Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed
Abstract
:1. Introduction
2. Results
2.1. Fish Diets’ Analysis
| Ingredient | OP diet | FO diet * |
|---|---|---|
| Crude protein | 44.95 ± 1.3 | 46 ± 4.3 |
| Fat | 19.4 ± 1.7 | 21 ± 2.1 |
| Moisture | 8.6 ± 0.6 | 9.1 ± 1.3 |
| Dietary fiber | 5.2 ± 0.3 † | 1.8 ± 0.3 † |
| Ash | 6.0 ± 0.9 | 8.3 ± 1.4 |
| Energy (MJ/kg) | 21.8 ± 2.1 | 23 ± 2.6 |
| Protein digestibility (%) | 89 ± 4.4 | 90 ± 6.2 |
| Vitamin Α (IU/kg) | 7000 ± 210 † | 20,000 ± 410 † |
| Vitamin D (IU/kg) | 3150 ± 110 | 3000 ± 120 |
| Vitamin E (mg/kg) | 180 ±17 † | 258 ± 19 † |
| Vitamin K3 (mg/kg) | 10 ± 0.7 † | 33 ± 7.3 † |
| Vitamin C (mg/kg) | 200 ± 20 | 168 ± 14 |
| Cu (mg/kg) | 7.5 ± 1.1 | 7.0 ± 1.1 |
2.2. Total Lipid (TL), Total Neutral Lipid (TNL), and Total Polar Lipid (TPL) Content of Samples
| TL (%) | TNL (%) | TPL (%) | |
|---|---|---|---|
| OP | 2.4 ± 0.2 | 0.2 ± 0.02 | 2.2 ± 0.2 |
| FO diet | 21 ± 2.1 | 11 ± 1.5 | 10 ± 0.9 |
| OP diet | 19 ± 1.3 | 8.4 ± 1.1 | 11 ± 0.9 |
| Fish fed with FO diet | 3.9 ± 0.3 † | 3.7 ± 0.3 † | 0.2 ± 0.03 † |
| Fish fed with OP diet | 5.4 ± 0.5 † | 4.9 ± 0.4 † | 0.5 ± 0.06 † |
2.3. HPLC TPL Purification—Biological Assay
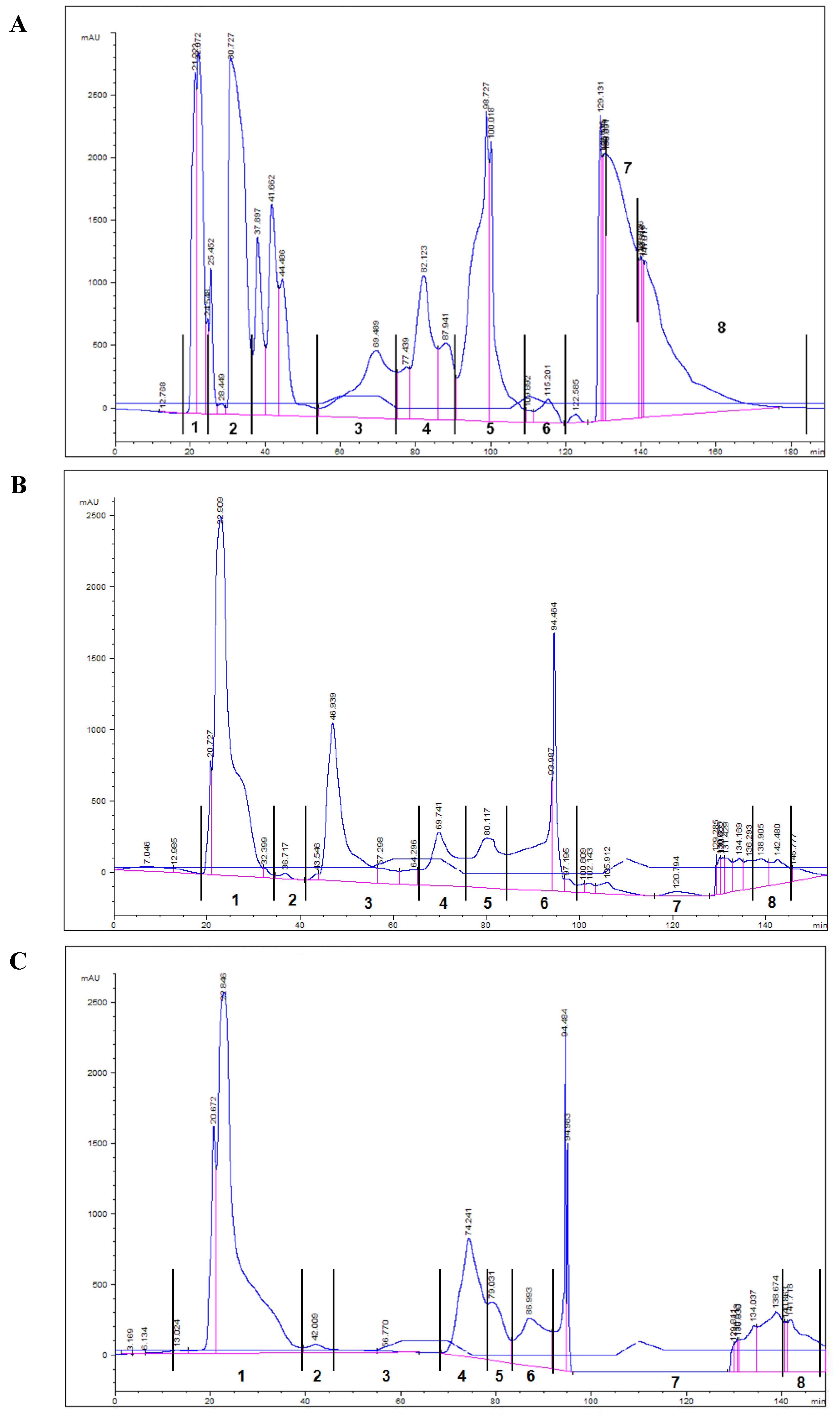
| HPLC lipid fractions | OP | FO diet | OP diet |
|---|---|---|---|
| 5 | 1.01 ± 0.05 | 0.04 ± 0.01 † | 1.24 ± 0.07 † |
| 6 | 0.43 ± 0.02 | n.a. | 0.56 ± 0.02 |
| 7 | 2.34 ± 0.12 | n.a. | n.a. |
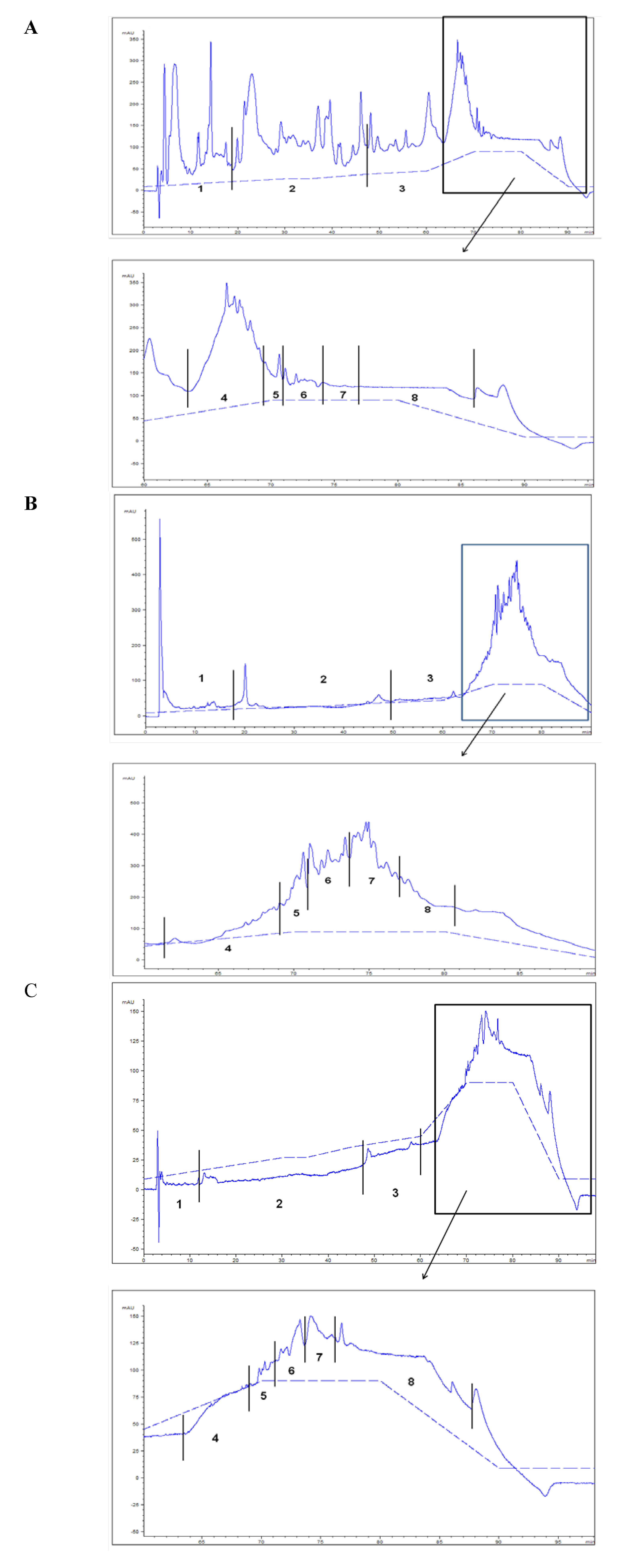
| HPLC lipid fractions | Aquacultured fish fed with FO diet | Aquacultured fish fed with OP diet |
|---|---|---|
| 1 | 0.13 ± 0.01 † | 0.30 ± 0.01 † |
| 2 | n.a. | n.a. |
| 3 | 0.04 ± 0.01 | n.a. |
| 4 | 0.11 ± 0.01 † | 0.25 ± 0.01 † |
| 5 | 0.11 ± 0.01 † | 0.24 ± 0.01 † |
| 6 | n.a. | 0.23 ± 0.01 |
| 7 | 0.11 ± 0.01 † | 0.26 ± 0.01 † |
| 8 | 0.16 ± 0.01 † | 0.28 ± 0.02 † |
3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Samples
4.3. Fish Diets’ Analysis
4.4. Instrumentation
4.5. Isolation of Lipids Extracts
4.6. HPLC Separation of TPL
4.7. Biological Assay
4.8. Statistical Analysis
5. Conclusions
Abbreviations
| HPLC | High Performance Liquid Chromatography |
| EFSA | European Food Safety Authority |
Acknowledgment
Conflict of Interest
References
- Hu, F.B.; Bronner, L.; Willett, W.C.; Stampfer, M.J.; Rexrode, K.M.; Albert, C.M.; Hunter, D.; Manson, J.E. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. J. Am. Med. Assoc. 2002, 14, 1821–1851. [Google Scholar]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: A population-based prospective study of middle-aged and elderly men. Eur. Heart J. 2009, 30, 1495–1500. [Google Scholar] [CrossRef]
- Mozaffarian, D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am. J. Clin. Nutr. 2008, 87, 19915–19965. [Google Scholar]
- Wang, C.; Harris, W.S.; Chung, M.; Lichtenstein, A.H.; Balk, E.M.; Kupelnick, B.; Jordan, H.S.; Lau, J. n-3 Fatty acids from fish or fish-oil supplements, but not α-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am. J. Clin. Nutr. 2006, 84, 5–17. [Google Scholar]
- Nasopoulou, C.; Zabetakis, I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds. A review. LWT Food Sci. Technol. 2012, 47, 217–224. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Zabetakis, I. Agricultural and Aquacultural Potential of Olive Pomace: A Review. J. Agric. Sci. 2013, 5, 1–12. [Google Scholar]
- Nasopoulou, C.; Stamatakis, G.; Demopoulos, C.A.; Zabetakis, I. Effects of olive pomace and olive pomace oil on growth performance, fatty acid composition and cardio protective properties of gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Food Chem. 2011, 129, 1108–1113. [Google Scholar] [CrossRef]
- Sioriki, E.; Nasopoulou, C.; Demopoulos, C.A.; Zabetakis, I. Comparison of sensory and cardioprotective properties of olive-pomace enriched and conventional gilthead sea bream (Sparus aurata): The effect of grilling. J. Aquat. Food Prod. Technol. 2013, in press. [Google Scholar]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.A.; Zabetakis, I. Comparison of antiatherogenic properties of lipids obtained from wild and cultured sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Cabarello, M.J.; Obach, A.; Rosenlund, G.; Montero, D.; Gisvold, M.; Izquiro, M.S. Impact of different dietary lipid sources on growth, lipid digestibility, tissue fatty acid composition and histology of rainbow trout, Oncorhynchus mykiss. Aquaculture 2002, 214, 253–271. [Google Scholar] [CrossRef]
- Piedecausa, M.A.; Mazόn, M.J.; García Carcía, B.; Hernández, M.D. Effect of total replacement of fish oil by vegetable oils in the diets of shardsnout seabream (Diplodus puntazzo). Aquaculture 2007, 263, 211–219. [Google Scholar] [CrossRef]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S. Food Ingredients and Lipid Mediators. Curr. Nutr. Food Sci. 2007, 3, 255–276. [Google Scholar] [CrossRef]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Atherosclerosis regression study in rabbits upon olive pomace polar lipid extract administration. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 740–747. [Google Scholar] [CrossRef]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theochari, S.E.; Iliopoulo, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and antiatherosclerotic properties of olive oil and pomace polar extracts in rabbits. Mediat. Inflamm. 2007, 2007, 1–11. [Google Scholar]
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem. 2013, in press. [Google Scholar]
- Rementzis, J.; Antonopoulou, S.; Demopoulos, C.A. Identification andstudy of gangliosides from Scomber scombrus muscle. J. Agric. Food Chem. 1997, 45, 611–615. [Google Scholar] [CrossRef]
- Stamatakis, G.; Tsantila, N.; Samiotaki, M.; Panayotou, G.N.; Demopoulos, A.C.; Halvadakis, C.P.; Demopoulos, C.A. Detection and isolation of antioxidant substances present in olive mill wastes by a novel filtration system. J. Agric. Food Chem. 2009, 57, 10554–10564. [Google Scholar] [CrossRef]
- Penna, C.; Bassino, E.; Alloatti, G. Platelet activating factor: the good and the bad in the ischemic/reperfused heart. Exp. Biol. Med. 2011, 236, 390–401. [Google Scholar] [CrossRef]
- Karantonis, H.C.; Antonopoulou, S.; Perrea, D.N.; Sokolis, D.P.; Theocharis, S.E.; Kavantzas, N.; Iliopoulos, D.G.; Demopoulos, C.A. In vivo antiatherogenic properties of olive oil and its constituent lipid classes in hyperlipidemic rabbits. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 174–185. [Google Scholar] [CrossRef]
- Gatlin, D.M. Principles of Fish Nutrition; Southern Regional Aquaculture Center: Stoneville, MI, USA, 2010. [Google Scholar]
- The European Communities. COMMISSION REGULATION (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L54, 1–130. [Google Scholar]
- van Leeuwen, P.; van Kleef, D.J.; van Kempen, G.J.M.; Huisman, J.; Verstegen, M.W.A. The post valve T-caecum cannulation technique in pigs applicated to determine the digestibility of amino acids in maize, groundnut and sunflower meal. J. Anim. Physiol. Anim. Nutr. 1991, 65, 183–193. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Galanos, D.S.; Kapoulas, V.M. Isolation of polar lipids from triglyceride mixtures. J. Lipid Res. 1962, 3, 134–137. [Google Scholar]
- Demopoulos, C.A.; Pinckard, R.N.; Hanahan, D.J. Platelet activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nasopoulou, C.; Gogaki, V.; Stamatakis, G.; Papaharisis, L.; Demopoulos, C.A.; Zabetakis, I. Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed. Mar. Drugs 2013, 11, 3676-3688. https://doi.org/10.3390/md11103676
Nasopoulou C, Gogaki V, Stamatakis G, Papaharisis L, Demopoulos CA, Zabetakis I. Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed. Marine Drugs. 2013; 11(10):3676-3688. https://doi.org/10.3390/md11103676
Chicago/Turabian StyleNasopoulou, Constantina, Vassiliki Gogaki, Giorgos Stamatakis, Leonidas Papaharisis, Constantinos A. Demopoulos, and Ioannis Zabetakis. 2013. "Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed" Marine Drugs 11, no. 10: 3676-3688. https://doi.org/10.3390/md11103676





