Antifreeze Peptides and Glycopeptides, and Their Derivatives: Potential Uses in Biotechnology
Abstract
:1. Introduction
2. Antifreeze Activity Assays
3. Type I Antifreeze Peptides

4. Structure-Activity Relationship (SAR) Studies of Type I AFP
5. Native Antifreeze Glycoproteins: Synthesis and Structure-Activity Relationship (SAR) Studies

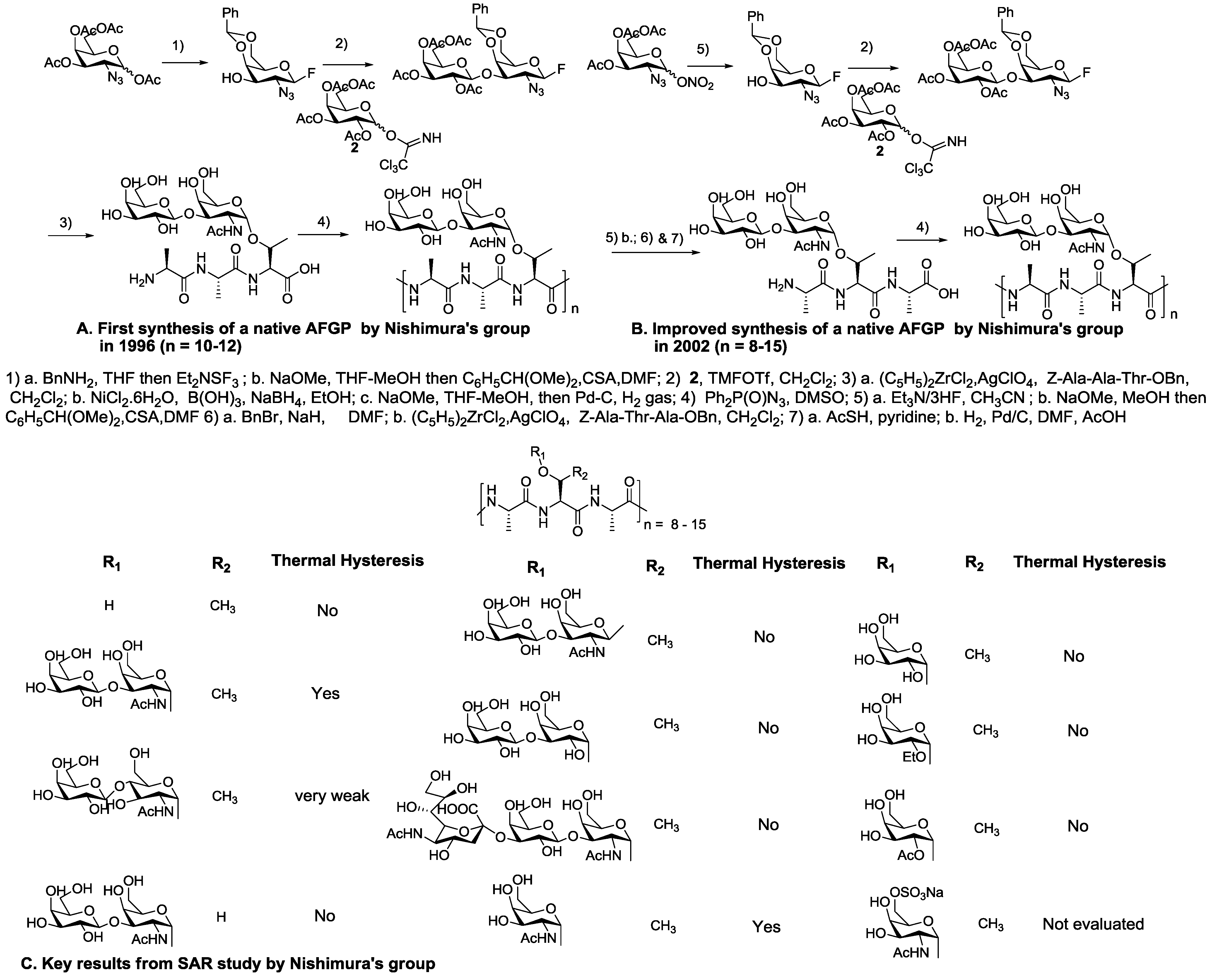

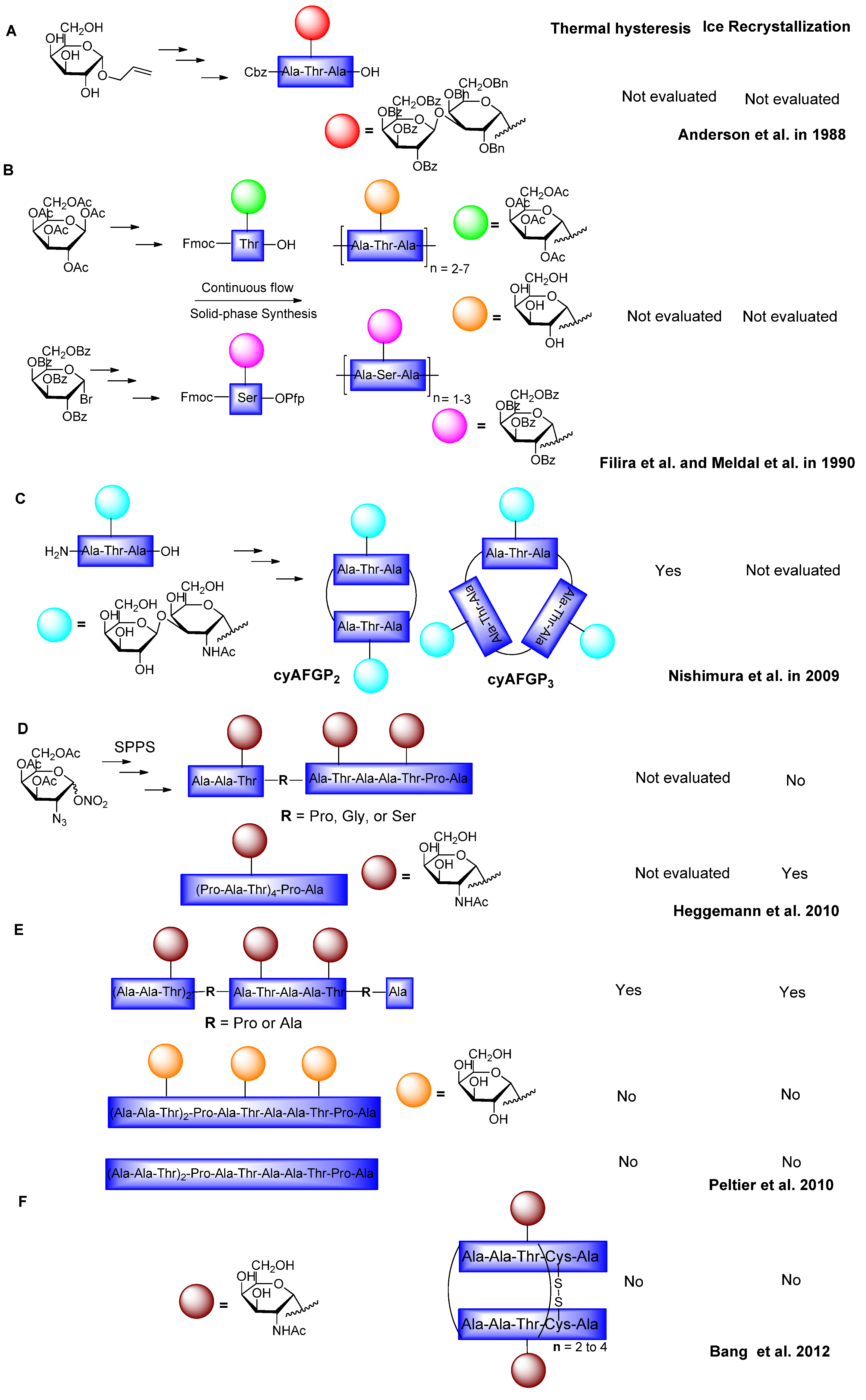
6. Synthesis of AFGP Analogues
6.1. Modification of Sugars and Peptide Backbone
6.2. C-Linked AFGP Derivative
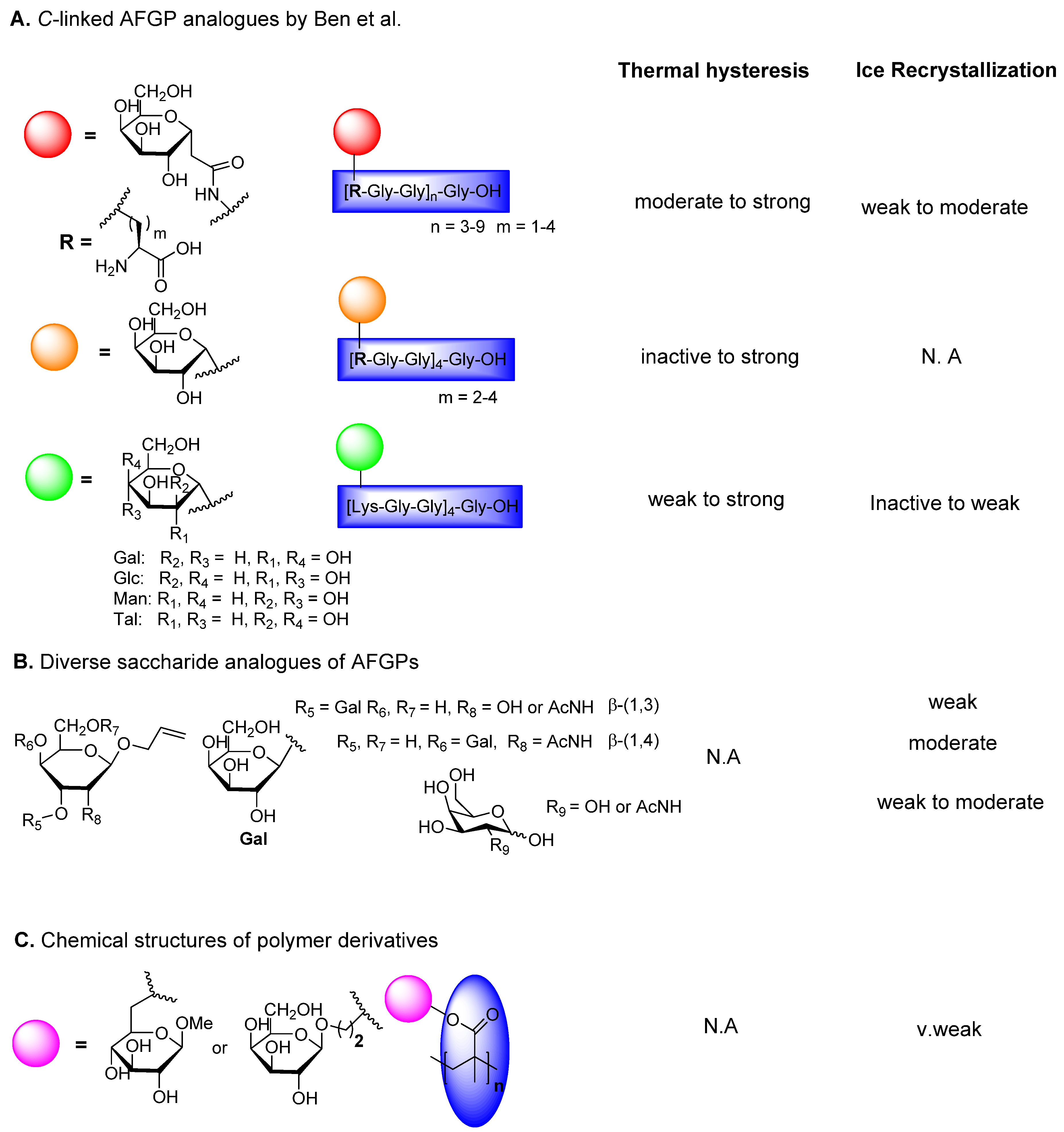
6.3. Triazole-Linked AFGP Derivatives
6.4. Peptoid Mimics
7. Application Studies
7.1. Cryopreservation
7.2. Cryosurgery & Food Preservation
7.3. Transgenic Approaches
8. Conclusions and Outlook
Acknowledgments
Conflict of Interest
References
- Jia, Z.; Davies, P.L. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem. Sci. 2002, 27, 101–106. [Google Scholar] [CrossRef]
- D’Amico, S.; Collins, T.; Marx, J.C.; Feller, G.; Gerday, C. Psychrophilic microorganisms: Challenges for life. EMBO Rep. 2006, 7, 385–389. [Google Scholar] [CrossRef]
- Davies, P.L.; Baardsnes, J.; Kuiper, M.J.; Walker, V.K.; Davies, P.L.; Baardsnes, J.; Kuiper, M.J.; Walker, V.K. Structure and function of antifreeze proteins. Philos. Trans. R. Soc. B 2002, 357, 927–935. [Google Scholar] [CrossRef]
- Venketesh, S.; Dayananda, C. Properties, potentials, and prospects of antifreeze proteins. Crit. Rev. Biotechnol. 2008, 28, 57–82. [Google Scholar] [CrossRef]
- Knight, C.; Cheng, C.; DeVries, A. Adsorption of α-helical antifreeze peptides on specific ice crystal surface planes. Biophys. J. 1991, 59, 409–418. [Google Scholar] [CrossRef]
- Haymet, A.; Ward, L.G.; Harding, M.M. Winter flounder “antifreeze” proteins: Synthesis and ice growth inhibition of analogues that probe the relative importance of hydrophobic and hydrogen-bonding interactions. J. Am. Chem. Soc. 1999, 121, 941–948. [Google Scholar] [CrossRef]
- Chao, H.; Houston, M.E., Jr.; Hodges, R.S.; Kay, C.M.; Sykes, B.D.; Loewen, M.C.; Davies, P.L.; Sönnichsen, F.D. A diminished role for hydrogen bonds in antifreeze protein binding to ice. Biochemistry 1997, 36, 14652–14660. [Google Scholar] [CrossRef]
- Haymet, A.; Ward, L.G.; Harding, M.M.; Knight, C.A. Valine substituted winter flounder “antifreeze”: Preservation of ice growth hysteresis. FEBS Lett. 1998, 430, 301–306. [Google Scholar] [CrossRef]
- Loewen, M.C.; Chao, H.; Houston, M.E., Jr.; Baardsnes, J.; Hodges, R.S.; Kay, C.M.; Sykes, B.D.; Sönnichsen, F.D.; Davies, P.L. Alternative roles for putative ice-binding residues in type I antifreeze protein. Biochemistry 1999, 38, 4743–4749. [Google Scholar] [CrossRef]
- Zhang, W.; Laursen, R.A. Structure-function relationships in a type I antifreeze polypeptide the role of threonine methyl and hydroxyl groups in antifreeze activity. J. Biol. Chem. 1998, 273, 34806–34812. [Google Scholar] [CrossRef]
- Wen, D.; Laursen, R. Structure-function relationships in an antifreeze polypeptide. The effect of added bulky groups on activity. J. Biol. Chem. 1993, 268, 16401–16405. [Google Scholar]
- Baardsnes, J.; Kondejewski, L.H.; Hodges, R.S.; Chao, H.; Kay, C.; Davies, P.L. New ice-binding face for type I antifreeze protein. FEBS Lett. 1999, 463, 87–91. [Google Scholar] [CrossRef]
- Gronwald, W.; Chao, H.; Reddy, D.V.; Davies, P.L.; Sykes, B.D.; Sönnichsen, F.D. NMR characterization of side chain flexibility and backbone structure in the type I antifreeze protein at near freezing temperatures. Biochemistry 1996, 35, 16698–16704. [Google Scholar] [CrossRef]
- Graether, S.P.; Slupsky, C.M.; Davies, P.L.; Sykes, B.D. Structure of type I antifreeze protein and mutants in supercooled water. Biophys. J. 2001, 81, 1677–1683. [Google Scholar] [CrossRef]
- Liepinsh, E.; Otting, G.; Harding, M.M.; Ward, L.G.; Mackay, J.P.; Haymet, A. Solution structure of a hydrophobic analogue of the winter flounder antifreeze protein. Eur. J. Biochem. 2002, 269, 1259–1266. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Vigh, L.; Meyer, J.D.; Manning, M.C.; Hincha, D.K.; Crowe, J.H. Lipid unsaturation determines the interaction of AFP type I with model membranes during thermotropic phase transitions. Cryobiology 2002, 45, 135–142. [Google Scholar] [CrossRef]
- Yang, D.; Sax, M.; Chakrabartty, A.; Hew, C. Crystal structure of an antifreeze polypeptide and its mechanistic implications. Nature 1988, 333, 232–237. [Google Scholar] [CrossRef]
- Sicheri, F.; Yang, D. Ice-binding structure and mechanism of an antifreeze protein from winter flounder. Nature 1995, 375, 427–431. [Google Scholar] [CrossRef]
- Graether, S.P.; Kuiper, M.J.; Gagne, S.M.; Walker, V.K.; Jia, Z.; Sykes, B.D.; Davies, P.L. β-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature 2000, 406, 325–328. [Google Scholar] [CrossRef]
- Leinala, E.K.; Davies, P.L.; Jia, Z. Crystal structure of β-helical antifreeze protein points to a general ice binding model. Structure 2002, 10, 619–627. [Google Scholar] [CrossRef]
- Graether, S.P.; Sykes, B.D. Cold survival in freeze-intolerant insects: The structure and function of β-helical antifreeze proteins. Eur. J. Biochem. 2004, 271, 3285–3296. [Google Scholar] [CrossRef]
- Liou, Y.C.; Tocilj, A.; Davies, P.L.; Jia, Z. Mimicry of ice structure by surface hydroxyls and water of a β-helix antifreeze protein. Nature 2000, 406, 322–324. [Google Scholar] [CrossRef]
- Xiao, N.; Suzuki, K.; Nishimiya, Y.; Kondo, H.; Miura, A.; Tsuda, S.; Hoshino, T. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS J. 2010, 277, 394–403. [Google Scholar] [CrossRef]
- Kondo, H.; Hanada, Y.; Sugimoto, H.; Hoshino, T.; Garnham, C.P.; Davies, P.L.; Tsuda, S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc. Natl. Acad. Sci. USA 2012, 109, 9360–9365. [Google Scholar]
- Raymond, J.A.; Janech, M.G. Ice-binding proteins from enoki and shiitake mushrooms. Cryobiology 2009, 58, 151–156. [Google Scholar] [CrossRef]
- Lee, J.K.; Park, K.S.; Park, S.; Park, H.; Song, Y.H.; Kang, S.H.; Kim, H.J. An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology 2010, 60, 222–228. [Google Scholar] [CrossRef]
- Park, K.S.; Do, H.; Lee, J.H.; Park, S.I.; Kim, S.-J.; Kang, S.-H.; Kim, H.J. Characterization of the ice-binding protein from Arctic yeast Leucosporidium sp. AY30. Cryobiology 2012, 64, 286–296. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, A.K.; Do, H.; Park, K.S.; Moh, S.H.; Chi, Y.M.; Kim, H.J. Structural basis for antifreeze activity of ice-binding protein from arctic yeast. J. Biol. Chem. 2012, 287, 11460–11468. [Google Scholar]
- Raymond, J.A.; Christner, B.C.; Schuster, S.C. A bacterial ice-binding protein from the Vostok ice core. Extremophiles 2008, 12, 713–717. [Google Scholar] [CrossRef]
- Garnham, C.P.; Campbell, R.L.; Davies, P.L. Anchored clathrate waters bind antifreeze proteins to ice. Proc. Natl. Acad. Sci. USA 2011, 108, 7363–7367. [Google Scholar] [CrossRef]
- Garnham, C.P.; Gilbert, J.A.; Hartman, C.P.; Campbell, R.L.; Laybourn-Parry, J.; Davies, P.L. A Ca2+-dependent bacterial antifreeze protein domain has a novel β-helical ice-binding fold. Biochem. J. 2008, 411, 171–180. [Google Scholar] [CrossRef]
- Middleton, A.J.; Brown, A.M.; Davies, P.L.; Walker, V.K. Identification of the ice-binding face of a plant antifreeze protein. FEBS Lett. 2009, 583, 815–819. [Google Scholar] [CrossRef]
- Middleton, A.J.; Marshall, C.B.; Faucher, F.; Bar-Dolev, M.; Braslavsky, I.; Campbell, R.L.; Walker, V.K.; Davies, P.L. Antifreeze protein from freeze-tolerant grass has a β-roll fold with an irregularly structured ice-binding site. J. Mol. Biol. 2012, 416, 713–724. [Google Scholar] [CrossRef]
- Pentelute, B.L.; Gates, Z.P.; Tereshko, V.; Dashnau, J.L.; Vanderkooi, J.M.; Kossiakoff, A.A.; Kent, S.B. X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers. J. Am. Chem. Soc. 2008, 130, 9695–9701. [Google Scholar] [CrossRef]
- Mok, Y.-F.; Lin, F.-H.; Graham, L.A.; Celik, Y.; Braslavsky, I.; Davies, P.L. Structural basis for the superior activity of the large isoform of snow flea antifreeze protein. Biochemistry 2010, 49, 2593–2603. [Google Scholar] [CrossRef]
- Patel, S.N.; Graether, S.P. Structures and ice-binding faces of the alanine-rich type I antifreeze proteins. Biochem. Cell Biol. 2010, 88, 223–229. [Google Scholar] [CrossRef]
- Fairley, K.; Westman, B.J.; Pham, L.H.; Haymet, A.D.; Harding, M.M.; Mackay, J.P. Type I shorthorn sculpin antifreeze protein: Recombinant synthesis, solution conformation, and ice growth inhibition studies. J. Biol. Chem. 2002, 277, 24073–24080. [Google Scholar]
- Kuduk, S.D.; Schwarz, J.B.; Chen, X.-T.; Glunz, P.W.; Sames, D.; Ragupathi, G.; Livingston, P.O.; Danishefsky, S.J. Synthetic and immunological studies on clustered modes of mucin-related Tn and TF O-linked antigens: The preparation of a glycopeptide-based vaccine for clinical trials against prostate cancer. J. Am. Chem. Soc. 1998, 120, 12474–12485. [Google Scholar] [CrossRef]
- Avanov, A.Y. Biological antifreezes and the mechanism of their activity. Mol. Biol. 1990, 24, 473–487. [Google Scholar]
- Wang, J.H. A comprehensive evaluation of the effects and mechanisms of antifreeze proteins during low-temperature preservation. Cryobiology 2000, 41, 1–9. [Google Scholar] [CrossRef]
- Zbikowska, H.M. Fish can be first—Advances in fish transgenesis for commercial applications. Transgenic Res. 2003, 12, 379–389. [Google Scholar] [CrossRef]
- Holmberg, N.; Lilius, G.; Bülow, L. Artificial antifreeze proteins can improve NaCl tolerance when expressed in E. coli. FEBS Lett. 1994, 349, 354–358. [Google Scholar] [CrossRef]
- Duncker, B.P.; Gauthier, S.Y.; Davies, P.L. Cystine-rich fish antifreeze is produced as an active proprotein precursor in fall armyworm cells. Biochem. Biophys. Res. Commun. 1994, 203, 1851–1857. [Google Scholar] [CrossRef]
- Loewen, M.; Liu, X.; Davies, P.; Daugulis, A. Biosynthetic production of type II fish antifreeze protein: Fermentation by Pichia pastoris. Appl. Microbiol. Biotechnol. 1997, 48, 480–486. [Google Scholar] [CrossRef]
- Gibson, M.I. Slowing the growth of ice with synthetic macromolecules: Beyond antifreeze (glyco) proteins. Polym. Chem. 2010, 1, 1141–1152. [Google Scholar] [CrossRef]
- Peltier, R.; Brimble, M.A.; Wojnar, J.M.; Williams, D.E.; Evans, C.W.; DeVries, A.L. Synthesis and antifreeze activity of fish antifreeze glycoproteins and their analogues. Chem. Sci. 2010, 1, 538–551. [Google Scholar] [CrossRef]
- Garner, J.; Harding, M.M. Design and synthesis of antifreeze glycoproteins and mimics. ChemBioChem 2010, 11, 2489–2498. [Google Scholar] [CrossRef]
- Chakrabartty, A.; Hew, C.L. The effect of enhanced α-helicity on the activity of a winter flounder antifreeze polypeptide. Eur. J. Biochem. 1991, 202, 1057–1063. [Google Scholar] [CrossRef]
- Knight, C.A.; Hallett, J.; DeVries, A. Solute effects on ice recrystallization: An assessment technique. Cryobiology 1988, 25, 55–60. [Google Scholar] [CrossRef]
- Hansen, T.N.; Baust, J.G. Differential scanning calorimetric analysis of antifreeze protein activity in the common mealworm, Tenebrio molitor. Biochim. Biophys. Acta 1988, 957, 217–221. [Google Scholar] [CrossRef]
- Wierzbicki, A.; Dalal, P.; Cheatham, T.E., III; Knickelbein, J.E.; Haymet, A.D.; Madura, J.D. Antifreeze proteins at the ice/water interface: Three calculated discriminating properties for orientation of type I proteins. Biophys. J. 2007, 93, 1442–1451. [Google Scholar] [CrossRef]
- Kwan, A.H.; Fairley, K.; Anderberg, P.I.; Liew, C.W.; Harding, M.M.; Mackay, J.P. Solution structure of a recombinant type I sculpin antifreeze protein. Biochemistry 2005, 44, 1980–1988. [Google Scholar]
- Chao, H.; Hodges, R.S.; Kay, C.M.; Gauthier, S.Y.; Davies, P.L. A natural variant of type I antifreeze protein with four ice-binding repeats is a particularly potent antifreeze. Protein Sci. 1996, 5, 1150–1156. [Google Scholar]
- Graham, L.A.; Marshall, C.B.; Lin, F.H.; Campbell, R.L.; Davies, P.L. Hyperactive antifreeze protein from fish contains multiple ice-binding sites. Biochemistry 2008, 47, 2051–2063. [Google Scholar] [CrossRef]
- Marshall, C.B.; Chakrabartty, A.; Davies, P.L. Hyperactive antifreeze protein from winter flounder is a very long rod-like dimer of α-helices. J. Biol. Chem. 2005, 280, 17920–17929. [Google Scholar]
- Wierzbicki, A.; Taylor, M.; Knight, C.; Madura, J.; Harrington, J.; Sikes, C. Analysis of shorthorn sculpin antifreeze protein stereospecific binding to (2–1 0) faces of ice. Biophys. J. 1996, 71, 8–18. [Google Scholar] [CrossRef]
- Baardsnes, J.; Davies, P.L. Sialic acid synthase: The origin of fish type III antifreeze protein? Trends Biochem. Sci. 2001, 26, 468–469. [Google Scholar] [CrossRef]
- Wu, Y.; Banoub, J.; Goddard, S.V.; Kao, M.H.; Fletcher, G.L. Antifreeze glycoproteins: Relationship between molecular weight, thermal hysteresis and the inhibition of leakage from liposomes during thermotropic phase transition. Comp. Biochem. Physiol. B 2001, 128, 265–273. [Google Scholar] [CrossRef]
- Devries, A.L. Glycoproteins as biological antifreeze agents in antarctic fishes. Science 1971, 172, 1152–1155. [Google Scholar]
- Komatsu, S.K.; DeVries, A.L.; Feeney, R.E. Studies of the structure of freezing point-depressing glycoproteins from an Antarctic fish. J. Biol. Chem. 1970, 245, 2909–2913. [Google Scholar]
- Vandenheede, J.R.; Ahmed, A.I.; Feeney, R.E. Structure and role of carbohydrate in freezing point-depressing glycoproteins from an Antarctic fish. J. Biol. Chem. 1972, 247, 7885–7889. [Google Scholar]
- Ahmed, A.; Yeh, Y.; Osuga, Y.; Feeney, R. Antifreeze glycoproteins from Antarctic fish. Inactivation by borate. J. Biol. Chem. 1976, 251, 3033–3036. [Google Scholar]
- Osuga, D.T.; Feather, M.S.; Shah, M.J.; Feeney, R.E. Modification of galactose and N-acetylgalactosamine residues by oxidation of C-6 hydroxyls to the aldehydes followed by reductive amination: Model systems and antifreeze glycoproteins. J. Protein Chem. 1989, 8, 519–528. [Google Scholar] [CrossRef]
- Schrag, J.D.; O’Grady, S.M.; Devries, A.L. Relationship of amino acid composition and molecular weight of antifreeze glycopeptides to non-colligative freezing point depression. Biochim. Biophys. Acta 1982, 717, 322–326. [Google Scholar] [CrossRef]
- Tsuda, T.; Nishimura, S.I. Synthesis of an antifreeze glycoprotein analogue: Efficient preparation of sequential glycopeptide polymers. Chem. Commun. 1996, 2779–2780. [Google Scholar] [CrossRef]
- Tachibana, Y.; Matsubara, N.; Nakajima, F.; Tsuda, T.; Tsuda, S.; Monde, K.; Nishimura, S.I. Efficient and versatile synthesis of mucin-like glycoprotein mimics. Tetrahedron 2002, 58, 10213–10224. [Google Scholar]
- Tachibana, Y.; Monde, K.; Nishimura, S.I. Sequential glycoproteins: Practical method for the synthesis of antifreeze glycoprotein models containing base labile groups. Macromolecules 2004, 37, 6771–6779. [Google Scholar] [CrossRef]
- Tachibana, Y.; Fletcher, G.L.; Fujitani, N.; Tsuda, S.; Monde, K.; Nishimura, S.I. Antifreeze glycoproteins: Elucidation of the structural motifs that are essential for antifreeze activity. Angew. Chem. Int. Ed. Engl. 2004, 43, 856–862. [Google Scholar] [CrossRef]
- Garner, J.; Jolliffe, K.A.; Harding, M.M.; Payne, R.J. Synthesis of homogeneous antifreeze glycopeptides via a ligation–desulfurisation strategy. Chem. Commun. 2009, 45, 6925–6927. [Google Scholar]
- Wilkinson, B.L.; Stone, R.S.; Capicciotti, C.J.; Thaysen-Andersen, M.; Matthews, J.M.; Packer, N.H.; Ben, R.N.; Payne, R.J. Total synthesis of homogeneous antifreeze glycopeptides and glycoproteins. Angew. Chem. Int. Ed. Engl. 2012, 51, 3606–3610. [Google Scholar] [CrossRef]
- Anisuzzaman, A.K.M.; Anderson, L.; Navia, J.L. Synthesis of a close analog of the repeating unit of the antifreeze glycoproteins of polar fish. Carbohydr. Res. 1988, 174, 265–278. [Google Scholar] [CrossRef]
- Filira, F.; Biondi, L.; Scolaro, B.; Foffani, M.; Mammi, S.; Peggion, E.; Rocchi, R. Solid phase synthesis and conformation of sequential glycosylated polytripeptide sequences related to antifreeze glycoproteins. Int. J. Biol. Macromol. 1990, 12, 41–49. [Google Scholar] [CrossRef]
- Hachisu, M.; Hinou, H.; Takamichi, M.; Tsuda, S.; Koshida, S.; Nishimura, S.-I. One-pot synthesis of cyclic antifreeze glycopeptides. Chem. Commun. 2009, 1641–1643. [Google Scholar] [CrossRef]
- Heggemann, C.; Budke, C.; Schomburg, B.; Majer, Z.; Wißbrock, M.; Koop, T.; Sewald, N. Antifreeze glycopeptide analogues: Microwave-enhanced synthesis and functional studies. Amino Acids 2010, 38, 213–222. [Google Scholar] [CrossRef]
- Peltier, R.; Evans, C.W.; DeVries, A.L.; Brimble, M.A.; Dingley, A.J.; Williams, D.E. Growth habit modification of ice crystals using antifreeze glycoprotein (AFGP) analogues. Cryst. GrowthDes. 2010, 10, 5066–5077. [Google Scholar] [CrossRef]
- Ahn, M.; Murugan, R.N.; Kim, E.; Lee, J.H.; Cheong, C.; Kang, S.W.; Park, H.J.; Shin, S.Y.; Kim, H.J.; Bang, J.K. Studies on the effect of number of sugar moiety in the antifreeze activity of homodimeric AFGPs. Bull. Korean Chem. Soc. 2012, 33, 2411–2414. [Google Scholar] [CrossRef]
- Meldal, M.; Jensen, K.J. Pentafluorophenyl esters for the temporary protection of the α-carboxy group in solid phase glycopeptide synthesis. Chem. Commun. 1990, 483–485. [Google Scholar] [CrossRef]
- Budke, C.; Heggemann, C.; Koch, M.; Sewald, N.; Koop, T. Ice recrystallization kinetics in the presence of synthetic antifreeze glycoprotein analogues using the framework of LSW theory. J. Phys. Chem. 2009, 113, 2865–2873. [Google Scholar] [CrossRef]
- Nagel, L.; Plattner, C.; Budke, C.; Majer, Z.; DeVries, A.L.; Berkemeier, T.; Koop, T.; Sewald, N. Synthesis and characterization of natural and modified antifreeze glycopeptides: Glycosylated foldamers. Amino Acids 2011, 41, 719–732. [Google Scholar] [CrossRef]
- Nagel, L.; Budke, C.; Dreyer, A.; Koop, T.; Sewald, N. Antifreeze glycopeptide diastereomers. Beilstein J. Org. Chem. 2012, 8, 1657–1667. [Google Scholar] [CrossRef]
- Nagel, L.; Budke, C.; Erdmann, R.S.; Dreyer, A.; Wennemers, H.; Koop, T.; Sewald, N. Influence of sequential modifications and carbohydrate variations in synthetic AFGP analogues on conformation and antifreeze activity. Chem. Eur. J. 2012, 18, 12783–12793. [Google Scholar]
- Ben, R.N.; Eniade, A.A.; Hauer, L. Synthesis of a C-linked antifreeze glycoprotein (AFGP) mimic: Probes for investigating the mechanism of action. Org. Lett. 1999, 1, 1759–1762. [Google Scholar] [CrossRef]
- Eniade, A.; Ben, R.N. Fully convergent solid phase synthesis of antifreeze glycoprotein analogues. Biomacromolecules 2001, 2, 557–561. [Google Scholar] [CrossRef]
- Eniade, A.; Murphy, A.V.; Landreau, G.; Ben, R.N. A general synthesis of structurally diverse building blocks for preparing analogues of C-linked antifreeze glycoproteins. Bioconjug. Chem. 2001, 12, 817–823. [Google Scholar] [CrossRef]
- Liu, S.; Ben, R.N. C-linked galactosyl serine AFGP analogues as potent recrystallization inhibitors. Org. Lett. 2005, 7, 2385–2388. [Google Scholar] [CrossRef]
- Tam, R.Y.; Rowley, C.N.; Petrov, I.; Zhang, T.; Afagh, N.A.; Woo, T.K.; Ben, R.N. Solution conformation of C-linked antifreeze glycoprotein analogues and modulation of ice recrystallization. J. Am. Chem. Soc. 2009, 131, 15745–15753. [Google Scholar]
- Leclère, M.; Kwok, B.K.; Wu, L.K.; Allan, D.S.; Ben, R.N. C-linked antifreeze glycoprotein (C-AFGP) analogues as novel cryoprotectants. Bioconjug. Chem. 2011, 22, 1804–1810. [Google Scholar] [CrossRef]
- Czechura, P.; Tam, R.Y.; Dimitrijevic, E.; Murphy, A.V.; Ben, R.N. The importance of hydration for inhibiting ice recrystallization with C-linked antifreeze glycoproteins. J. Am. Chem. Soc. 2008, 130, 2928–2929. [Google Scholar]
- Tam, R.Y.; Ferreira, S.S.; Czechura, P.; Chaytor, J.L.; Ben, R.N. Hydration index—A better parameter for explaining small molecule hydration in inhibition of ice recrystallization. J. Am. Chem. Soc. 2008, 130, 17494–17501. [Google Scholar]
- Balcerzak, A.K.; Ferreira, S.S.; Trant, J.F.; Ben, R.N. Structurally diverse disaccharide analogs of antifreeze glycoproteins and their ability to inhibit ice recrystallization. Bioorg. Med. Chem. Lett. 2011, 22, 1719–1721. [Google Scholar]
- Gibson, M.I.; Barker, C.A.; Spain, S.G.; Albertin, L.; Cameron, N.R. Inhibition of ice crystal growth by synthetic glycopolymers: Implications for the rational design of antifreeze glycoprotein mimics. Biomacromolecules 2008, 10, 328–333. [Google Scholar]
- Miller, N.; Williams, G.M.; Brimble, M.A. Synthesis of fish antifreeze neoglycopeptides using microwave-assisted “click chemistry”. Org. Lett. 2009, 11, 2409–2412. [Google Scholar] [CrossRef]
- Norgren, A.S.; Budke, C.; Majer, Z.; Heggemann, C.; Koop, T.; Sewald, N. On-resin click-glycoconjugation of peptoids. Synthesis 2009, 2009, 488–494. [Google Scholar]
- Capicciotti, C.J.; Trant, J.F.; Leclère, M.; Ben, R.N. Synthesis of C-linked triazole-containing AFGP analogues and their ability to inhibit ice recrystallization. Bioconjug. Chem. 2011, 22, 605–616. [Google Scholar] [CrossRef]
- Ahn, M.; Murugan, R.N.; Shin, S.Y.; Kim, H.J.; Bang, J.K. Peptoid-based positional scanning derivatives: Revealing the optimum residue required for ice recrystallization inhibition activity for every position in the AFGPs. Bull. Korean Chem. Soc. 2012, 33, 3931–3932. [Google Scholar] [CrossRef]
- Ahn, M.; Murugan, R.N.; Shin, S.Y.; Kim, E.; Lee, J.H.; Kim, H.J.; Bang, J.K. Synthesis of cyclic antifreeze glycopeptide and glycopeptoids and their ice recrystallization inhibition activity. Bull. Korean Chem. Soc. 2012, 33, 3565–3570. [Google Scholar] [CrossRef]
- Huang, M.L.; Ehre, D.; Jiang, Q.; Hu, C.; Kirshenbaum, K.; Ward, M.D. Biomimetic peptoid oligomers as dual-action antifreeze agents. Proc. Natl. Acad. Sci. USA 2012, 109, 19922–19927. [Google Scholar]
- Yeh, Y.; Feeney, R.E. Antifreeze roteins: Structures and mechanisms of function. Chem. Rev. 1996, 96, 601–618. [Google Scholar] [CrossRef]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryo Lett. 2004, 25, 375–388. [Google Scholar]
- Barrett, J. Thermal hysteresis proteins. Int. J. Biochem. Cell Biol. 2001, 33, 105–117. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G.L. Hypothermic protection—A fundamental property of “antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef]
- Arav, A.; Rubinsky, B.; Fletcher, G.; Seren, E. Cryogenic protection of oocytes with antifreeze proteins. Mol. Reprod. Dev. 1993, 36, 488–493. [Google Scholar] [CrossRef]
- O’Neil, L.; Paynter, S.; Fuller, B.; Shaw, R.; DeVries, A. Vitrification of mature mouse oocytes in a 6 M Me2SO solution supplemented with antifreeze glycoproteins: The effect of temperature. Cryobiology 1998, 37, 59–66. [Google Scholar] [CrossRef]
- Chen, L.R.; Huang, W.Y.; Luoh, Y.S.; Wu, M.C. Cryopreservation of porcine oocytes before and after polar body formation by antifreeze protein type III. J. Taiwan Livest. Res. 1995, 28, 169–180. [Google Scholar]
- Jo, J.W.; Jee, B.C.; Lee, J.R.; Suh, C.S. Effect of antifreeze protein supplementation in vitrification medium on mouse oocyte developmental competence. Fertil. Steril. 2011, 96, 1239–1245. [Google Scholar] [CrossRef]
- Koushafar, H.; Pham, L.; Lee, C.; Rubinsky, B. Chemical adjuvant cryosurgery with antifreeze proteins. J. Surg. Oncol. 1997, 66, 114–121. [Google Scholar] [CrossRef]
- Tursman, D.; Duman, J.G. Cryoprotective effects of thermal hysteresis protein on survivorship of frozen gut cells from the freeze tolerant centipede Lithobius forficatus. J. Exp. Zool. 1995, 272, 249–257. [Google Scholar] [CrossRef]
- Makarevich, A.; Kubovičová, E.; Popelková, M.; Fabian, D.; Čikoš, Š.; Pivko, J.; Chrenek, P. Several aspects of animal embryo cryopreservation: Anti-freeze protein (AFP) as a potential cryoprotectant. Zygote 2010, 18, 145–153. [Google Scholar] [CrossRef]
- Naidenko, T. Cryopreservative of Crassostrea gigas oocytes embryo and larvae using antioxidants echinochromes A and antifreeze protein AFP-1. Cryo Lett. 1997, 18, 375–382. [Google Scholar]
- Karanova, M.; Zsvetkova, L.; Petropavlov, N. Effect of antifreeze glycoproteins on quality of cryoconserved carp sperm. Biofizika 1997, 42, 725–728. [Google Scholar]
- Upreti, G.; Payne, S.; Duganzich, D.; Oliver, J.; Smith, J. Enzyme leakage during cryopreservation of ram spermatozoa. Anim. Reprod. Sci. 1996, 41, 27–36. [Google Scholar] [CrossRef]
- Baguisi, A.; Arav, A.; Crosby, T.F.; Roche, J.F.; Boland, M.P. Hypothermic storage of sheep embryos with antifreeze proteins: Development in vitro and in vivo. Theriogenology 1997, 48, 1017–1024. [Google Scholar] [CrossRef]
- Prathalingam, N.S.; Holt, W.V.; Revell, S.G.; Mirczuk, S.; Fleck, R.A.; Watson, P.F. Impact of antifreeze proteins and antifreeze glycoproteins on bovine sperm during freeze-thaw. Theriogenology 2006, 66, 1894–1900. [Google Scholar] [CrossRef]
- Robles, V.; Cabrita, E.; Anel, L.; Herráez, M. Microinjection of the antifreeze protein type III (AFPIII) in turbot Scophthalmus maximus embryos: Toxicity and protein distribution. Aquaculture 2006, 261, 1299–1306. [Google Scholar] [CrossRef]
- Younis, A.I.; Rooks, B.; Khan, S.; Gould, K.G. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J. Androl. 1998, 19, 207–214. [Google Scholar]
- Martínez-Páramo, S.; Pérez-Cerezales, S.; Robles, V.; Anel, L.; Herráez, M. Incorporation of antifreeze proteins into zebrafish embryos by a non-invasive method. Cryobiology 2008, 56, 216–222. [Google Scholar] [CrossRef]
- Wang, J.; Bian, R.; Zhang, Y.; Cheng, H. The dual effect of antifreeze protein on cryopreservation of rice (Oryza sativa I.) embryogenic suspension cells. Cryo Lett. 2001, 22, 175–182. [Google Scholar]
- Matsumoto, S.; Matsusita, M.; Morita, T.; Kamachi, H.; Tsukiyama, S.; Furukawa, Y.; Koshida, S.; Tachibana, Y.; Nishimura, S.; Todo, S. Effects of synthetic antifreeze glycoprotein analogue on islet cell survival and function during cryopreservation. Cryobiology 2006, 52, 90–98. [Google Scholar] [CrossRef]
- Tablin, F.; Oliver, A.E.; Walker, N.J.; Crowe, L.M.; Crowe, J.H. Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J. Cell. Physiol. 1996, 168, 305–313. [Google Scholar] [CrossRef]
- Kawahara, H.; Higa, S.; Tatsukawa, H.; Obata, H. Cryoprotection and cryosterilization effects of type I antifreeze protein on E. coli cells. Biocontrol Sci. 2009, 14, 49–54. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A.L. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Arav, A.; Rubinsky, B.; Seren, E.; Roche, J.F.; Boland, M.P. The role of thermal hysteresis proteins during cryopreservation of oocytes and embryos. Theriogenology 1994, 41, 107–112. [Google Scholar] [CrossRef]
- Negulescu, P.A.; Rubinsky, B.; Fletcher, G.L.; Machen, T.E. Fish antifreeze proteins block Ca entry into rabbit parietal cells. Am. J. Physiol. 1992, 263, C1310–C1313. [Google Scholar]
- Hays, L.M.; Feeney, R.E.; Crowe, L.M.; Crowe, J.H.; Oliver, A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA 1996, 93, 6835–6840. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Feeney, R.E.; Crowe, J.H. Antifreeze proteins differentially affect model membranes during freezing. Biochim. Biophys. Acta 2001, 1511, 255–263. [Google Scholar]
- Wu, Y.; Fletcher, G.L. Efficacy of antifreeze protein types in protecting liposome membrane integrity depends on phospholipid class. Biochim. Biophys. Acta 2001, 1524, 11–16. [Google Scholar]
- Hincha, D.K.; DeVries, A.L.; Schmitt, J.M. Cryotoxicity of antifreeze proteins and glycoproteins to spinach thylakoid membranes—Comparison with cryotoxic sugar acids. Biochim. Biophys. Acta 1993, 1146, 258–264. [Google Scholar] [CrossRef]
- Wang, J.H.; Bian, H.W.; Huang, C.N.; Ge, J.G. Studies on the application of antifreeze proteins in cryopreservation of rice suspension cells. Shi Yan Sheng Wu Xue Bao 1999, 32, 271–276. [Google Scholar]
- Wang, L.H.; Wusteman, M.C.; Smallwood, M.; Pegg, D.E. The stability during low-temperature storage of an antifreeze protein isolated from the roots of cold-acclimated carrots. Cryobiology 2002, 44, 307–310. [Google Scholar] [CrossRef]
- Ishiguro, H.; Rubinsky, B. Influence of fish antifreeze proteins on the freezing of cell suspensions with cryoprotectant penetrating cells. Int. J. Heat Mass Transfer 1998, 41, 1907–1915. [Google Scholar] [CrossRef]
- Payne, S.R.; Oliver, J.E.; Upreti, G.C. Effect of antifreeze proteins on the motility of ram spermatozoa. Cryobiology 1994, 31, 180–184. [Google Scholar] [CrossRef]
- Lagneaux, D.; Huhtinen, M.; Koskinen, E.; Palmer, E. Effect of anti-freeze protein (AFP) on the cooling and freezing of equine embryos as measured by DAPI-staining. Equine Vet. J. Suppl. 1997, 25, 85–87. [Google Scholar]
- Shaw, J.M.; Ward, C.; Trounson, A.O. Evaluation of propanediol, ethylene glycol, sucrose and antifreeze proteins on the survival of slow-cooled mouse pronuclear and 4-cell embryos. Hum. Reprod. 1995, 10, 396–402. [Google Scholar]
- Mezhevikina, L.M.; Karanova, M.V. The use of antifreeze glycoproteins in the freezing in liquid nitrogen of early mouse embryos. Izv. Akad. Nauk. Ser. Biol. 1995, 2, 172–177. [Google Scholar]
- Soltys, K.A.; Batta, A.K.; Koneru, B. Successful nonfreezing, subzero preservation of rat liver with 2,3-butanediol and type I antifreeze protein. J. Surg. Res. 2001, 96, 30–34. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Q.; Yang, X.; Layne, J.R., Jr.; Devries, A.L. Antifreeze glycoproteins from antarctic notothenioid fishes fail to protect the rat cardiac explant during hypothermic and freezing preservation. Cryobiology 1994, 31, 185–192. [Google Scholar] [CrossRef]
- Rous, P.; Turner, J.R. The preservation of living red blood cells in vitro: 1. Methods of preservation. J. Exp. Med. 1916, 23, 219–237. [Google Scholar] [CrossRef]
- Hess, J.R. An update on solutions for red cell storage. Vox Sang. 2006, 91, 13–19. [Google Scholar] [CrossRef]
- Hill, H.R.; Oliver, C.K.; Lippert, L.E.; Greenwalt, T.J.; Hess, J.R. The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sang. 2001, 81, 161–166. [Google Scholar] [CrossRef]
- Heaton, W.A.; Holme, S.; Smith, K.; Brecher, M.E.; Pineda, A.; AuBuchon, J.P.; Nelson, E. Effects of 3–5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. Br. J. Haematol. 1994, 87, 363–368. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenwalt, T.G. Storage of red blood cells: New approaches. Transfus. Med. Rev. 2002, 16, 283–295. [Google Scholar] [CrossRef]
- Chao, H.; Davies, P.L.; Carpenter, J.F. Effects of antifreeze proteins on red blood cell survival during cryopreservation. J. Exp. Biol. 1996, 199, 2071–2076. [Google Scholar]
- Carpenter, J.F.; Hansen, T.N. Antifreeze protein modulates cell survival during cryopreservation: Mediation through influence on ice crystal growth. Proc. Natl. Acad. Sci. USA 1992, 89, 8953–8957. [Google Scholar] [CrossRef]
- Kang, J.S.; Raymond, J.A. Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryo Lett. 2004, 25, 307–310. [Google Scholar]
- Lee, S.G.; Koh, H.Y.; Lee, J.H.; Kang, S.; Kim, H.J. Cryopreservative effects of the recombinant ice-binding protein from the Arctic yeast Leucosporidium sp. on red blood cells. Appl. Biochem. Biotechnol. 2012, 167, 824–834. [Google Scholar] [CrossRef]
- Meryman, H.T.; Hornblower, M. A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion 1972, 12, 145–156. [Google Scholar]
- Maathuis, M.H.J.; Leuvenink, H.G.D.; Ploeg, R.J. Perspectives in organ preservation. Transplantation 2007, 83, 1289–1298. [Google Scholar] [CrossRef]
- McAnulty, J.F. Hypothermic organ preservation by static storage methods: Current status and a view to the future. Cryobiology 2010, 60 (3 Suppl.), 13–19. [Google Scholar] [CrossRef]
- Lee, C.; Rubinsky, B.; Fletcher, G. Hypothermic preservation of whole mammalian organs with antifreeze proteins. Cryo Lett. 1992, 13, 59–66. [Google Scholar]
- Mugnano, J.A.; Wang, T.; Layne, J.R., Jr.; DeVries, A.L.; Lee, R.E., Jr. Antifreeze glycoproteins promote intracellular freezing of rat cardiomyocytes at high subzero temperatures. Am. J. Physiol. 1995, 269, R474–R479. [Google Scholar]
- Amir, G.; Rubinsky, B.; Kassif, Y.; Horowitz, L.; Smolinsky, A.K.; Lavee, J. Preservation of myocyte structure and mitochondrial integrity in subzero cryopreservation of mammalian hearts for transplantation using antifreeze proteins—An electron microscopy study. Eur. J. Cardiothorac. Surg. 2003, 24, 292–296, discussion 296–297. [Google Scholar] [CrossRef]
- Koushafar, H.; Rubinsky, B. Effect of antifreeze proteins on frozen primary prostatic adenocarcinoma cells. Urology 1997, 49, 421–425. [Google Scholar] [CrossRef]
- Muldrew, K.; Rewcastle, J.; Donnelly, B.J.; Saliken, J.C.; Liang, S.; Goldie, S.; Olson, M.; Baissalov, R.; Sandison, G. Flounder antifreeze peptides increase the efficacy of cryosurgery. Cryobiology 2001, 42, 182–189. [Google Scholar] [CrossRef]
- Pham, L.; Dahiya, R.; Rubinsky, B. An in vivo study of antifreeze protein adjuvant cryosurgery. Cryobiology 1999, 38, 169–175. [Google Scholar] [CrossRef]
- Griffith, M.; Ewart, K.V. Antifreeze proteins and their potential use in frozen foods. Biotechnol. Adv. 1995, 13, 375–402. [Google Scholar] [CrossRef]
- Feeney, R.E.; Yeh, Y. Antifreeze proteins: Current status and possible food uses. Trends Food Sci. Technol. 1998, 9, 102–106. [Google Scholar] [CrossRef]
- Payne, S.R.; Sandford, D.; Harris, A.; Young, O.A. The effects of antifreeze proteins on chilled and frozen meat. Meat Sci. 1994, 37, 429–438. [Google Scholar] [CrossRef]
- Payne, S.R.; Young, O.A. Effects of pre-slaughter administration of antifreeze proteins on frozen meat quality. Meat Sci. 1995, 41, 147–155. [Google Scholar] [CrossRef]
- Rothwell, J. Microbiology of Frozen Dairy Products; Elsevier Applied Science Publishers: London, UK, 1985. [Google Scholar]
- Hew, C.L.; Davies, P.L.; Fletcher, G. Antifreeze protein gene transfer in Atlantic salmon. Mol. Mar. Biol. Biotechnol. 1992, 1, 309–317. [Google Scholar]
- Hew, C.L.; Poon, R.; Xiong, F.; Gauthier, S.; Shears, M.; King, M.; Davies, P.; Fletcher, G. Liver-specific and seasonal expression of transgenic Atlantic salmon harboring the winter flounder antifreeze protein gene. Transgenic Res. 1999, 8, 405–414. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, P.; Gong, Z.; Hew, C.L. Expression of the antifreeze protein gene in transgenic goldfish (Carassius auratus) and its implication in cold adaptation. Mol. Mar. Biol. Biotechnol. 1995, 4, 20–26. [Google Scholar]
- Hightower, R.; Baden, C.; Penzes, E.; Lund, P.; Dunsmuir, P. Expression of antifreeze proteins in transgenic plants. Plant Mol. Biol. 1991, 17, 1013–1021. [Google Scholar] [CrossRef]
- Wallis, J.G.; Wang, H.; Guerra, D.J. Expression of a synthetic antifreeze protein in potato reduces electrolyte release at freezing temperatures. Plant Mol. Biol. 1997, 35, 323–330. [Google Scholar] [CrossRef]
- Khanna, H.K.; Daggard, G.E. Targeted expression of redesigned and codon optimised synthetic gene leads to recrystallisation inhibition and reduced electrolyte leakage in spring wheat at sub-zero temperatures. Plant Cell Rep. 2006, 25, 1336–1346. [Google Scholar] [CrossRef]
- Kenward, K.D.; Altschuler, M.; Hildebrand, D.; Davies, P.L. Accumulation of type I fish antifreeze protein in transgenic tobacco is cold-specific. Plant Mol. Biol. 1993, 23, 377–385. [Google Scholar] [CrossRef]
- Kenward, K.D.; Brandle, J.; McPherson, J.; Davies, P.L. Type II fish antifreeze protein accumulation in transgenic tobacco does not confer frost resistance. Transgenic Res. 1999, 8, 105–117. [Google Scholar] [CrossRef]
- Bagis, H.; Akkoç, T.; Tasş, A.; Aktoprakligil, D. Cryogenic effect of antifreeze protein on transgenic mouse ovaries and the production of live offspring by orthotopic transplantation of cryopreserved mouse ovaries. Mol. Reprod. Dev. 2008, 75, 608–613. [Google Scholar] [CrossRef]
- Bagis, H.; Aktoprakligil, D.; Mercan, H.O.; Yurdusev, N.; Turgut, G.; Sekmen, S.; Arat, S.; Cetin, S. Stable transmission and transcription of newfoundland ocean pout type III fish antifreeze protein (AFP) gene in transgenic mice and hypothermic storage of transgenic ovary and testis. Mol. Reprod. Dev. 2006, 73, 1404–1411. [Google Scholar] [CrossRef]
- Rubinsky, B.; Devries, A.L. Effects of ice crystal habit on the viability of glycerol protected red blood cells. Cryobiology 1989, 26, 580. [Google Scholar] [CrossRef]
- Macouzet, M.; Simpson, B.K.; Lee, B.H. Cloning of fish enzymes and other fish protein genes. Crit. Rev. Biotechnol. 1999, 19, 179–196. [Google Scholar] [CrossRef]
- Crevel, R.; Fedyk, J.; Spurgeon, M. Antifreeze proteins: Characteristics, occurrence and human exposure. Food Chem. Toxicol. 2002, 40, 899–903. [Google Scholar] [CrossRef]
- Hall-Manning, T.; Spurgeon, M.; Wolfreys, A.; Baldrick, A. Safety evaluation of ice-structuring protein (ISP) type III HPLC 12 preparation. Lack of genotoxicity and subchronic toxicity. Food Chem. Toxicol. 2004, 42, 321–333. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; von Moos, E.; Jackman, J.; Mealing, G.; Monette, R.; Ben, R.N. In vitro studies of antifreeze glycoprotein (AFGP) and a C-linked AFGP analogue. Biomacromolecules 2007, 8, 1456–1462. [Google Scholar] [CrossRef]
- Goel, R.; Anderson, K.; Slaton, J.; Schmidlin, F.; Vercellotti, G.; Belcher, J.; Bischof, J.C. Adjuvant approaches to enhance cryosurgery. J. Biomech. Eng. 2009, 131, 074003. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bang, J.K.; Lee, J.H.; Murugan, R.N.; Lee, S.G.; Do, H.; Koh, H.Y.; Shim, H.-E.; Kim, H.-C.; Kim, H.J. Antifreeze Peptides and Glycopeptides, and Their Derivatives: Potential Uses in Biotechnology. Mar. Drugs 2013, 11, 2013-2041. https://doi.org/10.3390/md11062013
Bang JK, Lee JH, Murugan RN, Lee SG, Do H, Koh HY, Shim H-E, Kim H-C, Kim HJ. Antifreeze Peptides and Glycopeptides, and Their Derivatives: Potential Uses in Biotechnology. Marine Drugs. 2013; 11(6):2013-2041. https://doi.org/10.3390/md11062013
Chicago/Turabian StyleBang, Jeong Kyu, Jun Hyuck Lee, Ravichandran N. Murugan, Sung Gu Lee, Hackwon Do, Hye Yeon Koh, Hye-Eun Shim, Hyun-Cheol Kim, and Hak Jun Kim. 2013. "Antifreeze Peptides and Glycopeptides, and Their Derivatives: Potential Uses in Biotechnology" Marine Drugs 11, no. 6: 2013-2041. https://doi.org/10.3390/md11062013
APA StyleBang, J. K., Lee, J. H., Murugan, R. N., Lee, S. G., Do, H., Koh, H. Y., Shim, H.-E., Kim, H.-C., & Kim, H. J. (2013). Antifreeze Peptides and Glycopeptides, and Their Derivatives: Potential Uses in Biotechnology. Marine Drugs, 11(6), 2013-2041. https://doi.org/10.3390/md11062013






