Limited Impact of 2 g/day Omega-3 Fatty Acid Ethyl Esters (Omacor®) on Plasma Lipids and Inflammatory Markers in Patients Awaiting Carotid Endarterectomy
Abstract
:1. Introduction
2. Patients, Materials, and Methods
2.1. Study Design
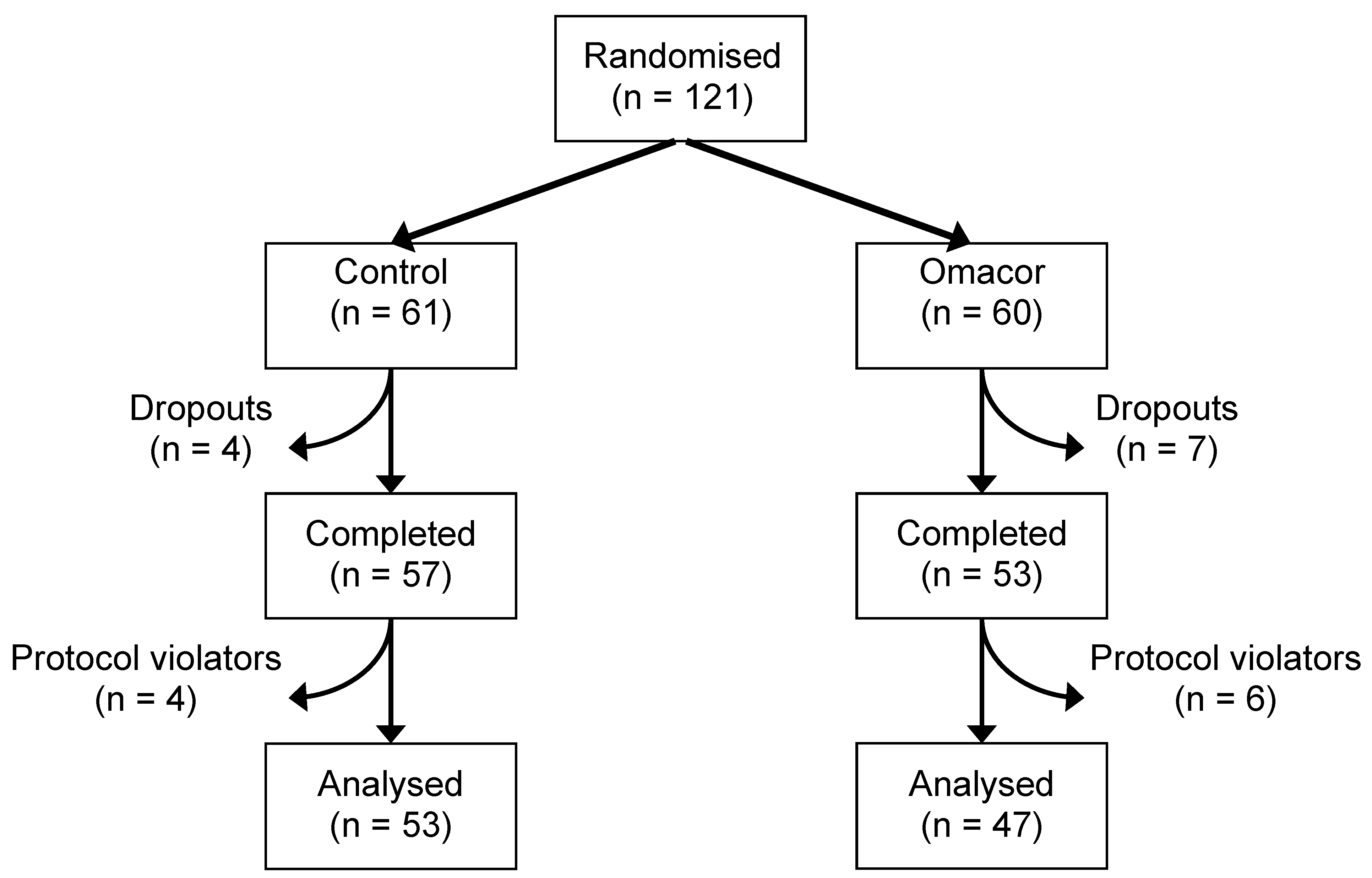
2.2. Measurement of Plasma Lipid Concentrations
2.3. Measurement of Plasma Inflammatory Marker Concentrations
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Patients
| Omacor® (n = 47) | Placebo (n = 53) | |
|---|---|---|
| Sex (n) | ||
| Male | 32 | 36 |
| Female | 15 | 17 |
| Smoking status (n) | ||
| Yes | 8 | 8 |
| No | 8 | 11 |
| Ex-smokers | 31 | 34 |
| Medication use (n) | ||
| Aspirin | 41 | 38 |
| Anti-coagulant | 13 | 5 |
| Beta-blocker | 17 | 16 |
| Calcium channel blocker | 18 | 16 |
| ACE-inhibitors | 28 | 27 |
| Statin | 45 | 39 |
| Diuretics | 26 | 25 |
| Nitrates | 13 | 8 |
| Oral hypoglycaemic agents | 10 | 13 |
| Insulin | 2 | 1 |
| Age (year) | 72.0 (10.7) | 73.0 (8.3) |
| BMI (kg/m2) | 27.1 (4.9) | 26.5 (3.7) |
| Systolic blood pressure (mm Hg) | 155.3 (27.9) | 155.2 (22.1) |
| Diastolic blood pressure (mm Hg) | 80.6 (13.9) | 82.0 (13.3) |
| Total cholesterol (mmol/L) | 4.8 (1.1) | 4.9 (1.2) |
| LDL-cholesterol (mmol/L) | 2.5 (1.6–4.3) | 2.7 (1.5–4.4) |
| HDL-cholesterol (mmol/L) | 1.3 (0.9–2.4) | 1.2 (0.9–1.9) |
| Triglycerides (mmol/L) | 1.3 (0.7–2.2) | 1.3 (0.7–2.6) |
| Total cholesterol:HDL-cholesterol ratio | 3.2 (2.4–4.8) | 3.8 (2.6–5.0) |
| LDL-cholesterol:HDL-cholesterol ratio | 1.8 (1.0–3.6) | 2.2 (1.3–3.2) |
| sICAM-1 (ng/mL) | 167 (73–426) | 216 (65–445) |
| sVCAM-1(ng/mL) | 673 (226–1578) | 594 (272–1131) |
| sE-selectin (ng/mL) | 92.0 (33.0–234.4) | 91.4 (15.6–290.0) |
| IL-6 (pg/mL) | 1.2 (0.4–4.0) | 1.2 (0.1–4.8) |
| IL-10 (pg/mL) | 1.5 (0–5.24) | 0.5 (0–2.9) |
| MMP-2 (ng/mL) | 192 (129-290) | 191 (129–294) |
| MMP-9 (ng/mL) | 167 (47–421) | 138 (29–389) |
| TGF-β1 (ng/mL) | 9308 (2394–19170) | 9788 (3356–16844) |
| CRP (mg/L) | 1.0 (1.0–31.7) | 1.0 (1.0–9.1) |
| sCD40L (pg/mL) | 776 (243–2239) | 774 (213–3140) |
| IP-10 (pg/mL) | 103.9 (51.6–273.3) | 102.8 (47.8–289.4) |
| MIG (pg/mL) | 119.3 (37.9–360.7) | 107.4 (30.6–294.3) |
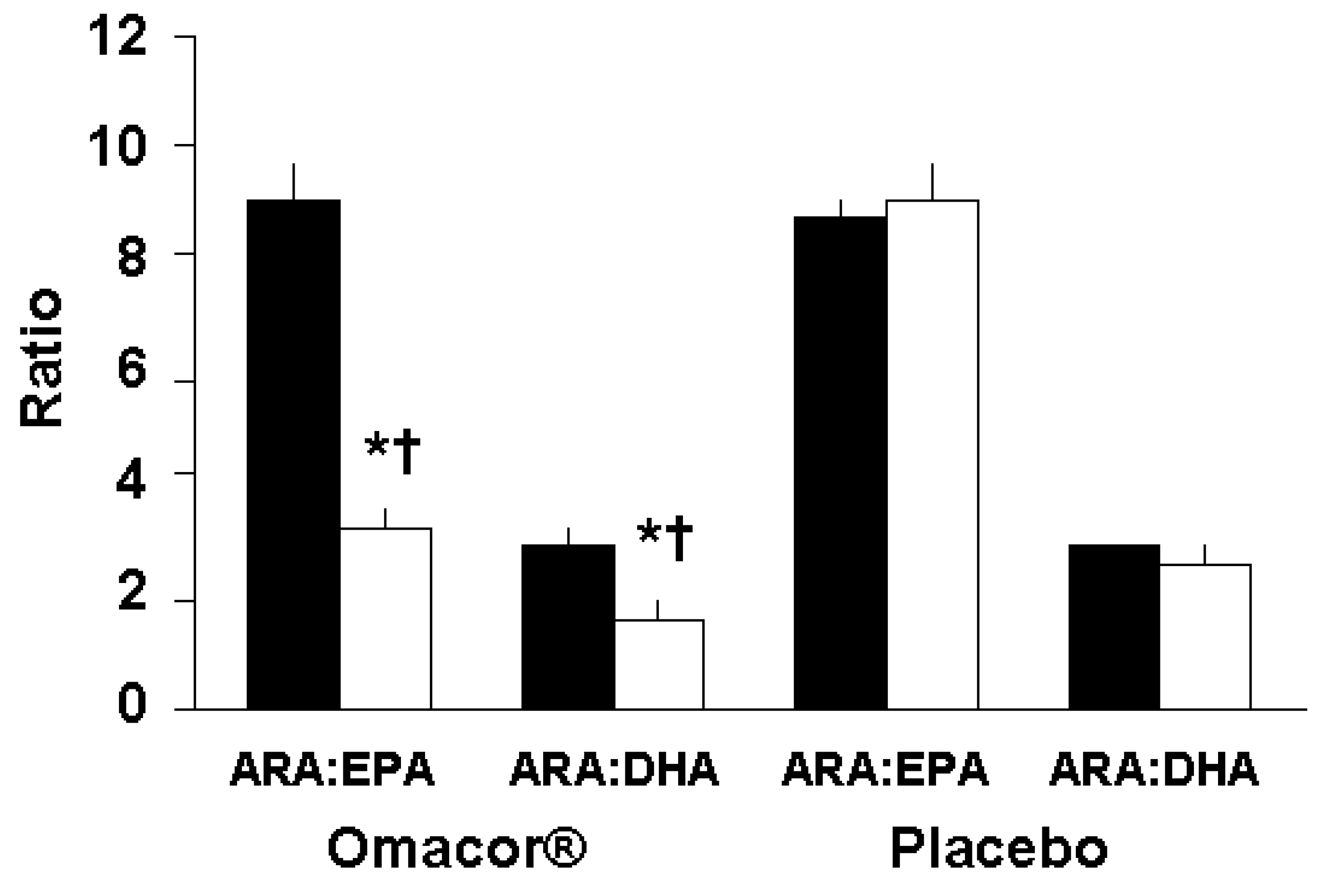
3.2. Effect of Supplementation on BMI, Blood Pressure, and Plasma Lipid Profile
3.3. Effect of Supplementation on Plasma Inflammatory Markers
| Omacor® (n = 47) | Placebo (n = 53) | ||||
|---|---|---|---|---|---|
| Before | After | Baseline | After | ||
| BMI (kg/m2) | 27.1 (4.9) | 27.0 (4.8) | 26.5 (3.7) | 26.3 (3.9) | |
| SBP (mm Hg) | 155.3 (27.9) | 142.7 * (23.6) | 155.2 (22.1) | 142.0 ** (19.2) | |
| DBP (mm Hg) | 80.6 (13.9) | 72.4 ** (10.2) | 82.0 (13.3) | 73.8 ** (11.3) | |
| TAG (mmol/L) | 1.31 (0.70–2.22) | 1.00 *** (0.56–1.62) | 1.30 (0.74–2.60) | 1.10 ** (0.60–2.40) | |
| Total cholesterol (mmol/L) | 4.8 (0.2) | 4.3 ** (0.2) | 4.9 (0.2) | 4.3 ** (0.2) | |
| HDL-cholesterol (mmol/L) | 1.31 (0.93–2.41) | 1.25 *** (0.85–2.06) | 1.23 (0.88–1.88) | 1.07 *** (0.70–1.77) | |
| LDL-cholesterol (mmol/L) | 2.50 (1.62–4.28) | 2.46 * (1.56–3.67) | 2.68 (1.50–4.37) | 2.50 * (1.47–3.89) | |
| Total:HDL-cholesterol ratio | 3.2 (2.4–4.8) | 3.4 (2.5–5.1) | 3.8 (2.6–5.0) | 3.9 (2.4–5.4) | |
| LDL-cholesterol:HDL-cholesterol | 1.8 (1.0–3.6) | 2.0 (1.2–3.4) | 2.2 (1.3–3.2) | 2.4 (1.3–3.7) | |
| Omacor® (n = 47) | Placebo (n = 53) | |||
|---|---|---|---|---|
| Baseline | After | Baseline | After | |
| CRP (mg/L) | 1.0 (1.0–31.7) | 1.0 (1.0–20.0) | 1.0 (1.0–9.1) | 1.0 (1.0–23.0) |
| sE-selectin (ng/mL) | 92.0 (33.0–234.4) | 60.3 ** (17.4-240.7) | 91.4 (15.6–290.0) | 95.4 * (16.4–274.9) |
| sICAM-1 (ng/mL) | 167.1 (73.2–425.7) | 146.3 (69.2–383.3) | 216.1 (64.9–444.7) | 202.0 (74.2–345.5) |
| sVCAM-1 (ng/mL) | 673 (226–1578) | 544 *** (252–1146) | 594 (272–1131) | 489 ** (236–933) |
| IL-6 (pg/mL) | 1.2 (0.2–4.0) | 1.0 (0.2–4.1) | 1.2 (0.1–4.8) | 0.9 (0–3.7) |
| IL-10 (pg/mL) | 1.5 (0–5.2) | 0.9 (0–6.2) | 0.5 (0–2.9) | 0.8 (0–3.5) |
| MMP-2 (ng/mL) | 192.2 (129.2–290.3) | 165.7 *** (100.8–248.4) | 191.2 (129.0–293.7) | 155.3 *** (71.8–233.2) |
| MMP-9 (ng/mL) | 167.2 (46.6–421.1) | 163.3 (32.0–558.3) | 138.0 (28.8–389.3) | 152.1 (34.2–403.2) |
| TGF-β1 (ng/mL) | 9308 (2394–19170) | 7908 (2956–13690) | 9788 (3356–16844) | 7516 (3484–17388) |
| sCD40-L (ng/mL) | 776 (243–2239) | 655 (179–1677) | 774 (193–2906) | 659 (248–2285) |
| IP-10 (pg/mL) | 103.9 (51.6–273.3) | 100.2 (55.4–353.1) | 102.8 (47.8–289.4) | 114.9 (35.9–295.9) |
| MIG (pg/mL) | 119.3 (37.9–360.7) | 107.2 (30.6–320.6) | 107.4 (30.6–294.3) | 97.4 (16.9–327.2) |
4. Discussion
5. Conclusions
Acknowledgements
Conflicts of Interest
References
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; van Horn, L.; Dyer, A.R.; Greenland, P. Accumulated evidence on fish consumption and coronary heart disease mortality: A meta-analysis of cohort studies. Circulation 2004, 109, 2705–2711. [Google Scholar] [CrossRef]
- He, K.; Song, Y.; Daviglus, M.L.; Liu, K.; van Horn, L.; Dyer, A.R.; Goldbourt, U.; Greenland, P. Fish consumption and incidence of stroke: A meta-analysis of cohort studies. Stroke 2004, 35, 1538–1542. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Fatty acids and cardiovascular disease: Evidence explained and mechanisms explored. Clin. Sci. 2004, 107, 1–11. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. American Heart Association Nutrition Committee. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.A.; Willett, W.C.; Ma, J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Eng. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef]
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. J. Am. Med. Assoc. 2002, 287, 1815–1821. [Google Scholar] [CrossRef]
- Morris, M.C.; Sacks, F.; Rosner, B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation 1993, 88, 523–533. [Google Scholar] [CrossRef]
- Geleijnse, J.M.; Giltay, E.J.; Grobbee, D.E.; Donders, A.R.T.; Kok, F.J. Blood pressure response to fish oil supplementation: Meta-regression analysis of randomized trials. J. Hypertens. 2002, 20, 1493–1499. [Google Scholar] [CrossRef]
- Harris, W.S. n-3 Fatty acids and serum lipoproteins: Human studies. Am. J. Clin. Nutr. 1997, 65, 1645S–1654S. [Google Scholar]
- Roche, H.M. Unsaturated fatty acids. Proc. Nutr. Soc. 1999, 58, 397–401. [Google Scholar] [CrossRef]
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar]
- Calder, P.C. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food. Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef]
- Scientific Advisory Committee on Nutrition/Committee on Toxicity, Advice on Fish Consumption: Benefits and Risks; The Stationary Office: Norwich, UK, 2004.
- Abe, Y.; El Masri, B.; Kimball, K.T.; Pownall, H.; Reilly, C.F.; Osmundsen, K.; Smith, C.W.; Ballantyne, C.M. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 723–731. [Google Scholar] [CrossRef]
- Johansen, O.; Seljeflot, I.; Hostmark, A.T.; Arnesen, H. The effect of supplementation with omega-3 fatty acids on soluble markers of endothelial function in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1681–1686. [Google Scholar] [CrossRef]
- Davidson, M.H.; Stein, E.A.; Bays, H.E.; Maki, K.C.; Doyle, R.T.; Shalwitz, R.A.; Ballantyne, C.M.; Ginsberg, H.N.; Combination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: An 8-week, randomized, double-blind, placebo-controlled study. Clin. Ther. 2007, 29, 1354–1367. [Google Scholar] [CrossRef]
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [CrossRef]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; di Gregorio, D.; di Mascio, R.; Franzosi, M.G.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef]
- Cawood, A.L.; Ding, R.; Napper, F.L.; Young, R.H.; Williams, J.A.; Ward, M.J.; Gudmundsen, O.; Vige, R.; Payne, S.P.; Ye, S.; et al. Eicosapentanoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 252–259. [Google Scholar] [CrossRef]
- British Nutrition Foundation, Unsaturated Fatty Acids: Report of the British Nutrition Foundation Task Force; Blackwell Science: Oxford, UK, 1992.
- Mayer, K.; Merfels, M.; Muhly-Reinholz, M.; Gokorsch, S.; Rosseau, S.; Lohmeyer, J.; Schwarzer, N.; Krüll, M.; Suttorp, N.; Grimminger, F.; et al. Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: Role of endothelial PAF generation. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H811–H818. [Google Scholar]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.D.; Beilin, L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar]
- Durrington, P.N.; Bhatnagar, D.; Mackness, M.I.; Morgan, J.; Julier, K.; Khan, M.A.; France, M. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart 2001, 85, 544–548. [Google Scholar] [CrossRef]
- Harris, W.S.; Sands, S.A.; Windsor, S.L.; Ali, H.A.; Stevens, T.L.; Magalski, A.; Porter, C.B.; Borkon, A.M. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 2004, 110, 1645–1649. [Google Scholar]
- Hjerkinn, E.M.; Seljeflot, I.; Ellingse, I.; Berstad, P.; Hjermann, I.; Sandvik, L.; Arnesen, H. Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am. J. Clin. Nutr. 2005, 81, 583–589. [Google Scholar]
- Eschen, O.; Christensen, J.H.; de Caterina, R.; Schmidt, E.B. Soluble adhesion molecules in healthy subjects: A dose-response study using n-3 fatty acids. Nutr. Metab. Cardiovasc. Dis. 2004, 14, 180–185. [Google Scholar] [CrossRef]
- Miles, E.A.; Thies, F.; Wallace, F.A.; Powell, J.R.; Hirst, T.L.; Newsholme, E.A.; Calder, P.C. Influence of age and dietary fish oil on plasma soluble adhesion molecule concentrations. Clin. Sci. 2001, 100, 91–100. [Google Scholar] [CrossRef]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 year. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar]
- Balk, E.M.; Lichtenstein, A.H.; Chung, M.; Kupelnick, B.; Chew, P.; Lau, J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: A systematic review. Atherosclerosis 2006, 189, 19–30. [Google Scholar] [CrossRef]
- Carrero, J.J.; Fonolla, J.; Marti, J.L.; Jimenez, J.; Boza, J.J.; Lopez-Huertas, E. Intake of fish oil, oleic acid, folic acid, and vitamins B6 and E for one year decreases plasma C-reactive protein, and reduces coronary heart disease risk factors in male patients in a cardiac rehabilitation program. J. Nutr. 2007, 137, 384–390. [Google Scholar]
- Lee, K.W.; Blann, A.D.; Lip, G.Y. Effects of omega-3 polyunsaturated fatty acids on plasma indices of thrombogenesis and inflammation in patients post-myocardial infarction. Thromb. Res. 2006, 118, 305–312. [Google Scholar] [CrossRef]
- Aarsetøy, H.; Brügger-Andersen, T.; Hetland, Ø.; Grundt, H.; Nilsen, D.W. Long term influence of regular intake of high dose n-3 fatty acids on CD40-ligand, pregnancy-associated plasma protein A and matrix metalloproteinase-9 following acute myocardial infarction. Thromb. Haemost. 2006, 95, 329–336. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yusof, H.M.; Cawood, A.L.; Ding, R.; Williams, J.A.; Napper, F.L.; Shearman, C.P.; Grimble, R.F.; Payne, S.P.K.; Calder, P.C. Limited Impact of 2 g/day Omega-3 Fatty Acid Ethyl Esters (Omacor®) on Plasma Lipids and Inflammatory Markers in Patients Awaiting Carotid Endarterectomy. Mar. Drugs 2013, 11, 3569-3581. https://doi.org/10.3390/md11093569
Yusof HM, Cawood AL, Ding R, Williams JA, Napper FL, Shearman CP, Grimble RF, Payne SPK, Calder PC. Limited Impact of 2 g/day Omega-3 Fatty Acid Ethyl Esters (Omacor®) on Plasma Lipids and Inflammatory Markers in Patients Awaiting Carotid Endarterectomy. Marine Drugs. 2013; 11(9):3569-3581. https://doi.org/10.3390/md11093569
Chicago/Turabian StyleYusof, Hayati M., Abbie L. Cawood, Ren Ding, Jennifer A. Williams, Frances L. Napper, Clifford P. Shearman, Robert F. Grimble, Simon P.K. Payne, and Philip C. Calder. 2013. "Limited Impact of 2 g/day Omega-3 Fatty Acid Ethyl Esters (Omacor®) on Plasma Lipids and Inflammatory Markers in Patients Awaiting Carotid Endarterectomy" Marine Drugs 11, no. 9: 3569-3581. https://doi.org/10.3390/md11093569





