Nano- and Microdelivery Systems for Marine Bioactive Lipids
Abstract
:1. Fatty Acids in Health & Disease
2. Nano- and Microencapsulation
3. Case Studies: Micro- and Nanodelivery Systems for Fatty Acids
3.1. Lipidic Systems
3.1.1. Liposomes
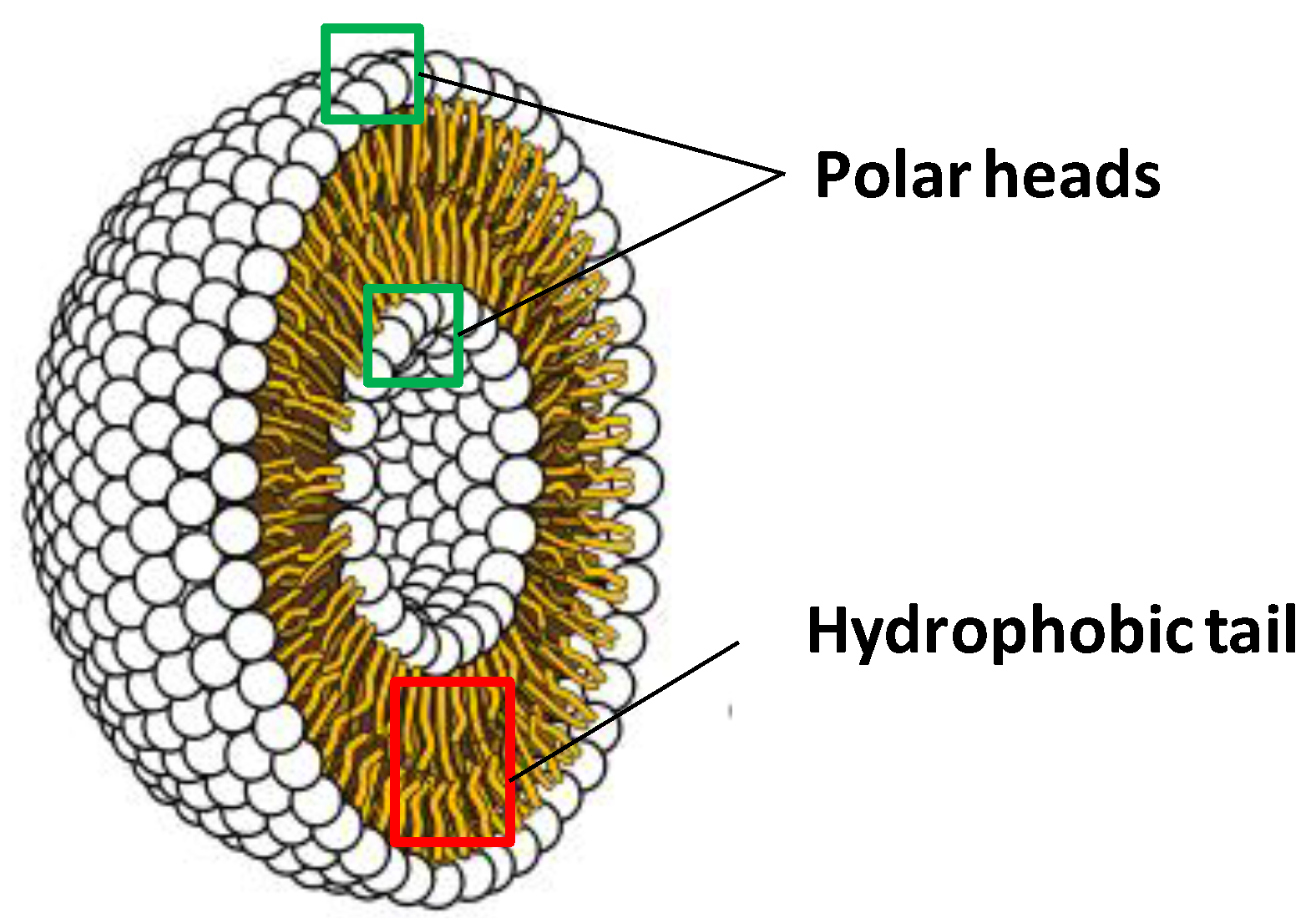
3.1.2. Multi-Layered Emulsions
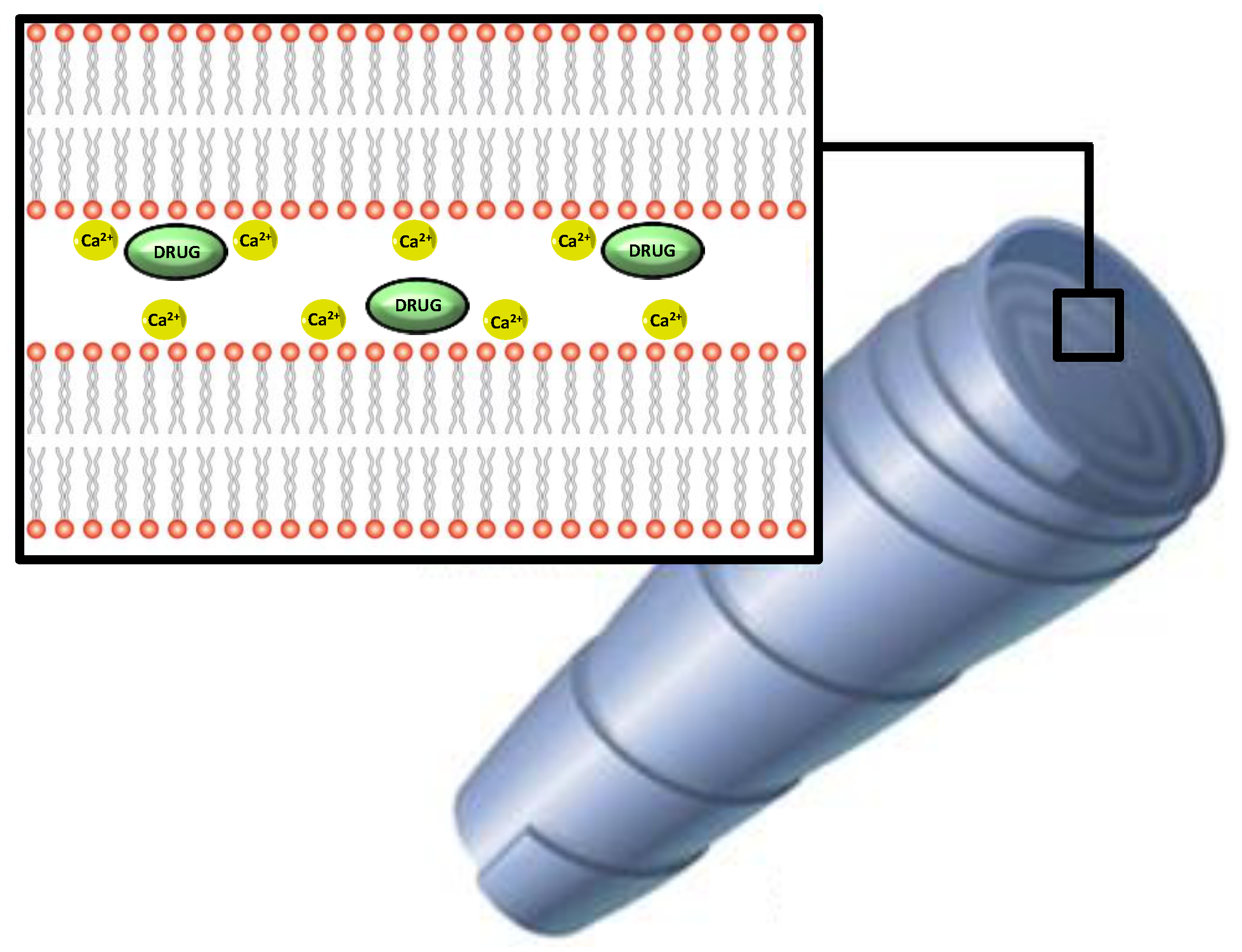
3.1.3. Cochleates
3.1.4. Simple Emulsion-Based Systems
3.2. Non-Lipidic Systems
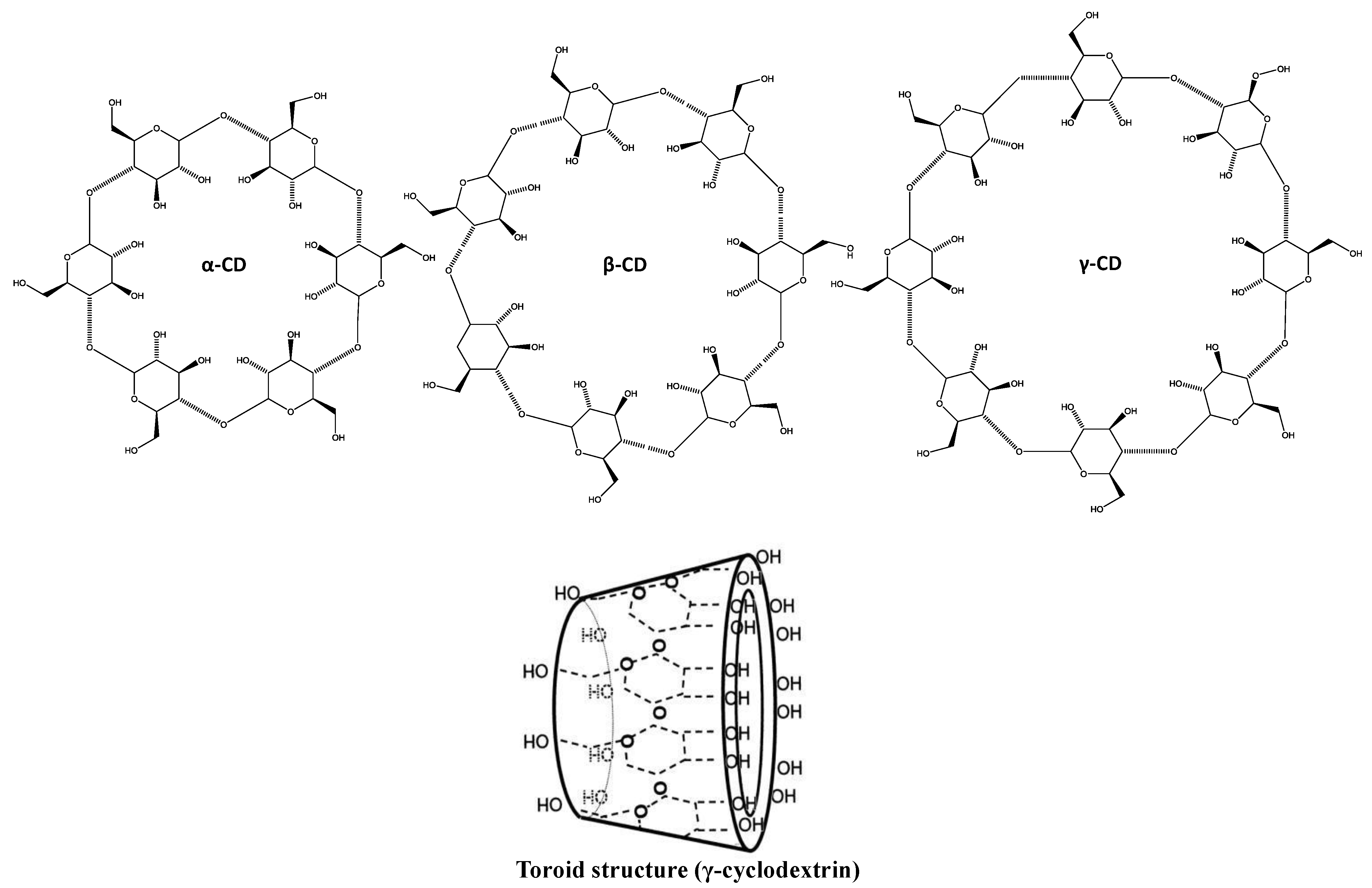
3.2.1. Cyclodextrins
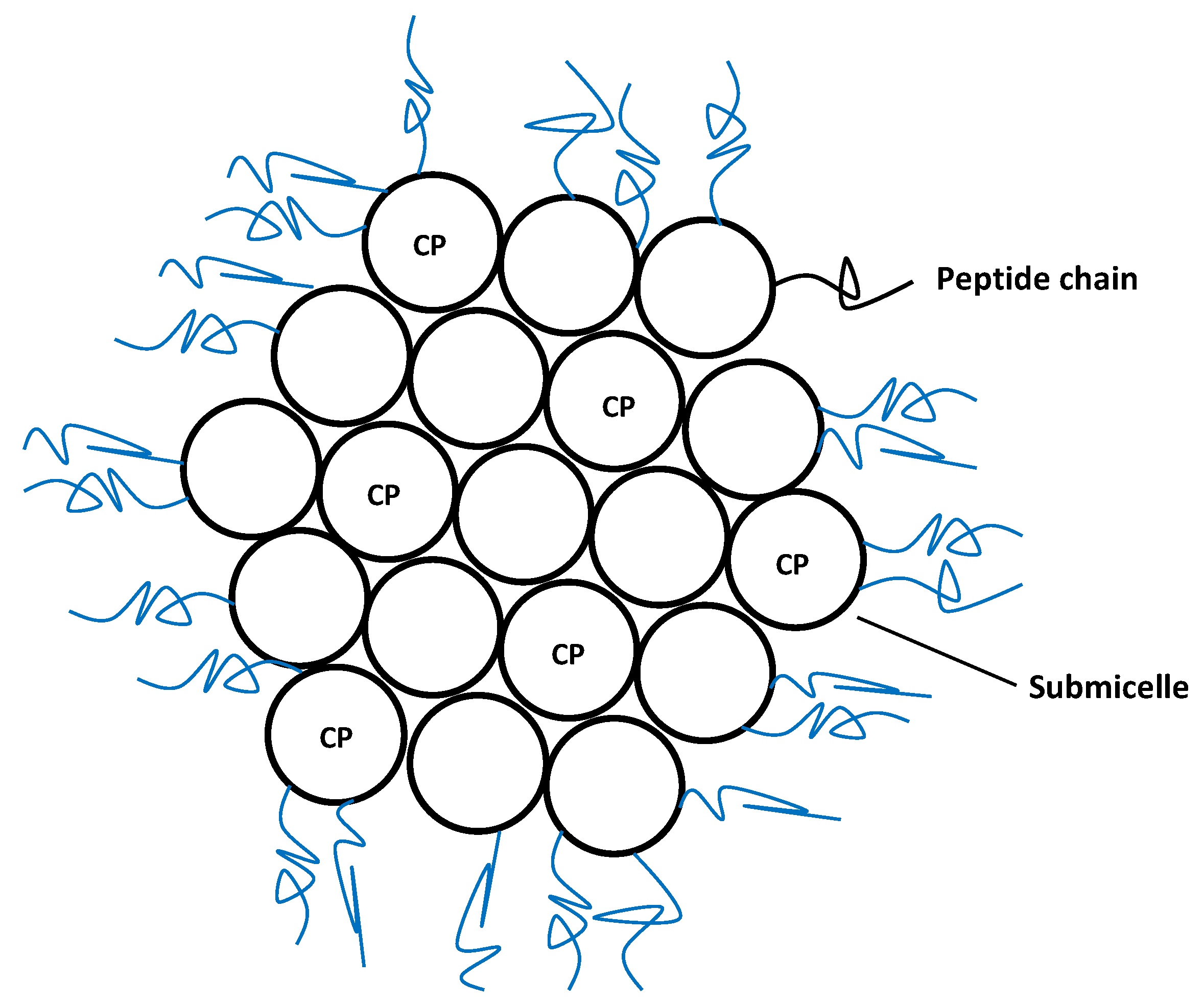
3.2.2. Caseins
3.2.3. Zein Nanoparticles
4. Conclusions
Conflicts of Interest
References
- Pereira, D.M.; Vinholes, J.; Correia-da-Silva, G.; Valentao, P.; Teixeira, N.; Andrade, P.B. Fatty acids in marine organisms: In the pursuit of bioactive agents. Curr. Pharm. Anal. 2011, 7, 108–119. [Google Scholar] [CrossRef]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.; Capps, N.E. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002, 287, 1815–1821. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Omega-3 fatty acids and cardiovascular disease new recommendations from the American Heart Association. Arterioscl. Throm. Vas. 2003, 23, 151–152. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar]
- Simopoulos, A.P. Importance of the ratio of omega-6/omega-3 essential fatty acids: Evolutionary aspects. World Rev. Nutr. Diet. 2003, 92, 1–22. [Google Scholar] [PubMed]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Essential fatty acids in health and chronic diseases. Forum Nutr. 2003, 56, 67–70. [Google Scholar] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Lessons from the sea: Distribution, SAR and molecular mechanisms of anti-inflammatory drugs from marine organisms. In Studies in Natural Products Chemistry (Bioactive Natural Products); Atta-ur-Rahman, Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Choi, M.-J.; Ruktanonchai, U.; Min, S.-G.; Chun, J.-Y.; Soottitantawat, A. Physical characteristics of fish oil encapsulated by β-cyclodextrin using an aggregation method or polycaprolactone using an emulsion-diffusion method. Food Chem. 2010, 119, 1694–1703. [Google Scholar] [CrossRef]
- Katsuda, M.S.; McClements, D.J.; Miglioranza, L.H.S.; Decker, E.A. Physical and oxidative stability of fish oil-in-water emulsions stabilized with β-lactoglobulin and pectin. J. Agric. Food Chem. 2008, 56, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, W.; Jaworska, D.; Weißbrodt, J.; Kunz, B. Sensory assessment of microencapsulated fish oil powder. J. Am. Oil Chem. Soc. 2007, 84, 37–45. [Google Scholar] [CrossRef]
- Kolanowski, W.; Ziolkowski, M.; Weißbrodt, J.; Kunz, B.; Laufenberg, G. Microencapsulation of fish oil by spray drying-impact on oxidative stability. Part 1. Eur. Food Res. Technol. 2006, 222, 336–342. [Google Scholar] [CrossRef]
- Serfert, Y.; Drusch, S.; Schwarz, K. Sensory odour profiling and lipid oxidation status of fish oil and microencapsulated fish oil. Food Chem. 2010, 123, 968–975. [Google Scholar] [CrossRef]
- Szente, L.; Szejtli, J.; Szemán, J.; Kató, L. Fatty acid-cyclodextrin complexes: Properties and applications. J. Incl. Phenom. Macro. 1993, 16, 339–354. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Ocio, M.J.; Lagaron, J.M. Stabilization of a nutraceutical omega-3 fatty acid by encapsulation in ultrathin electrosprayed zein prolamine. J. Food Sci. 2010, 75, N69–N79. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hasegawa, N.; Doi, U.; Umekawa, H.; Takahashi, T.; Suzuki, K.; Furuichi, Y. Biological availability of docosahexaenoic acid from fish oil encapsulated in zein-coated porous starch granules in rats. Food Sci. Technol. Res. 2000, 6, 87–93. [Google Scholar] [CrossRef]
- Zimet, P.; Rosenberg, D.; Livney, Y.D. Re-assembled casein micelles and casein nanoparticles as nano-vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2011, 25, 1270–1276. [Google Scholar] [CrossRef]
- Choi, M.-J.; Ruktanonchai, U.; Soottitantawat, A.; Min, S.-G. Morphological characterization of encapsulated fish oil with β-cyclodextrin and polycaprolactone. Food. Res. Int. 2009, 42, 989–997. [Google Scholar] [CrossRef]
- Kwak, H.-S. Nano- and Microencapsulation for Foods; Wiley-Blackwell: Oxford, UK, 2014. [Google Scholar]
- Ahmad, M.; Madni, A.; Usman, M.; Munir, A.; Akhtar, N.; Khan, H.S. Pharmaceutical micro encapsulation technology for development of controlled release drug delivery systems. WASET 2011, 75, 384–387. [Google Scholar]
- Lam, P.; Gambari, R. Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. J. Control. Release 2014, 178, 25–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hemant, K.; Ram, M.; Shivakumar, H. Microencapsulation: A promising technique for controlled drug delivery. Res. Pharm. Sci. 2010, 5, 65–77. [Google Scholar] [PubMed]
- Garg, M.; Wood, L.; Singh, H.; Moughan, P. Means of delivering recommended levels of long chain n-3 polyunsaturated fatty acids in human diets. J. Food Sci. 2006, 71, R66–R71. [Google Scholar] [CrossRef]
- Taneja, A.; Singh, H. Challenges for the delivery of long-chain n-3 fatty acids in functional foods. Annu. Rev. Food Sci. T. 2012, 3, 105–123. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent developments in microencapsulation of food ingredients. Dry Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Anton, N.; Benoit, J.-P.; Saulnier, P. Design and production of nanoparticles formulated from nano-emulsion templates—A review. J. Control. Release 2008, 128, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Mozafari, M.; Mohebbi, M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends Food Sci. Tech. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloid Surf. B 2010, 75, 356–362. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Mustafa, G.; Akhter, S.; Iqbal, Z. Design and development of oral oil-in-water nanoemulsion formulation bearing atorvastatin: In vitro assessment. J. Disper. Sci. Technol. 2010, 31, 690–701. [Google Scholar] [CrossRef]
- Gutiérrez, J.; González, C.; Maestro, A.; Sole, I.; Pey, C.; Nolla, J. Nano-emulsions: New applications and optimization of their preparation. Curr. Opin. Colloid Interface Sci. 2008, 13, 245–251. [Google Scholar] [CrossRef]
- Let, M.B.; Jacobsen, C.; Frankel, E.N.; Meyer, A.S. Oxidative flavour deterioration of fish oil enriched milk. Eur. J. Lipid Sci. Tech. 2003, 105, 518–528. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; Oily Press Lipid Library Series, Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Anwar, S.H.; Kunz, B. The influence of drying methods on the stabilization of fish oil microcapsules: Comparison of spray granulation, spray drying, and freeze drying. J. Food Eng. 2011, 105, 367–378. [Google Scholar] [CrossRef]
- Arunagirinathan, G.H.S.M.; Bellare, J.R. Self-assembled surfactant nano-structures important in drug delivery: A review. Indian J. Exp. Biol. 2007, 45, 133–159. [Google Scholar] [PubMed]
- Kaya-Celiker, H.; Mallikarjunan, K. Better nutrients and therapeutics delivery in food through nanotechnology. Food. Eng. Rev. 2012, 4, 114–123. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, N.R.; Sevrin, C.; Lespineux, D.; Bovin, N.V.; Vodovozova, E.L.; Mészáros, T.; Szebeni, J.; Grandfils, C. Hemocompatibility of liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the lipid bilayer. J. Control. Release 2012, 160, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Mufamadi, M.S.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Modi, G.; Naidoo, D.; Ndesendo, V.M. A review on composite liposomal technologies for specialized drug delivery. J. Drug Deliv. 2011, 2011. [Google Scholar] [CrossRef]
- Chen, H.; Weiss, J.; Shahidi, F. Nanotechnology in nutraceuticals and functional foods. Food Technol. 2006, 60, 30–36. [Google Scholar]
- Alves, M.P.; Raffin, R.P.; Fagan, S.B. Rheological behavior of semisolid formulations containing nanostructured systems. In Nanocosmetics and Nanomedicines; Springer: Berlin Heidelberg, Germany, 2011; pp. 37–45. [Google Scholar]
- Lu, T.; Zhao Wang, Y.M.; Zhang, Y.; Chen, T. Influence of polymer size, liposomal composition, surface charge, and temperature on the permeability of pH-sensitive liposomes containing lipid-anchored poly (2-ethylacrylic acid). Int. J. Nanomed. 2012, 7, 4917–4926. [Google Scholar] [CrossRef]
- Taylor, T.M.; Weiss, J.; Davidson, P.M.; Bruce, B.D. Liposomal nanocapsules in food science and agriculture. Crit. Rev. Food Sci. Nutr. 2005, 45, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Cansell, M.; Nacka, F.; Combe, N. Marine lipid-based liposomes increase in vivo FA bioavailability. Lipids 2003, 38, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Gudipati, V.; Sandra, S.; McClements, D.J.; Decker, E.A. Oxidative stability and in vitro digestibility of fish oil-in-water emulsions containing multilayered membranes. J. Agric. Food Chem. 2010, 58, 8093–8099. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.A.; McClements, D.J.; Decker, E.A. Spray-dried multilayered emulsions as a delivery method for ω-3 fatty acids into food systems. J. Agric. Food Chem. 2007, 55, 3112–3119. [Google Scholar] [CrossRef] [PubMed]
- Zarif, L.; Graybill, J.R.; Perlin, D.; Mannino, R.J. Cochleates: new lipid-based drug delivery system. J. Liposome Res. 2000, 10, 523–538. [Google Scholar] [CrossRef]
- Evans, C.C.; Zasadzinski, J. Encapsulating vesicles and colloids from cochleate cylinders. Langmuir 2003, 19, 3109–3113. [Google Scholar] [CrossRef]
- Zarif, L. Drug delivery by lipid cochleates. Methods Enzymol. 2005, 391, 314–329. [Google Scholar] [PubMed]
- Santangelo, R.; Paderu, P.; Delmas, G.; Chen, Z.-W.; Mannino, R.; Zarif, L.; Perlin, D.S. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 2000, 44, 2356–2360. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S. Sugar beet pectin: A novel emulsifying wall component for microencapsulation of lipophilic food ingredients by spray-drying. Food Hydrocoll. 2007, 21, 1223–1228. [Google Scholar] [CrossRef]
- Klaypradit, W.; Huang, Y.-W. Fish oil encapsulation with chitosan using ultrasonic atomizer. LWT—Food Sci. Technol. 2008, 41, 1133–1139. [Google Scholar] [CrossRef]
- Habib, S.M.; Amr, A.S.; Hamadneh, I.M. Nanoencapsulation of alpha-linolenic acid with modified emulsion diffusion method. J. Am. Oil Chem. Soc. 2012, 89, 695–703. [Google Scholar] [CrossRef]
- Saenger, W.; Jacob, J.; Gessler, K.; Steiner, T.; Hoffmann, D.; Sanbe, H.; Koizumi, K.; Smith, S.M.; Takaha, T. Structures of the common cyclodextrins and their larger analogues beyond the doughnut. Chem. Rev. 1998, 98, 1787–1802. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M.; Brewster, M.E. Self-association of cyclodextrins and cyclodextrin complexes. J. Pharm. Sci.-US 2004, 93, 1091–1099. [Google Scholar] [CrossRef]
- Na, H.-S.; Kim, J.-N.; Kim, J.-M.; Lee, K.-Y. Encapsulation of fish oil using cyclodextrin and whey protein concentrate. Biotechnol. Bioproc. E. 2011, 16, 1077–1082. [Google Scholar] [CrossRef]
- Dickinson, E.; Singh, H. Protein functionality and aggregation. Milk protein functionality in food colloids. In Food Colloids: Interactions, Microstructure and Processing; Royal Society of Chemistry: Cambridge, UK, 2005. [Google Scholar]
- Zhang, Z.; Decker, E.A.; McClements, D.J. Encapsulation, protection, and release of polyunsaturated lipids using biopolymer-based hydrogel particles. Food Res. Int. 2014, 64, 520–526. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Zhu, F.; Kale, A.V.; Cheryan, M. Fractionation of zein by size exclusion chromatography. J. Agric. Food Chem. 2007, 55, 3843–3849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, Q.; Wang, H.; Zhang, L.; Wang, J.-Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials 2005, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Lin, Z.-X.; Liu, X.-M.; Sheng, S.-Y.; Wang, J.-Y. Heparin-loaded zein microsphere film and hemocompatibility. J. Control. Release 2005, 105, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Tian, H.; Zivanovic, S. Encapsulation of fish oil in solid zein particles by liquid-liquid dispersion. J. Food Process. Pres. 2009, 33, 255–270. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, D.M.; Valentão, P.; Andrade, P.B. Nano- and Microdelivery Systems for Marine Bioactive Lipids. Mar. Drugs 2014, 12, 6014-6027. https://doi.org/10.3390/md12126014
Pereira DM, Valentão P, Andrade PB. Nano- and Microdelivery Systems for Marine Bioactive Lipids. Marine Drugs. 2014; 12(12):6014-6027. https://doi.org/10.3390/md12126014
Chicago/Turabian StylePereira, David M., Patrícia Valentão, and Paula B. Andrade. 2014. "Nano- and Microdelivery Systems for Marine Bioactive Lipids" Marine Drugs 12, no. 12: 6014-6027. https://doi.org/10.3390/md12126014




