Adverse Effect of Antifouling Compounds on the Reproductive Mechanisms of the Ascidian Ciona intestinalis
Abstract
:1. Introduction
2. Results
2.1. Effects of TBT and Diuron on the Plasma Membrane Conductance
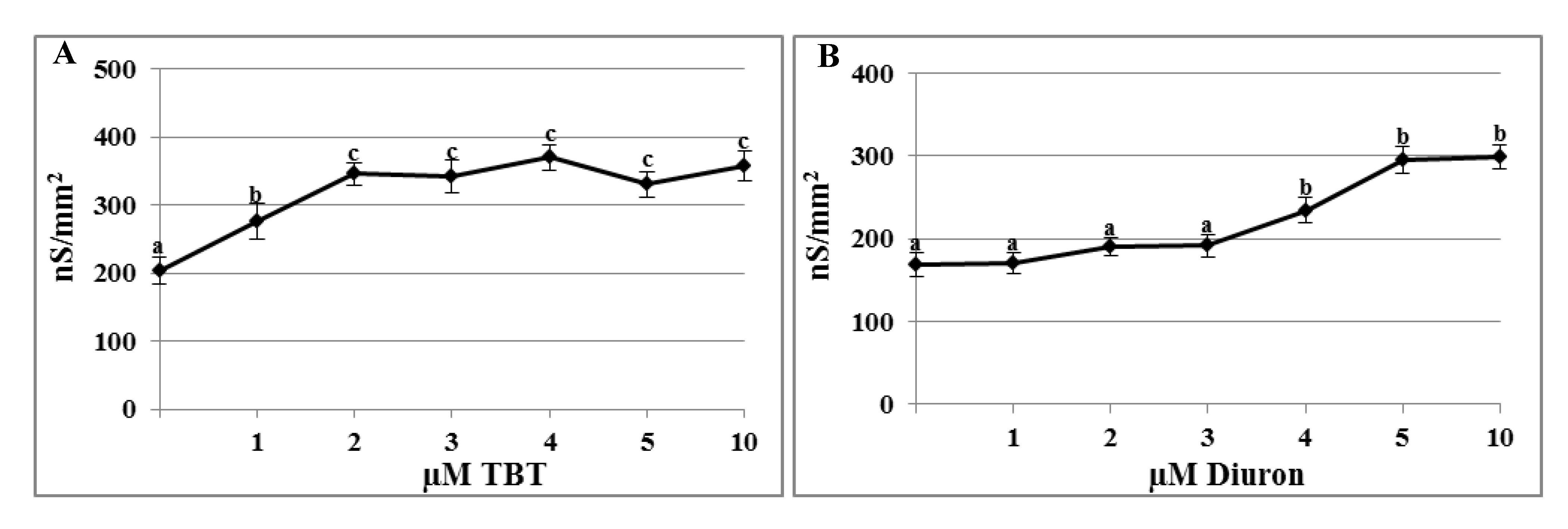
2.2. Effects of TBT and Diuron on Na+ Currents
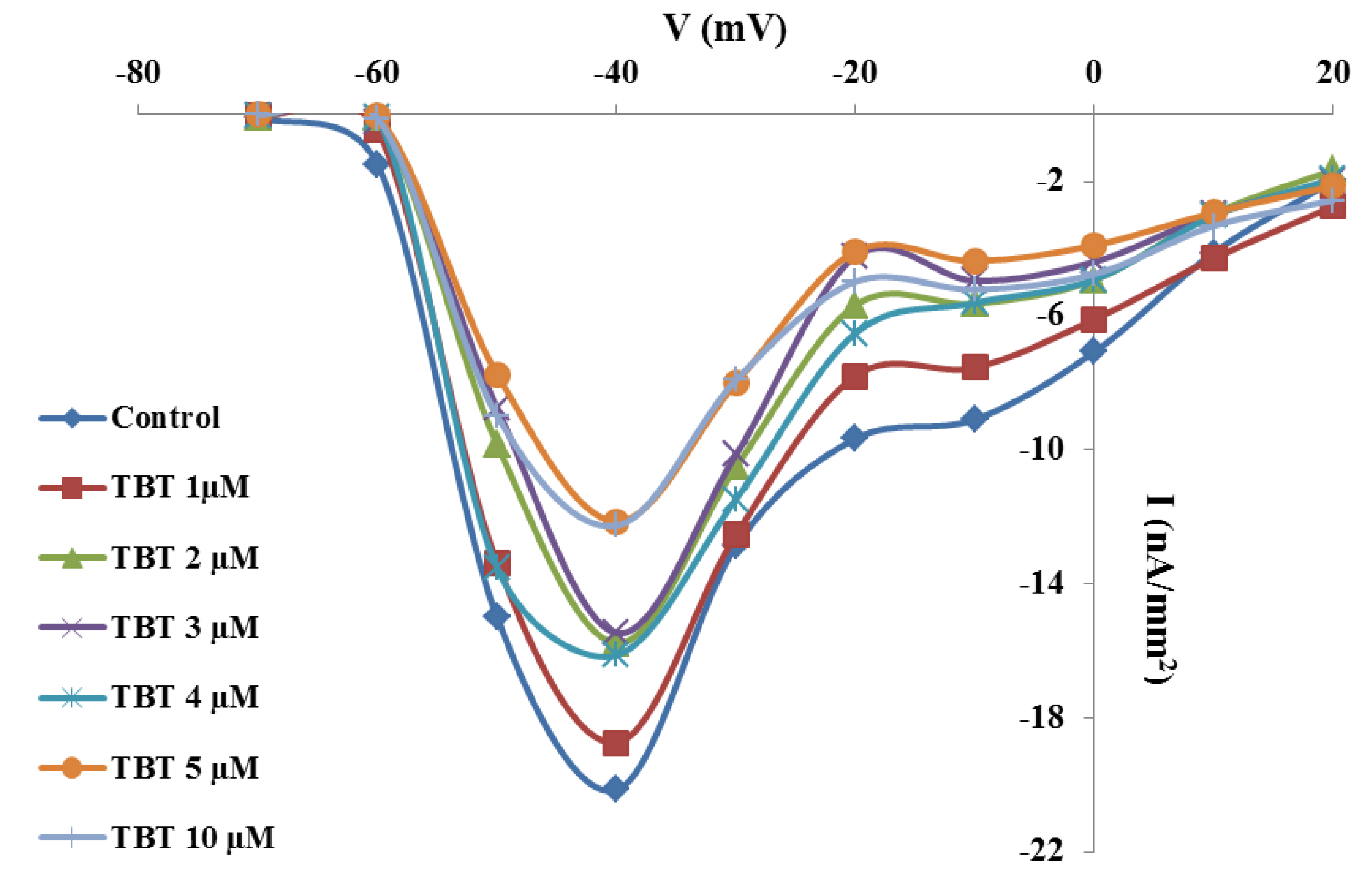
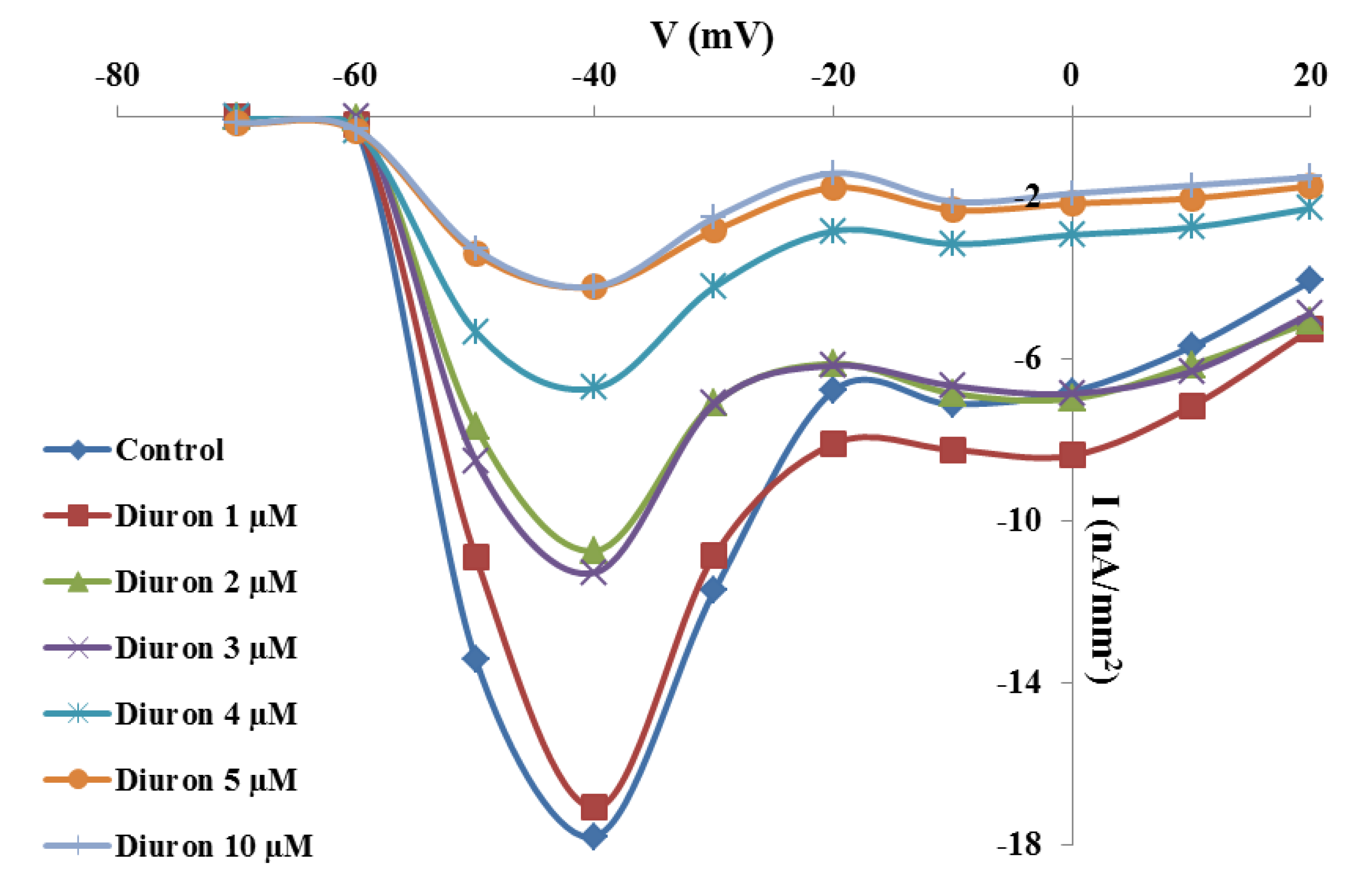
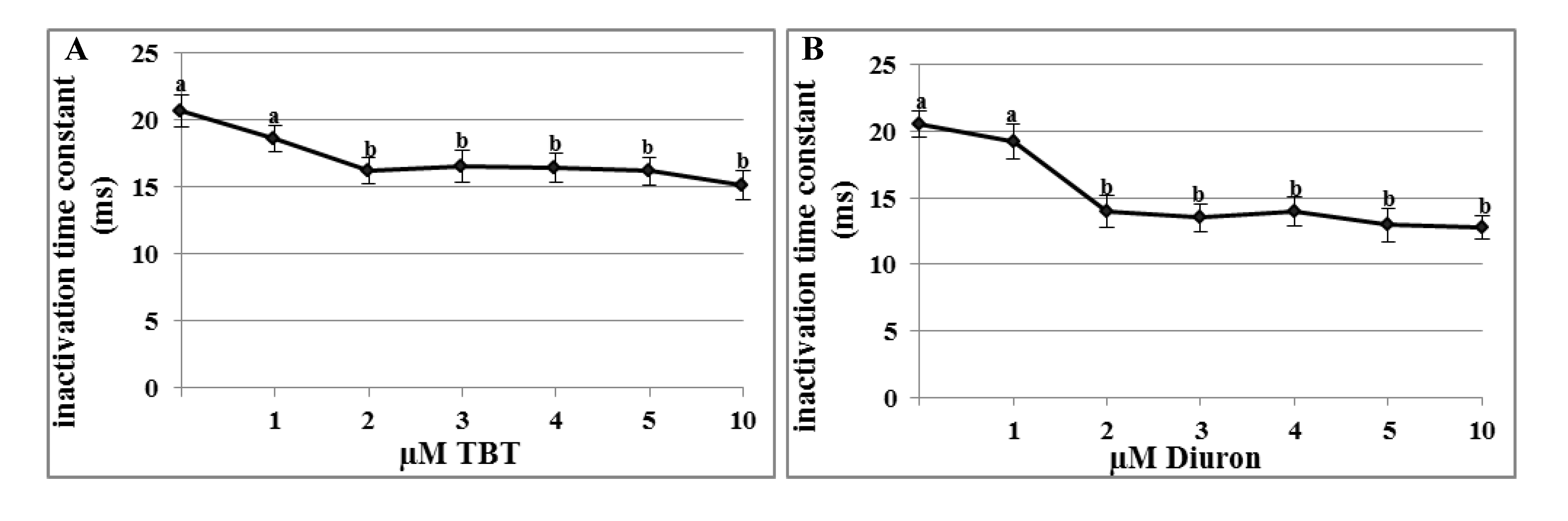
2.3. Effects of TBT and Diuron on Na+ Channel Time Constants
2.4. Effects of TBT and Diuron on the Fertilization Current
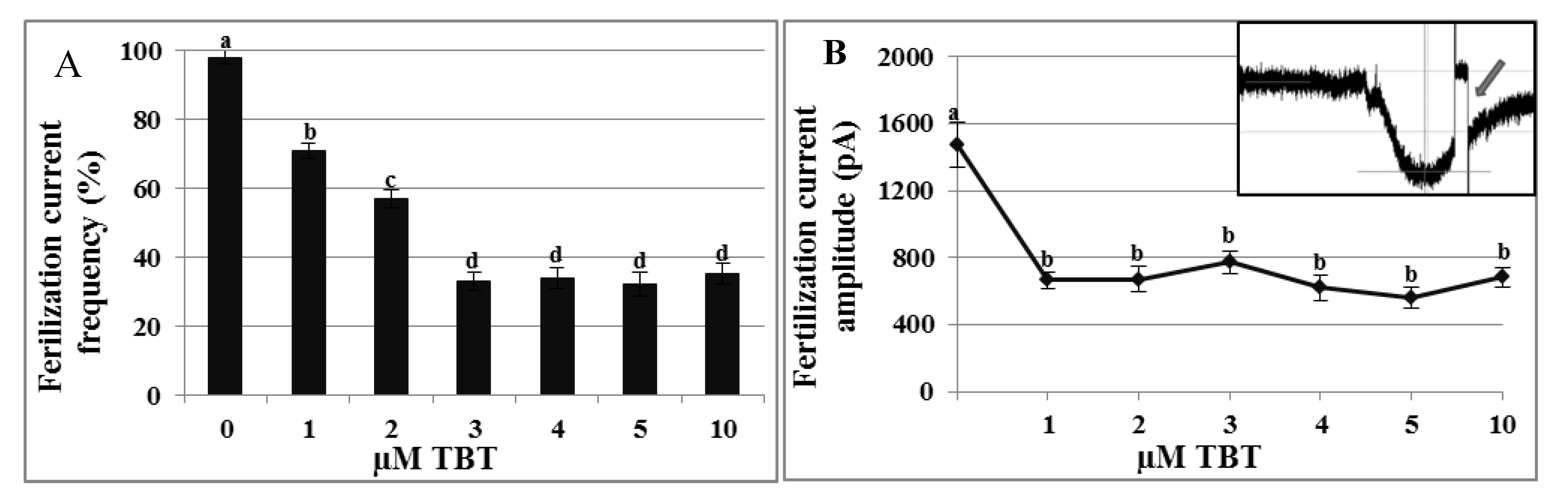
2.5. Toxicity Assays
2.5.1. Exposure of Spermatozoa to TBT and Diuron
2.5.2. Exposure of Oocytes to TBT and Diuron
2.5.3. Fertilization Test

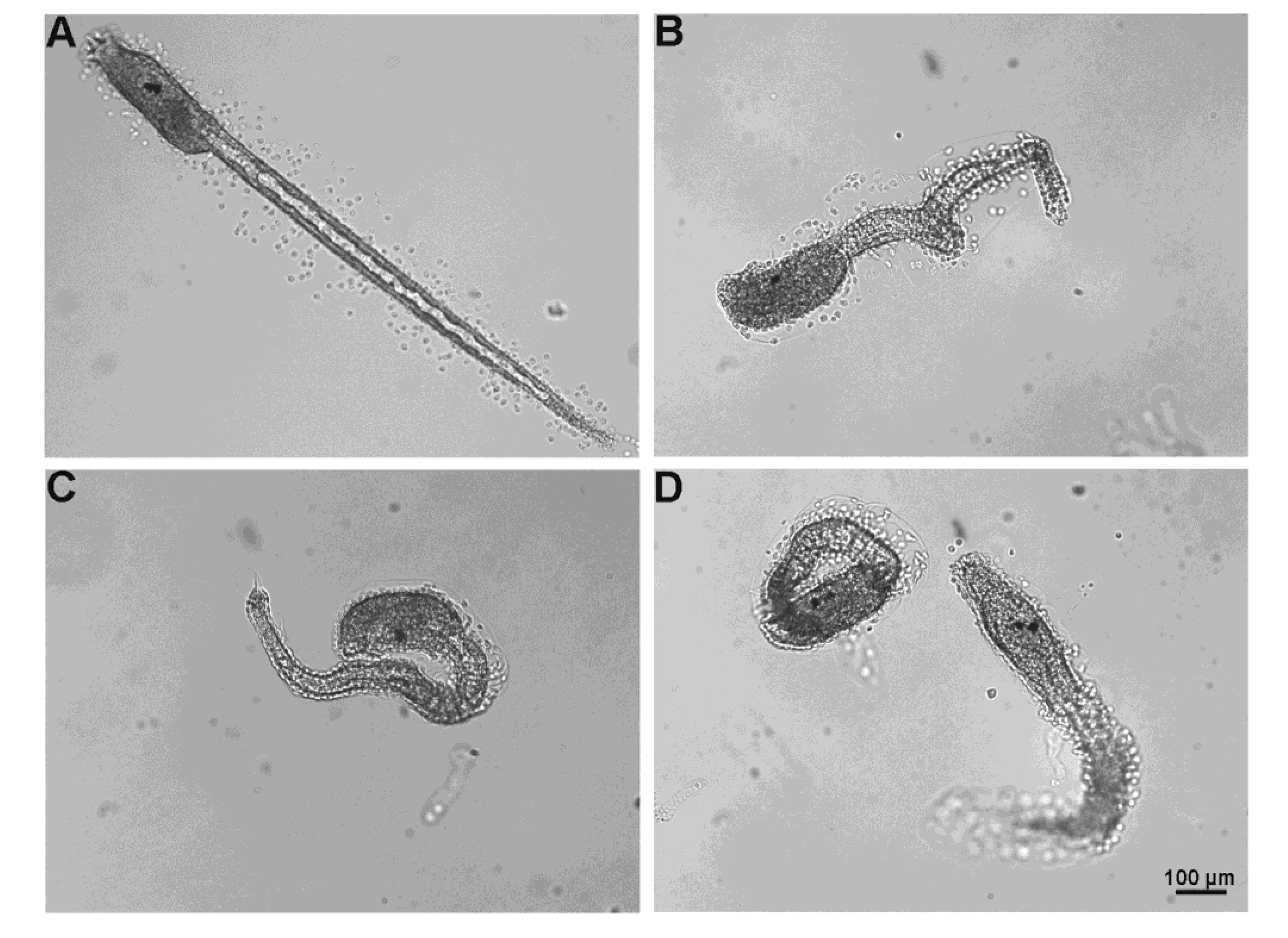
3. Discussion
4. Experimental Section
4.1. Animals and Gametes
4.2. Test Solutions
4.3. Electrophysiology
4.4. Toxicity Assays
4.4.1. Exposure of Spermatozoa to TBT and Diuron
4.4.2. Exposure of Oocytes to TBT and Diuron
4.4.3. Fertilization Test
4.4.4. Fertilization and Normal Larvae Rate
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wurl, O.; Obbard, J.P. A review of pollutants in the sea-surface microlayer (SML): A unique habitat for marine organisms. Mar. Pollut. Bull. 2004, 48, 1016–1030. [Google Scholar] [CrossRef]
- Danzo, B.J. Environmental xenobiotics may disrupt normal endocrine function by interfering with the binding of physiological ligands to steroid receptors and binding proteins. Environ. Health Perspect. 1997, 105, 294–301. [Google Scholar] [CrossRef]
- Gallo, A.; Silvestre, F.; Cuomo, A.; Papoff, F.; Tosti, E. The impact of metals on the reproductive mechanisms of the ascidian Ciona intestinalis. Mar. Ecol. 2011, 32, 222–231. [Google Scholar] [CrossRef]
- Henson, M.C.; Chedrese, P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004, 229, 383–392. [Google Scholar]
- Mills, L.J.; Chichester, C. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Sci. Total Environ. 2005, 343, 1–34. [Google Scholar] [CrossRef]
- Pennati, R.; Groppelli, S.; Zega, G.; Biggiogero, M.; de Bernardi, F.; Sotgia, C. Toxic effects of two pesticides, Imazalil and Triadimefon, on the early development of the ascidian Phallusia mammillata (Chordata, Ascidiacea). Aquat. Toxicol. 2006, 79, 205–212. [Google Scholar] [CrossRef]
- Zega, G.; Pennati, R.; Candiani, S.; Pestarino, M.; de Bernardi, F. Solitary ascidians embryos (Chordata, Tunicata) as model organisms for testing coastal pollutant toxicity. Invertebr. Surviv. J. 2009, 6, S29–S34. [Google Scholar]
- Blaber, S.J. The occurrence of a penis-like outgrowth behind the right tentacle in spent females of Nucella lapillus (L.). J. Molluscan Stud. 1970, 39, 231–233. [Google Scholar]
- Smith, B. Sexuality in the American mud snail, Nassarius obsoletus Say. J. Molluscan Stud. 1971, 39, 377–378. [Google Scholar]
- Oehlmann, J.; Stroben, E.; Fioroni, P. The morphological expression of imposex in Nucella lapillus (Linnaeus)(Gastropoda: Muricidae). J. Molluscan Stud. 1991, 57, 375–390. [Google Scholar] [CrossRef]
- Smith, B.S. Tributyltin compounds induce male characteristics on female mud snails Nassarius obsoletus = Ilyanassa obsoleta. J. Appl. Toxicol. 1981, 1, 141–144. [Google Scholar] [CrossRef]
- Matthiessen, P.; Gibbs, P.E. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ. Toxicol. Chem. 1998, 17, 37–43. [Google Scholar] [CrossRef]
- Fent, K. Worldwide Occurrence of Organotins from Antifouling Paints and Effects in the Aquatic Environment. In Antifouling Paint Biocides; Konstantinou, I., Ed.; Springer-Verlag: Berlin-Heidelberg, Germany, 2006; pp. 71–100. [Google Scholar]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological activity studies on organotin(IV)n+ complexes and parent compounds. J. Organomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Gray, B.H.; Porvaznik, M.; Flemming, C.; Lee, L.H. tri-n-Butyltin: A membrane toxicant. Toxicology 1987, 47, 35–54. [Google Scholar] [CrossRef]
- International Marine Organization, International Convention on the Control of Harmful Anti-Fouling Systems on Ships; International Marine Organization: London, UK, 2001.
- Graceli, J.B.; Sena, G.C.; Lopes, P.F.; Zamprogno, G.C.; da Costa, M.B.; Godoi, A.F.; Dos Santos, D.M.; de Marchi, M.R.; Dos Santos Fernandez, M.A. Organotins: A review of their reproductive toxicity, biochemistry, and environmental fate. Reprod. Toxicol. 2013, 36, 40–52. [Google Scholar] [CrossRef]
- Readman, J.W. Development, Occurrence and Regulationof Antifouling Paint Biocides: Historical Review and Future Trends. In Antifouling Paint Biocides; Konstantinou, I., Ed.; Springer-Verlag: Berlin-Heidelberg, Germany, 2006; Volume 5, pp. 1–15. [Google Scholar]
- Giacomazzi, S.; Cochet, N. Environmental impact of diuron transformation: A review. Chemosphere 2004, 56, 1021–1032. [Google Scholar] [CrossRef]
- Hoch, M. Organotin compounds in the environment—An overview. Appl. Geochem. 2001, 16, 719–743. [Google Scholar] [CrossRef]
- Ramirez Llodra, E. Fecundity and life-history strategies in marine invertebrates. Adv. Mar. Biol. 2002, 43, 87–170. [Google Scholar] [CrossRef]
- Cangialosi, M.V.; Mansueto, V.; Puccia, E.; Corsi, I.; Bonacci, S.; Focardi, S.; Mazzola, A. A biochemical study of the effects of tributyltin on unfertilized eggs, embryos and larvae of the sea squirt Ciona intestinalis. Caryologia 2009, 62, 309–315. [Google Scholar]
- Cima, F.; Ballarin, L.; Bressa, G.; Martinucci, G.; Burighel, P. Toxicity of organotin compounds on embryos of a marine invertebrate (Styela plicata; tunicata). Ecotoxicol. Environ. Saf. 1996, 35, 174–182. [Google Scholar] [CrossRef]
- Mansueto, C.; Villa, L.; D’Agati, P.; Marcianò, V.; Pellerito, C.; Fiore, T.; Scopelliti, M.; Nagy, L.; Pellerito, L. Effects of tributyltin (IV) chloride on fertilization of Styela plicata (Ascidiacea: Tunicata): II. Scanning and transmission electron microscopy studies. Appl. Organomet. Chem. 2003, 17, 553–560. [Google Scholar] [CrossRef]
- Patricolo, E.; Mansueto, C.; D’Agati, P.; Pellerito, L. Organometallic complexes with biological molecules: XVI. Endocrine disruption effects of tributyltin (IV) chloride on metamorphosis of the ascidian larva. Appl. Organomet. Chem. 2001, 15, 916–923. [Google Scholar] [CrossRef]
- Cuomo, A.; Silvestre, F.; de Santis, R.; Tosti, E. Ca2+ and Na+ current patterns during oocyte maturation, fertilization, and early developmental stages of Ciona intestinalis. Mol. Reprod. Dev. 2006, 73, 501–511. [Google Scholar] [CrossRef]
- Silvestre, F.; Cuomo, A.; Tosti, E. Ion current activity and molecules modulating maturation and growth stages of ascidian (Ciona intestinalis) oocytes. Mol. Reprod. Dev. 2009, 76, 1084–1093. [Google Scholar] [CrossRef]
- Tosti, E.; Gallo, A.; Silvestre, F. Ion currents involved in oocyte maturation, fertilization and early developmental stages of the ascidian Ciona intestinalis. Mol. Reprod. Dev. 2011, 78, 854–860. [Google Scholar] [CrossRef]
- Dale, B. Oocyte activation in invertebrates and humans. Zygote 1994, 2, 373–377. [Google Scholar]
- Dale, B.; de Felice, L. Sperm-activated channels in ascidian oocytes. Dev. Biol. 1984, 101, 235–239. [Google Scholar] [CrossRef]
- Grumetto, L.; Wilding, M.; de Simone, M.L.; Tosti, E.; Galione, A.; Dale, B. Nitric oxide gates fertilization channels in ascidian oocytes through nicotinamide nucleotide metabolism. Biochem. Biophys. Res. Commun. 1997, 239, 723–728. [Google Scholar] [CrossRef]
- Hagiwara, S.; Jaffe, L.A. Electrical properties of egg cell membranes. Annu. Rev. Biophys. Bioeng. 1979, 8, 385–416. [Google Scholar] [CrossRef]
- Tosti, E.; Boni, R. Electrical events during gamete maturation and fertilization in animals and humans. Hum. Reprod. Update 2004, 10, 53–65. [Google Scholar] [CrossRef]
- Tosti, E.; Boni, R.; Gallo, A.; Silvestre, F. Ion currents modulating oocyte maturation in animals. Syst. Biol. Reprod. Med. 2013, 59, 61–68. [Google Scholar] [CrossRef]
- Tosti, E. Calcium ion currents mediating oocyte maturation events. Reprod. Biol. Endocr. 2006, 4, 26. [Google Scholar] [CrossRef]
- Dale, B.; Wilding, M. Ionic Events at Fertilization. In Oocyte Maturation and Fertilization: A Long History for a Short Event; Tosti, E., Boni, R., Eds.; Bentham Science Publisher: Dubai, UAE, 2011; pp. 104–120. [Google Scholar]
- Girard, J.-P.; Ferrua, C.; Pesando, D. Effects of tributyltin on Ca2+ homeostasis and mechanisms controlling cell cycling in sea urchin eggs. Aquat. Toxicol. 1997, 38, 225–239. [Google Scholar] [CrossRef]
- Mech, A.; Orynbayeva, Z.; Irgebayev, K.; Kolusheva, S.; Jelinek, R. Screening membrane interactions of pesticides by cells decorated with chromatic polymer nanopatches. Chem. Res. Toxicol. 2008, 22, 90–96. [Google Scholar]
- Franchet, C.; Goudeau, M.; Goudeau, H. Tributyltin impedes early sperm-egg interactions at the egg coat level in the ascidian Phallusia mammillata but does not prevent sperm-egg fusion in naked eggs. Aquat. Toxicol. 1998, 44, 213–228. [Google Scholar] [CrossRef]
- Arizza, V.; di Fazio, G.; Celi, M.; Parrinello, N.; Vazzana, M. Cadmium, Copper and Tributyltin effects on fertilization of Paracentrotus lividus (Echinodermata). Ital. J. Anim. Sci. 2010, 8, 839–841. [Google Scholar]
- Villa, L.; Agati, P.; Mansueto, C.; Pellerito, C.; Scopelliti, M.; Fiore, T.; Nagy, L.; Pellerito, L. Effects of tributyltin (IV) chloride on the gametes and fertilization of Ascidia malaca (Ascidiacea: Tunicata). Appl. Organomet. Chem. 2003, 17, 106–112. [Google Scholar]
- Satoh, N. Developmental Biology of Ascidians; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Tosti, E.; Romano, G.; Buttino, I.; Cuomo, A.; Ianora, A.; Miralto, A. Bioactive aldehydes from diatoms block the fertilization current in ascidian oocytes. Mol. Reprod. Dev. 2003, 66, 72–80. [Google Scholar] [CrossRef]
- Antizar-Ladislao, B. Environmental levels, toxicity and human exposure to tributyltin (TBT)-contaminated marine environment. Environ. Int. 2008, 34, 292–308. [Google Scholar] [CrossRef]
- Di Landa, G.; Ansanelli, G.; Ciccoli, R.; Cremisini, C. Occurrence of antifouling paint booster biocides in selected harbors and marinas inside the Gulf of Napoli: A preliminary survey. Mar. Pollut. Bull. 2006, 52, 1541–1546. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Goldberg, E.D. TBT: An environmental dilemma. Environ. Sci. Policy 1986, 28, 17–44. [Google Scholar]
- Sasakura, Y.; Inaba, K.; Satoh, N.; Kondo, M.; Akasaka, K. Ciona intestinalis and Oxycomanthus japonicus, representatives of marine invertebrates. Exp. Anim. 2009, 58, 459–469. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Gallo, A.; Tosti, E. Adverse Effect of Antifouling Compounds on the Reproductive Mechanisms of the Ascidian Ciona intestinalis. Mar. Drugs 2013, 11, 3554-3568. https://doi.org/10.3390/md11093554
Gallo A, Tosti E. Adverse Effect of Antifouling Compounds on the Reproductive Mechanisms of the Ascidian Ciona intestinalis. Marine Drugs. 2013; 11(9):3554-3568. https://doi.org/10.3390/md11093554
Chicago/Turabian StyleGallo, Alessandra, and Elisabetta Tosti. 2013. "Adverse Effect of Antifouling Compounds on the Reproductive Mechanisms of the Ascidian Ciona intestinalis" Marine Drugs 11, no. 9: 3554-3568. https://doi.org/10.3390/md11093554




