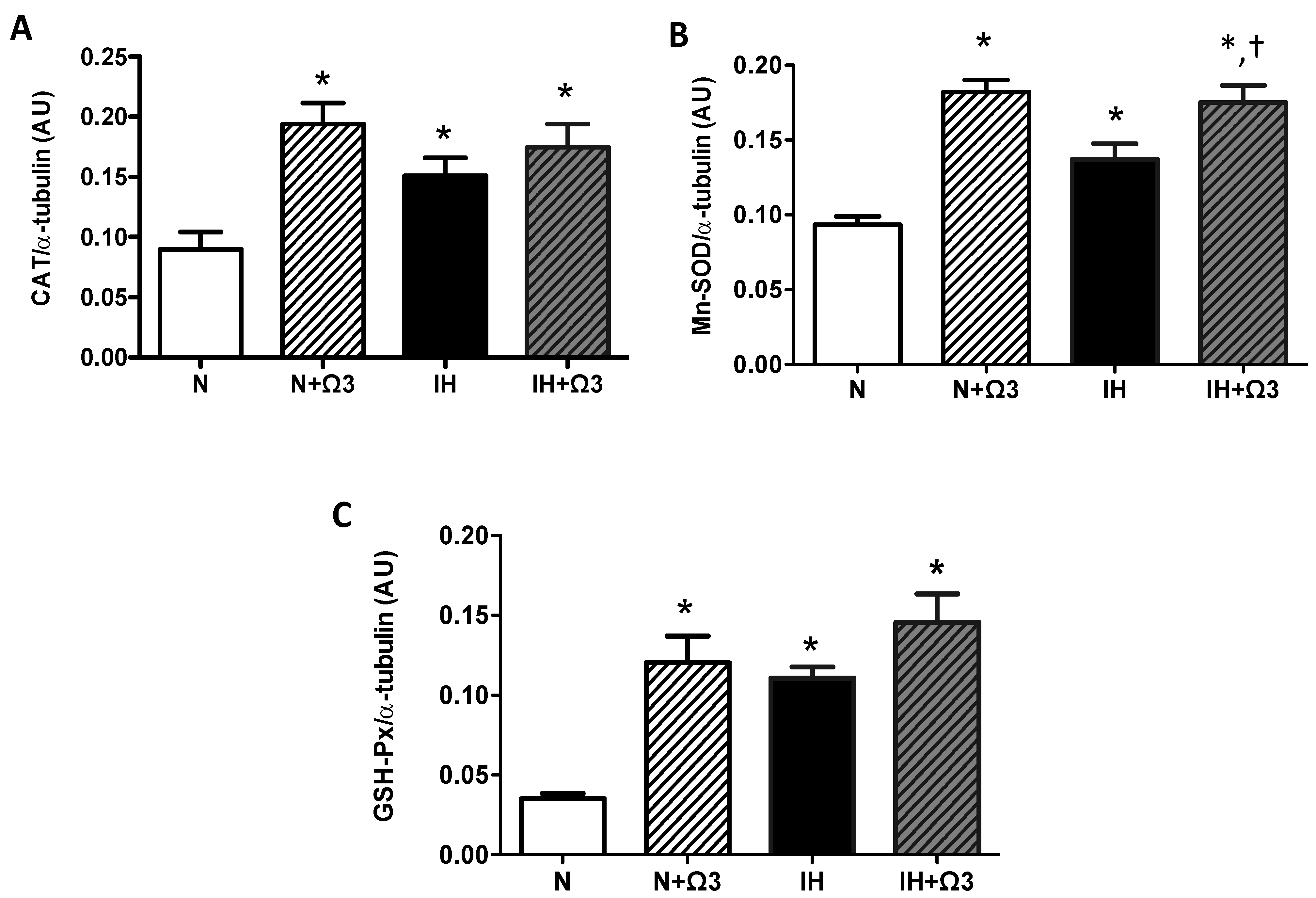
2.7. Pro-Inflammatory Markers

3. Discussion
3.1. Cardioprotective Mechanisms Induced by Chronic IHH
3.2. Cardioprotective Effects Derived from Ω3 Supplementation
3.3. Previous Studies on Chronic IHH
4. Materials and Methods
4.1. Animals
4.2. Ex Vivo Heart—Langendorff Setup
4.3. Left Ventricular Function
4.4. Energy Consumption
4.5. Cellular Injury, Hypoxia, Oxidative Stress and Pro-Inflammatory Markers
4.5.1. Plasmatic Lactate Dehydrogenase
4.5.2. Cardiac HIF-1α
4.5.3. Cardiac ATP
4.5.4. Cardiac Malondialdehyde and Nitrotyrosine
4.5.5. Cardiac Antioxidant Enzymes
4.5.6. Cardiac NF-kappaB Transcription Factor
4.5.7. Cardiac Myeloperoxidase Activity
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antezana, A. Cardiovascular changes in chronic intermittent hypoxia. In Health & Height; Viscor, G., Ricart, A., Leal, C., Eds.; Publicacions Universitat de Barcelona: Barcelona, Spain, 2003; pp. 151–155. [Google Scholar]
- Siqués, P.; Brito, J.; León-Velarde, F.; Barrios, L.; Cruz, J.J.; López, V.; Herruzo, R. Time course of cardiovascular and hematological responses in rats exposed to chronic intermittent hypobaric hypoxia (4600 m). High Alt. Med. Biol. 2006, 7, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Escudero, E.; Pando, J.; Sharma, S.; Johnson, R.J. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol. Dial. Transplant. 2012, 27, 11–16. [Google Scholar] [CrossRef]
- Beguin, P.C.; Belaidi, E.; Godin-Ribuot, D.; Lévy, P.; Ribuot, C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 map kinase and Erk1/2. J. Mol. Cell. Cardiol. 2007, 42, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.G.; Jimenez, D.; Osorio, J.; Zepeda, A.B.; Figueroa, C.A.; Pulgar, V.M. Acclimatization to chronic intermittent hypoxia in mine workers: A challenge to mountain medicine in Chile. Biol. Res. 2013, 46, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, B.; Kolar, F. Cardiac adaptation to chronic high-altitude hypoxia: Beneficial and adverse effects. Respir. Physiol. Neurobiol. 2007, 158, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Knock, G.A.; Ward, J.P. Redox regulation of protein kinases as a modulator of vascular function. Antioxid. Redox. Signal. 2011, 15, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, N.V.; Pinge-Filho, P.; Panis, C.; Silva, B.R.; Pernomian, L.; Grando, M.D.; Cecchini, R.; Bendhack, L.M.; Martins-Pinge, M.C. Decreased endothelial nitric oxide, systemic oxidative stress, and increased sympathetic modulation contribute to hypertension in obese rats. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1472–H1480. [Google Scholar]
- Bailey, D.M.; Evans, K.A.; James, P.E.; McEneny, J.; Young, I.S.; Fall, L.; Gutowski, M.; Kewley, E.; McCord, J.M.; Møller, K.; et al. Altered free radical metabolism in acute mountain sickness: Implications for dynamic cerebral autoregulation and blood-brain barrier function. J. Physiol. 2009, 587, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Dehnert, C.; Luks, A.M.; Menold, E.; Castell, C.; Schendler, G.; Faoro, V.; Gutowski, M.; Evans, K.A.; Taudorf, S.; et al. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J. Physiol. 2010, 588, 4837–4847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Gao, Y.; Liao, W.; Huang, J.; Gao, W. Hypoxia affects mitochondrial protein expression in rat skeletal muscle. OMICS 2012, 16, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; López de Pablo, A.L.; Brito, J.; Arribas, S.M.; Flores, K.; Arriaza, K.; Naveas, N.; González, M.C.; Hoorntje, A.; León-Velarde, F.; et al. Nitric oxide and superoxide anion balance in rats exposed to chronic and long term intermittent hypoxia. Biomed. Res. Int. 2014, 2014, 610474. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Lee, K.; Choi, W.; Sunoo, S.; Kizaki, T.; Oh-ishi, S.; Suzuki, K.; Taniguchi, N.; Ohno, H.; Asano, K. Oxidative stress induced by intermittent exposure at a simulated altitude of 4000 m decreases mitochondrial superoxide dismutase content in soleus muscle of rats. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Ma, H.P.; Jing, L.L.; Li, L.; Jia, Z.P. The antioxidative effect of a novel free radical scavenger 4′-hydroxyl-2-substituted phenylnitronyl nitroxide in acute high-altitude hypoxia mice. Biol. Pharm. Bull. 2013, 36, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.L.; Zhu, H.F.; Dong, J.W.; Zhu, W.Z.; Yang, W.W.; Yang, H.T.; Zhou, Z.N. Inducible nitric oxide synthase contributes to intermittent hypoxia against ischemia/reperfusion injury. Acta Pharmacol. Sin. 2005, 26, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Shamsuzzaman, A.S.; Gersh, B.J.; Somers, V.K. Obstructive sleep apnea: Implications for cardiac and vascular disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Lewicka, J.; Narkiewicz, K. Obstructive sleep apnea: An update on mechanisms and cardiovascular consequences. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.T.; Zhou, Z.N. Cardioprotection of intermittent hypoxia. Acta Physiol. Sin. 2007, 59, 601–613. [Google Scholar]
- Zhuang, J.; Zhou, Z.N. Protective effects of intermittent hypoxic adaptation on myocardium and its mechanism. Biol. Signals Recept. 1999, 8, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhong, N.; Zhu, H.F.; Zhou, Z.N. Antiarrhythmic and antioxidative effects of intermittent hypoxia exposure on rat myocardium. Acta Physiol. Sin. 2000, 52, 89–92. [Google Scholar]
- Zong, P.; Setty, S.; Sun, W.; Martinez, R.; Tune, J.D.; Ehrenburg, I.V.; Tkatchouk, E.N.; Mallet, R.T.; Downey, H.F. Intermittent hypoxic training protects canine myocardium from infarction. Exp. Biol. Med. (Maywood) 2004, 229, 806–812. [Google Scholar]
- Zhu, H.F.; Dong, J.W.; Zhu, W.Z.; Ding, H.L.; Zhou, Z.N. ATP-dependent potassium channels involved in the cardiac protection induced by intermittent hypoxia against ischemia/reperfusion injury. Life Sci. 2003, 25, 1275–1287. [Google Scholar] [CrossRef]
- Kolár, F.; Ostádal, B. Molecular mechanisms of cardiac protection by adaptation to chronic hypoxia. Physiol. Res. 2004, 53 (Suppl. S1), S3–S13. [Google Scholar]
- Gorjao, R.; Azevedo-Martins, A.K.; Rodrigues, H.G.; Abdulkader, F.; Arcisio-Miranda, M.; Procopio, J.; Curi, R. Comparative effects of DHA and EPA on cell function. Pharmacol. Ther. 2009, 122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, P.; Taneja, G. Fish oil and vascular endothelial protection: Bench to bedside. Free Radic. Biol. Med. 2012, 53, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalifa, A.; Maddaford, T.G.; Chahine, M.N.; Austria, J.A.; Edel, A.L.; Richard, M.N.; Ander, B.P.; Gavel, N.; Kopilas, M.; Ganguly, R.; et al. Effect of dietary hempseed intake on cardiac ischemia-reperfusion injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1198–R1203. [Google Scholar] [CrossRef] [PubMed]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; West, S.G. Effects of marine-derived omega-3 fatty acids on systemic hemodynamics at rest and during stress: A dose-response study. Ann. Behav. Med. 2012, 44, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Makazan, Z.; Saini, H.K.; Dhalla, N.S. Role of oxidative stress in alterations of mitochondrial function in ischemic-reperfused hearts. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1986–H1994. [Google Scholar] [CrossRef] [PubMed]
- Kourie, J.I. Interaction of reactive oxygen species with ion transport mechanisms. Am. J. Physiol. 1998, 275, C1–C24. [Google Scholar] [PubMed]
- Eigel, B.N.; Gursahani, H.; Hadley, R.W. ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H955–H963. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Tsai, S.Y.; Ma, M.C.; Wu, M.S. Hypoxic preconditioning enhances renal superoxide dismutase levels in rats. J. Physiol. 2003, 552, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Tajima, F.; Nakamura, A.; Yagura, S.; Ookawara, T.; Yamashita, H.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J. Physiol. 1995, 489, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Z.; Dong, J.W.; Ding, H.L.; Yang, H.T.; Zhou, Z.N. Postnatal development in intermittent hypoxia enhances resistance to myocardial ischemia/reperfusion in male rats. Eur. J. Appl. Physiol. 2004, 91, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Kolar, F.; Jezkova, J.; Balkova, P.; Breh, J.; Neckar, J.; Novak, F.; Nováková, O.; Tomásová, H.; Srbová, M.; Ost’ádal, B.; et al. Role of oxidative stress in PKC-delta upregulation and cardioprotection induced by chronic intermittent hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H224–H230. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Chen, L.; Lu, Z.Z.; Gao, H.; Wu, L.; Chen, Y.X.; Zhang, C.M.; Jiang, Y.K.; Jing, Q.; Zhang, Y.Y.; et al. Activation of α1B-adrenoceptors contributes to intermittent hypobaric hypoxia-improved postischemic myocardial performance via inhibiting MMP-2 activation. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1569–H1581. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Z.; Xie, Y.; Chen, L.; Yang, H.T.; Zhou, Z.N. Intermittent high altitude hypoxia inhibits opening of mitochondrial permeability transition pores against reperfusion injury. J. Mol. Cell. Cardiol. 2006, 40, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Park, A.M.; Suzuki, Y.J. Effects of intermittent hypoxia on oxidative stress-induced myocardial damage in mice. J. Appl. Physiol. 2007, 102, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Polotsky, V.Y.; Savransky, V.; Bevans-Fonti, S.; Reinke, C.; Li, J.; Grigoryev, D.N.; Shimoda, L.A. Intermittent and sustained hypoxia induce a similar gene expression profile in human aortic endothelial cells. Physiol. Genomics 2010, 41, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Yeung, S.C.; Ip, M.S.; Mak, J.C. Intermittent hypoxia-induced NF-κB and HO-1 regulation in human endothelial EA.hy926 cells. Cell Biochem. Biophys. 2013, 66, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Howden, R. Nrf2 and cardiovascular defense. Oxidative Med. Cell. Longev. 2013, 2013, 104308. [Google Scholar]
- Shukla, D.; Saxena, S.; Jayamurthy, P.; Sairam, M.; Singh, M.; Jain, S.K.; Bansal, A.; Ilavazaghan, G. Hypoxic preconditioning with cobalt attenuates hypobaric hypoxia-induced oxidative damage in rat lungs. High Alt. Med. Biol. 2009, 10, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Balková, P.; Hlaváčková, M.; Milerová, M.; Neckář, J.; Kolář, F.; Novák, F.; Nováková, O. N-acetylcysteine treatment prevents the up-regulation of MnSOD in chronically hypoxic rat hearts. Physiol. Res. 2011, 60, 467–474. [Google Scholar] [PubMed]
- Huang, X.S.; Chen, H.P.; Yu, H.H.; Yan, Y.F.; Liao, Z.P.; Huang, Q.R. Nrf2-dependent upregulation of antioxidative enzymes: A novel pathway for hypoxic preconditioning-mediated delayed cardioprotection. Mol. Cell. Biochem. 2014, 385, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zhang, Y.; Fang, Q.Z.; Zhou, Z.N. Intermittent hypoxia exposure-induced heat-shock protein 70 expression increases resistance of rat heart to ischemic injury. Acta Pharmacol. Sin. 2000, 21, 467–472. [Google Scholar] [PubMed]
- Ding, H.L.; Zhu, H.F.; Dong, J.W.; Zhu, W.Z.; Zhou, Z.N. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci. 2004, 75, 2587–2603. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.M.; Farías, J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Manalo, D.J.; Wei, G.; Rodriguez, E.R.; Fox-Talbot, K.; Lu, H.; Zweier, J.L.; Semenza, G.L. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia reperfusion injury. Circulation 2003, 108, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhong, H.; Bosch-Marce, M.; Fox-Talbot, K.; Wang, L.; Wei, C.; Trush, M.A.; Semenza, G.L. Complete loss of ischemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc. Res. 2008, 77, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Salloum, F.N.; Fisher, B.J.; Kukreja, R.C.; Fowler, A.A. Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circ. Res. 2006, 98, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Salloum, F.N.; Fisher, B.J.; Ownby, E.D.; Kukreja, R.C.; Fowler, A.A., 3rd. Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1571–H1580. [Google Scholar] [CrossRef] [PubMed]
- Tekin, D.; Dursun, A.D.; Xi, L. Hypoxia inducible factor 1 (HIF-1) and cardioprotection. Acta Pharmacol. Sin. 2010, 31, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Salloum, F.N.; Fisher, B.J.; Smithson, L.; Almenara, J.; Fowler, A.A., 3rd. Prolyl hydroxylase inhibition attenuates post-ischemic cardiac injury via induction of endoplasmic reticulum stress genes. Vasc. Pharmacol. 2009, 51, 110–118. [Google Scholar] [CrossRef]
- Afshordel, S.; Hagl, S.; Werner, D.; Röhner, N.; Kögel, D.; Bazan, N.G.; Eckert, G.P. Omega-3 polyunsaturated fatty acids improve mitochondrial dysfunction in brain aging—Impact of Bcl-2 and NPD-1 like metabolites. Prostaglandins Leukot. Essent. Fatty Acids 2014. [Google Scholar] [CrossRef]
- Flachs, P.; Horakova, O.; Brauner, P.; Rossmeisl, M.; Pecina, P.; Franssen-van Hal, N.; Ruzickova, J.; Sponarova, J.; Drahota, Z.; Vlcek, C.; et al. Polyunsaturated fatty acids of marine origin upregulate mitochondrial biogenesis and induce beta-oxidation in white fat. Diabetologia 2005, 48, 2365–2375. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, X.; Liu, Y.; Xia, M. Dietary supplementation with fish oil alters the expression levels of proteins governing mitochondrial dynamics and prevents high-fat diet-induced endothelial dysfunction. Br. J. Nutr. 2014, 112, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.T.; Royse, C.; Royse, A.G. The mitochondrial permeability transition pore and its role in anaesthesia-triggered cellular protection during ischaemia-reperfusion injury. Anaesth. Intensive Care 2012, 40, 46–70. [Google Scholar] [PubMed]
- Mozaffarian, D.; Willett, W.C. Trans fatty acids and cardiovascular risk: A unique cardiometabolic imprint? Curr. Atheroscler. Rep. 2007, 9, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.; Oszust, F.; Guillaume, C.; Millart, H.; Laurent-Maquin, D.; Brou, C.; Bausero, P.; Visioli, F. Infusion of docosahexaenoic acid protects against myocardial infarction. Prostaglandins Leukot. Essent. Fatty Acids 2014, 90, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Arias, C.; Farías, J.G. Omega 3 chronic supplementation attenuates myocardial ischaemia-reperfusion injury through reinforcement of antioxidant defense system in rats. Cell Biochem. Funct. 2014, 32, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Abdukeyum, G.G.; Owen, A.J.; McLennan, P.L. Dietary (n-3) long-chain polyunsaturated fatty acids inhibit ischemia and reperfusion arrhythmias and infarction in rat heart not enhanced by ischemic preconditioning. J. Nutr. 2008, 138, 1902–1909. [Google Scholar] [PubMed]
- Erdogan, H.; Fadillioglu, E.; Ozgocmen, S.; Sogut, S.; Ozyurt, B.; Akyol, O.; Ardicoglu, O. Effect of fish oil supplementation on plasma oxidant/antioxidant status in rats. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Sekhar, K.R.; Yin, H.; Yared, N.F.; Schneider, S.N.; Sasi, S.; Dalton, T.P.; Anderson, M.E.; Chan, J.Y.; et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007, 282, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Leifert, W.R.; Kind, K.L.; McMurchie, E.J. Dietary fish oil alters cardiomyocyte Ca2+ dynamics and antioxidant status. Free Radic. Biol. Med. 2006, 40, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Giannakou, M.E.; Partridge, L. The interaction between FOXO and SIRT1: Tipping the balance towards survival. Trends Cell Biol. 2004, 14, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Von Schacky, C. N-3 PUFA in CVD: Influence of cytokine polymorphism. Proc. Nutr. Soc. 2007, 66, 166–170. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Ruzmetov, N.; Miller, S.J.; Zaloga, G.P. N-3 fatty acids prevent whereas trans-fatty acids induce vascular inflammation and sudden cardiac death. Br. J. Nutr. 2009, 102, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, K.; Yang, R.; Porter, T.F.; Agrawal, N.; Petasis, N.A.; Irimia, D.; Toner, M.; Serhan, C.N. Rapid appearance of resolvin precursors in inflammatory exudates: Novel mechanisms in resolution. J. Immunol. 2008, 181, 8677–8687. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; McGuinness, J.; Chen, G.; Hill, A.D.; Redmond, M.J. Intravenous omega-3, a technique to prevent an excessive innate immune response to cardiac surgery in a rodent gut ischemia model. J. Thorac. Cardiovasc. Surg. 2011, 141, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Rodrigo, R.; Cereceda, M.; Asenjo, R.; Zamorano, J.; Navarrete, R.; Villalabeitia, E.; Sanz, J.; Baeza, C.; Aguayo, R. Antioxidant therapy reduces oxidative and inflammatory tissue damage in patients subjected to cardiac surgery with extracorporeal circulation. Bas. Clin. Pharmacol. Toxicol. 2011, 108, 256–262. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Z.; Wang, X.; Shi, H. Omega-3 polyunsaturated fatty acids antagonize macrophage inflammation via activation of AMPK/SIRT1 pathway. PLoS One 2012, 7, e45990. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. The salutary effects of dha dietary supplementation on cognition, neuroplasticity, and membrane homeostasis after brain trauma. J. Neurotrauma 2011, 28, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Schenk, S.; Imamura, T.; Babendure, J.L.; Sonoda, N.; Bae, E.J.; Oh, D.Y.; Lu, M.; Milne, J.C.; Westphal, C.; et al. Sirt1 inhibits inflammatory pathways in macrophages and modulates insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E419–E428. [Google Scholar] [CrossRef] [PubMed]
- Tähepõld, P.; Vaage, J.; Starkopf, J.; Valen, G. Hyperoxia elicits myocardial protection through a nuclear factor kappaB-dependent mechanism in the rat heart. J. Thorac. Cardiovasc. Surg. 2003, 125, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Guo, F.; Zhang, L.N.; Zhang, R.; Chen, Q.; Li, J.X.; Yin, J.; Wang, Y.L. Enhancement of Na/K pump activity by chronic intermittent hypobaric hypoxia protected against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2280–H2287. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Falcão-Pires, I.; Gonçalves, I.O.; Lumini-Oliveira, J.; Marques-Aleixo, I.; Dos Passos, E.; Rocha-Rodrigues, S.; Machado, N.G.; Moreira, A.C.; Miranda-Silva, D.; et al. Synergistic impact of endurance training and intermittent hypobaric hypoxia on cardiac function and mitochondrial energetic and signaling. Int. J. Cardiol. 2013, 168, 5363–5371. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Li, Q.; Ma, H.J.; Guan, Y.; Shi, M.; Yang, J.; Li, D.P.; Zhang, Y. Chronic intermittent hypobaric hypoxia ameliorates ischemia/reperfusion-induced calcium overload in heart via Na/Ca2+ exchanger in developing rats. Cell. Physiol. Biochem. 2014, 34, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, J.; Gonçalves, I.O.; Lumini-Oliveira, J.; Marques-Aleixo, I.; Passos, E.; Rocha-Rodrigues, S.; Machado, N.G.; Moreira, A.C.; Rizo, D.; Viscor, G.; et al. Modulation of cardiac mitochondrial permeability transition and apoptotic signaling by endurance training and intermittent hypobaric hypoxia. Int. J. Cardiol. 2014, 173, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.C.; Zhang, Z.; Zhang, L.N.; Xiong, C.; Feng, C.; Liu, Q.; Liu, X.; Shi, X.L.; Wang, Y.L. Ischemia/reperfusion injury through upregulation of antioxidant enzymes in adult guinea pigs. Chronic intermittent hypobaric hypoxia protects the heart against. Acta Pharmacol. Sin. 2009, 30, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.L.; Farías, J.G.; Herrera, E.A.; Álvarez, P.I.; Short, S.E.; Tapia, L.; Carrasco, R.; Sotomayor-Zárate, R. Effects of Chronic Intermittent Hypoxia and Polyunsaturated Fatty Acids on Infarct Size and Oxidative Stress Markers in Cardiac Ischemia Reperfusion. Exp. Clin. Cardiol. 2014, 20, 3833–3858. [Google Scholar]
- Panisello, P.; Torrella, J.R.; Esteva, S.; Pagés, T.; Viscor, G. Capillary supply, fibre types and fibre morphometry in rat tibialis anterior and diaphragm muscles after intermittent exposure to hypobaric hypoxia. Eur. J. Appl. Physiol. 2008, 103, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.L.; Chou, S.W.; Cho, Y.M.; Ho, H.Y.; Ivy, J.L.; Hunt, D.; Wang, P.S.; Kuo, C.H. Effect of prolonged intermittent hypoxia and exercise training on glucose tolerance and muscle GLUT4 protein expression in rats. J. Biomed. Sci. 2004, 11, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Siques, P.; Brito, J.; Naveas, N.; Pulido, R.; De la Cruz, J.J.; Mamani, M.; León-Velarde, F. Plasma and liver lipid profiles in rats exposed to chronic hypobaric hypoxia: Changes in metabolic pathways. High Alt. Med. Biol. 2014, 15, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Zeghichi-Hamri, S.; de Lorgeril, M.; Salen, P.; Chibane, M.; de Leiris, J.; Boucher, F.; Laporte, F. Protective effect of dietary n-3 polyunsaturated fatty acids on myocardial resistance to ischemia-reperfusion injury in rats. Nutr. Res. 2010, 30, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Rodrigo, R.; Orellana, M.; Rivera, G. Red wine raises plasma HDL and preserves long-chain polyunsaturated fatty acids in rat kidney and erythrocytes. Br. J. Nutr. 2001, 86, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Farias, J.G.; Puebla, M.; Acevedo, A.; Tapia, P.J.; Gutierrez, E.; Zepeda, A.; Calaf, G.; Juantok, C.; Reyes, J.G. Oxidative stress in rat testis and epididymis under intermittent hypobaric hypoxia: Protective role of ascorbate supplementation. J. Androl. 2010, 31, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Trimble, E.R. Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Ann. Clin. Biochem. 1991, 28, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.A.; Reyes, R.V.; Giussani, D.A.; Riquelme, R.A.; Sanhueza, E.M.; Ebensperger, G.; Casanello, P.; Mendez, N.; Ebensperger, R.; Sepulveda-Kattan, E.; et al. Carbon monoxide: A novel pulmonary artery vasodilator in neonatal llamas of the Andean altiplano. Cardiovasc. Res. 2008, 77, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Speisky, H.; Brunser, O.; Pastene, E.; Gotteland, M. Apple peel polyphenols protect against gastrointestinal mucosa alterations induced by indomethacin in rats. J. Agric. Food Chem. 2011, 59, 6459–6466. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, E.A.; Farías, J.G.; González-Candia, A.; Short, S.E.; Carrasco-Pozo, C.; Castillo, R.L. Ω3 Supplementation and Intermittent Hypobaric Hypoxia Induce Cardioprotection Enhancing Antioxidant Mechanisms in Adult Rats. Mar. Drugs 2015, 13, 838-860. https://doi.org/10.3390/md13020838
Herrera EA, Farías JG, González-Candia A, Short SE, Carrasco-Pozo C, Castillo RL. Ω3 Supplementation and Intermittent Hypobaric Hypoxia Induce Cardioprotection Enhancing Antioxidant Mechanisms in Adult Rats. Marine Drugs. 2015; 13(2):838-860. https://doi.org/10.3390/md13020838
Chicago/Turabian StyleHerrera, Emilio A., Jorge G. Farías, Alejandro González-Candia, Stefania E. Short, Catalina Carrasco-Pozo, and Rodrigo L. Castillo. 2015. "Ω3 Supplementation and Intermittent Hypobaric Hypoxia Induce Cardioprotection Enhancing Antioxidant Mechanisms in Adult Rats" Marine Drugs 13, no. 2: 838-860. https://doi.org/10.3390/md13020838






