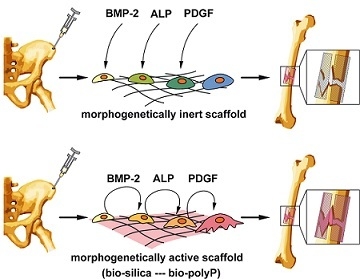
The Deep-Sea Natural Products, Biogenic Polyphosphate (Bio-PolyP) and Biogenic Silica (Bio-Silica), as Biomimetic Scaffolds for Bone Tissue Engineering: Fabrication of a Morphogenetically-Active Polymer
Abstract
:1. Introduction
2. Two Novel Bio-Inorganic Polymers from Deep-Sea Sponges: Candidate Molecules for Biomimetic Bone Substitution Materials
2.1. Biogenic Polyphosphate (Bio-PolyP)

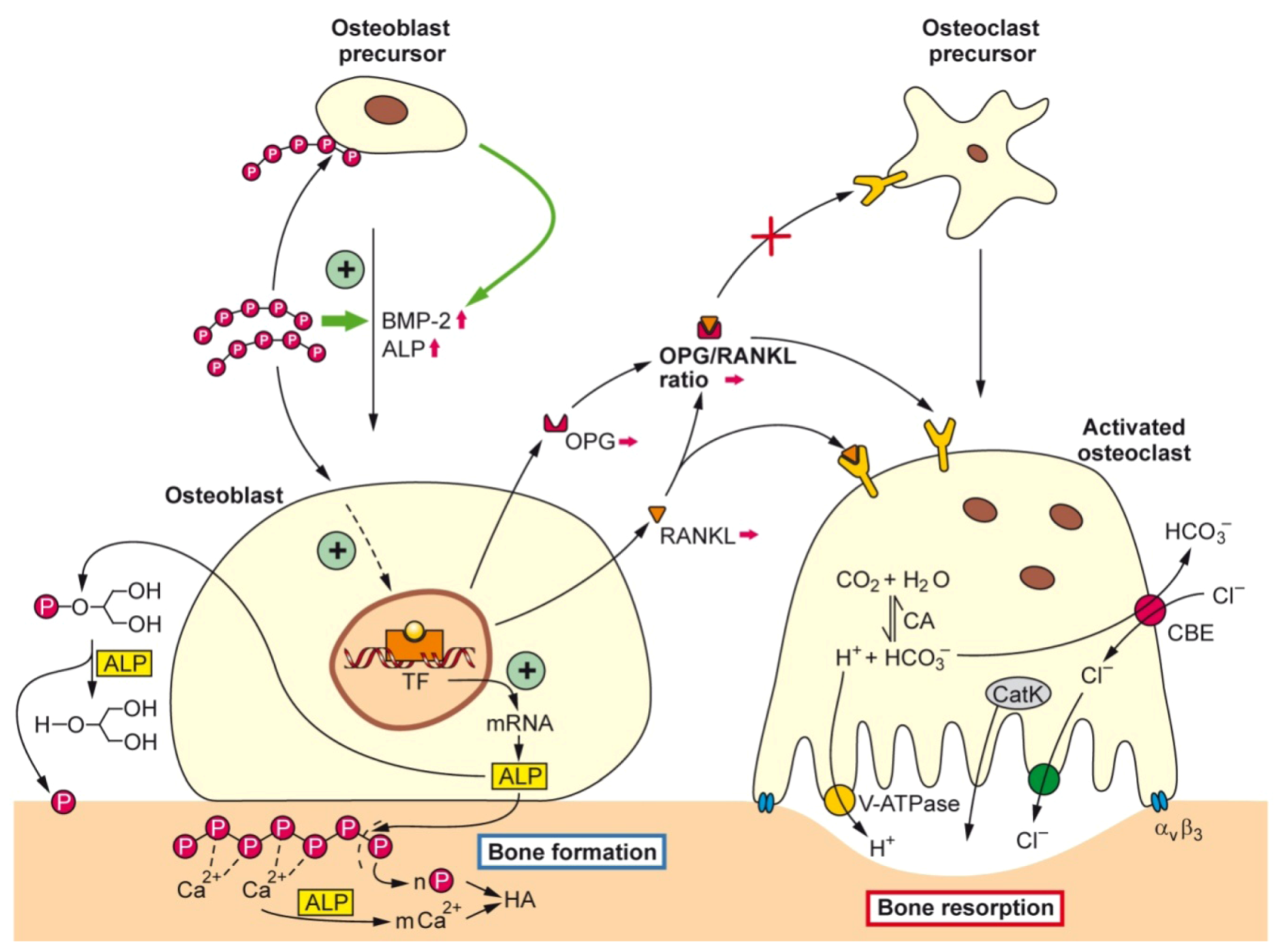
2.2. Biogenic Silica (Bio-Silica)
3. Silica as an Essential Nutrient
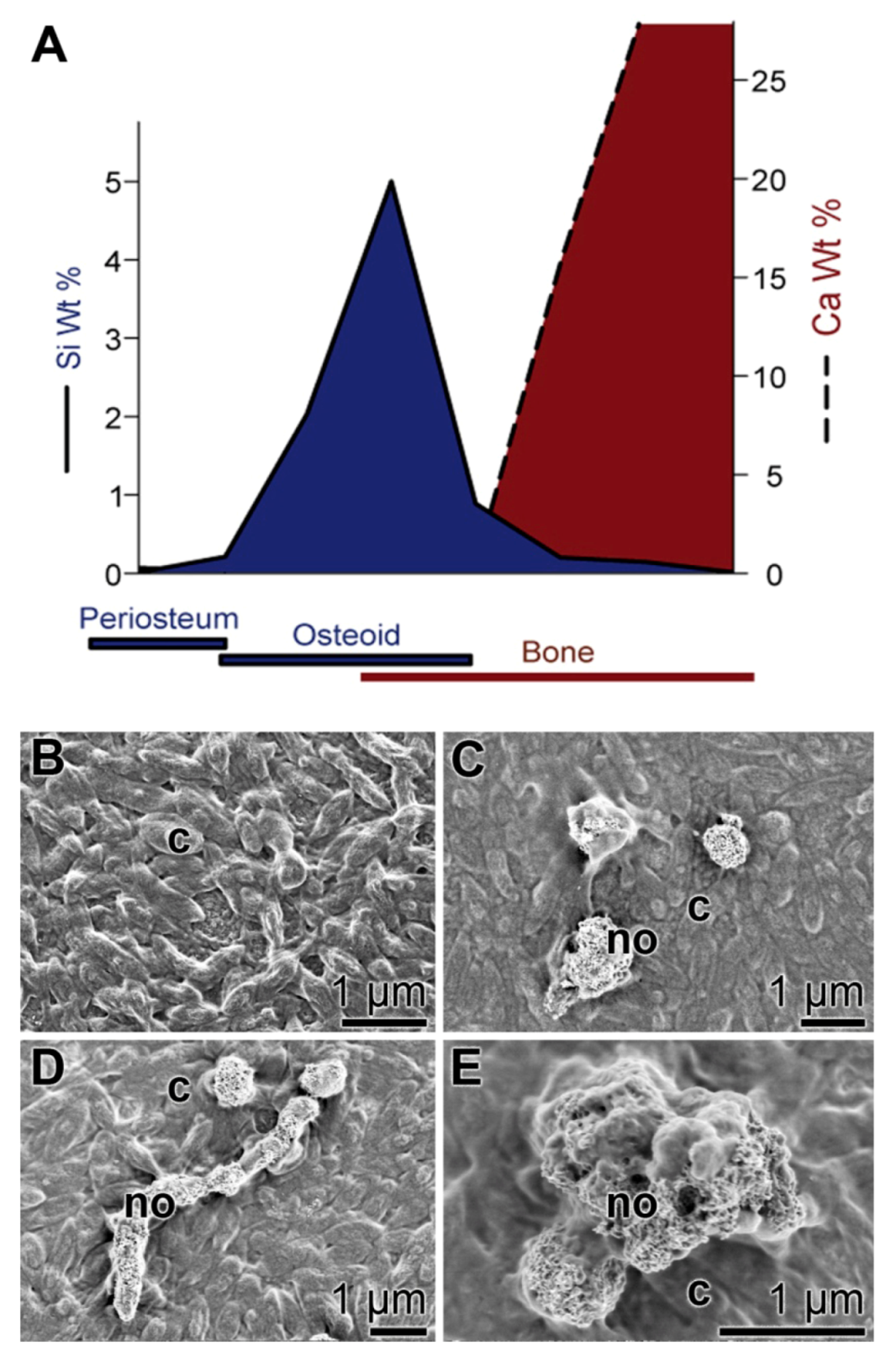
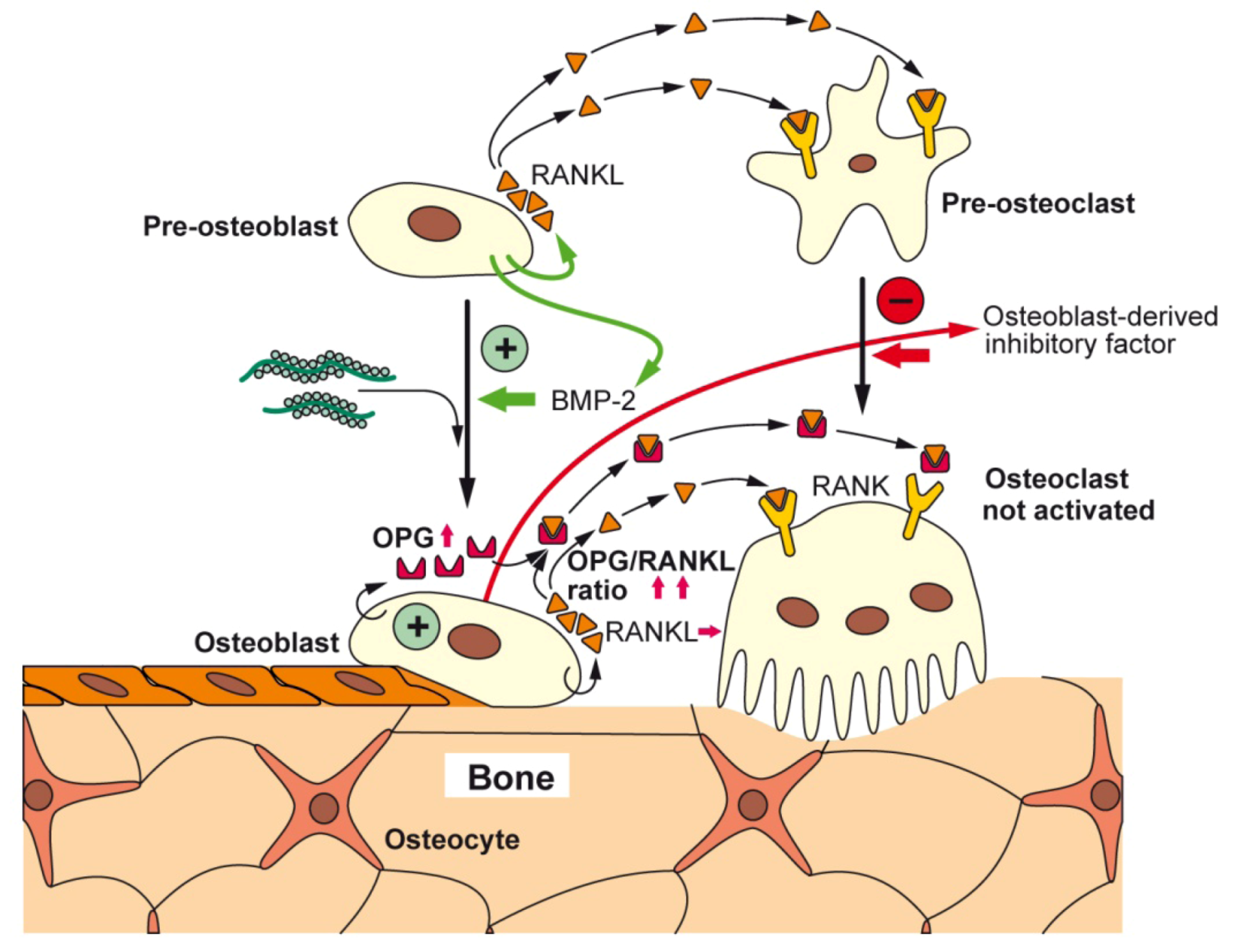
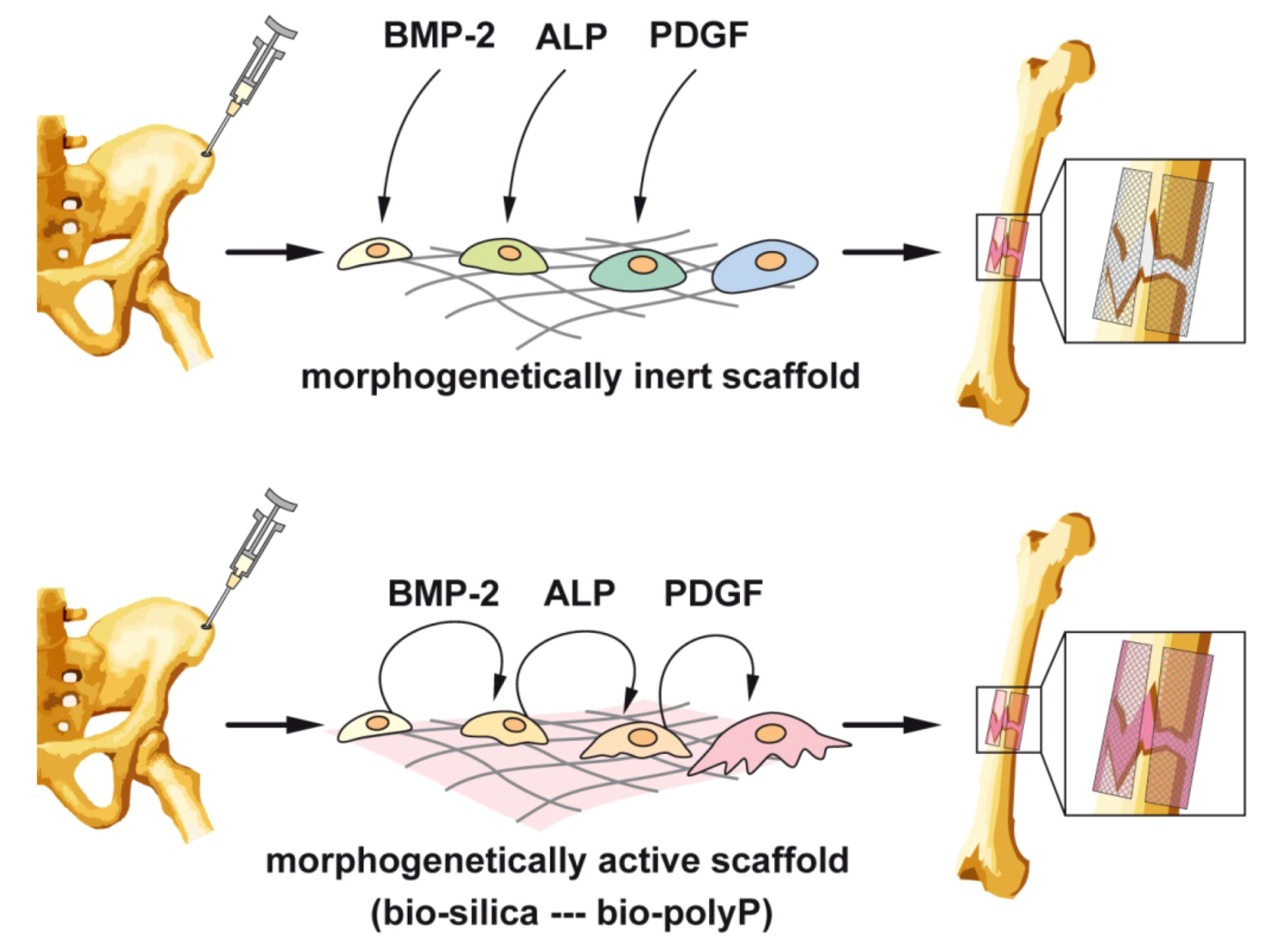
4. Moldable Implants
5. Scaffold
5.1. Organic Scaffold: Osteoconductive/Osteoinductive Properties
5.2. Inorganic Scaffold: Space-Filling Properties
5.3. Bio-Inorganic Scaffold: Osteoinductive Properties of Bio-PolyP and Bio-Silica

5.4. Bio-Inorganic Scaffold: Formation of a Flexible Structure
5.5. Bio-Inorganic Bio-Silica Scaffold

5.6. Biocompatibility of the Bio-Silica Scaffold
5.7. Bio-Inorganic Bio-PolyP Scaffold
6. Future Direction: Application of Bio-Silica and Bio-PolyP in Bone Tissue Engineering
Acknowledgments
Conflict of Interest
References
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar]
- Wagner, W.; Wein, F.; Roderburg, C.; Saffrich, R.; Faber, A.; Krause, U.; Schubert, M.; Benes, V.; Eckstein, V.; Maul, H.; et al. Adhesion of hematopoietic progenitor cells to human mesenchymal stem cells as a model for cell-cell interaction. Exp. Hematol. 2007, 35, 314–325. [Google Scholar] [CrossRef]
- Low, K.; Noblin, J.D.; Browne, J.E.; Barnthouse, C.D.; Scott, A.R. Jones fractures in the elite football player. J. Surg. Orthop. Adv. 2004, 13, 156–160. [Google Scholar]
- Nishida, J.; Shimamura, T. Methods of reconstruction for bone defect after tumor excision: A review of alternatives. Med. Sci. Monit. 2008, 14, RA107–RA113. [Google Scholar]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopedic surgery. J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar]
- Epple, M. Biomimetic Bone Substitution Materials. In Biomineralisation: Medical and Clinical Aspects; Epple, M., Baeuerlein, E., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 81–95. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. SpineJ. 2001, 10, S96–S101. [Google Scholar] [CrossRef]
- Wang, X.H.; Schröder, H.C.; Wiens, M.; Ushijima, H.; Müller, W.E.G. Bio-silica and bio-polyphosphate: Applications in biomedicine (bone formation). Curr. Opin. Biotechnol. 2012, 23, 570–578. [Google Scholar] [CrossRef]
- Kulakovskaya, T.V.; Vagabov, V.M.; Kulaev, I.S. Inorganic polyphosphate in industry, agriculture and medicine: Modern state and outlook. Process. Biochem. 2012, 47, 1–10. [Google Scholar] [CrossRef]
- Müller, W.E.G. Silicon Biomineralization: Biology—Biochemistry—Molecular Biology—Biotechnology (Progress in Molecular and Subcellular Biology); Springer Press: Berlin, Germany, 2003. [Google Scholar]
- Kulaev, I.S.; Vagabov, V.; Kulakovskaya, T. The Biochemistry of Inorganic Polyphosphates; John Wiley & Sons Inc.: New York, NY, USA, 2004. [Google Scholar]
- Rao, N.N.; Gómez-García, M.R.; Kornberg, A. Inorganic polyphosphate: Essential for growth and survival. Annu. Rev. Biochem. 2009, 78, 605–647. [Google Scholar] [CrossRef]
- Laitinen, M.; Jortikka, L.; Halttunen, T.; Böhling, T.; Marttinen, A.; Lindholm, T.S. Soluble factors from human Saos-2 osteosarcoma cells induce ectopic bone formation and osteoblastic differentiation of cultured mesenchymal cells. J. Musculoskelet. Res. 1997, 1, 21–32. [Google Scholar] [CrossRef]
- Hausser, H.J.; Brenner, R.E. Phenotypic instability of SaOS-2 cells in long-term culture. Biochem. Biophys. Res. Commun. 2005, 333, 216–222. [Google Scholar] [CrossRef]
- Kelly, S.E.; di Benedetto, A.; Greco, A.; Howard, C.M.; Sollars, V.E.; Primerano, D.A.; Valluri, J.V.; Claudio, P.P. Rapid selection and proliferation of CD133(+) cells from cancer cell lines: Chemotherapeutic implications. PLoS One 2010, 5, e10035. [Google Scholar] [CrossRef]
- Vincent, C.; Kogawa, M.; Findlay, D.M.; Atkins, G.J. The generation of osteoclasts from RAW 264.7 precursors in defined, serum-free conditions. J. BoneMiner. Metab. 2009, 27, 114–119. [Google Scholar] [CrossRef]
- Lymperi, S.; Ersek, A.; Ferraro, F.; Dazzi, F.; Horwood, N.J. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood 2011, 117, 1540–1549. [Google Scholar] [CrossRef]
- Omelon, S.J.; Grynpas, M.D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef]
- Griffith, E.J. Phosphate Fibers (Topics inAppliedChemistry); Springer Press: Berlin, Germany, 1995. [Google Scholar]
- Katchman, B.J.; Smith, H.E. Diffusion of synthetic and natural polyphosphates. Arch. Biochem. Biophys. 1958, 76, 396–402. [Google Scholar] [CrossRef]
- Van Wazer, J.R. Phosphorus and Its Compounds: Chemistry; Interscience Publishers Inc.: New York, NY, USA, 1958; Volume 1, pp. 1–125. [Google Scholar]
- Omoto, M.; Imai, T.; Seki, K.; Nomura, R.; Otahara, Y. The effect on the bones of condensed phosphate when used as food additives: Its importance in relation to preventive medicine. Environ. HealthPrev. Med. 1997, 2, 105–116. [Google Scholar]
- Lee, B.H.; Kim, M.C.; Choi, S.H.; Lee, Y.K. Amorphous calcium polyphosphate bone regenerative materials based on calcium phosphate glass. Key Eng. Mater 2009, 396–398, 209–212. [Google Scholar] [CrossRef]
- Shahidi, F.; Rubin, L.J.; Diosady, L.L.; Kassam, N.; Fong, J.C.; Li, S.; Wood, D.F. Effect of sequestering agents on lipid oxidation in cooked meats. Food Chem. 1986, 21, 145–152. [Google Scholar] [CrossRef]
- Wood, H.G.; Clark, J.E. Biological aspects of inorganic polyphosphates. Annu. Rev. Biochem. 1988, 57, 235–260. [Google Scholar] [CrossRef]
- Schröder, H.C.; Müller, W.E.G. Inorganic Polyphosphates: Biochemistry, Biology, Biotechnology; Springer Press: Berlin, Germany, 1999. [Google Scholar]
- Leyhausen, G.; Lorenz, B.; Zhu, H.; Geurtsen, W.; Bohnensack, R.; Müller, W.E.G.; Schröder, H.C. Inorganic polyphosphate in human osteoblast-like cells. J. Bone Miner. Res. 1998, 13, 803–812. [Google Scholar]
- Schröder, H.C.; Kurz, L.; Müller, W.E.G.; Lorenz, B. Polyphosphate in bone. Biochemistry (Moscow) 2000, 65, 296–303. [Google Scholar]
- Usui, Y.; Uematsu, T.; Uchihashi, T.; Takahashi, M.; Takahashi, M.; Ishizuka, M.; Doto, R.; Tanaka, H.; Komazaki, Y.; Osawa, M.; et al. Inorganic polyphosphate induces osteoblastic differentiation. J. Dent. Res. 2010, 89, 504–509. [Google Scholar] [CrossRef]
- Sinha, K.M.; Yasuda, H.; Coombes, M.M.; Dent, S.Y.R.; de Crombrugghe, B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBOJ. 2010, 29, 68–79. [Google Scholar]
- Sun, L.; Blair, H.C.; Peng, Y.; Zaidi, N.; Adebanjo, O.A.; Wu, X.B.; Wu, X.Y.; Iqbal, J.; Epstein, S.; Abe, E.; et al. Calcineurin regulates bone formation by the osteoblast. Proc. Natl. Acad. Sci. USA 2005, 102, 17130–17135. [Google Scholar] [CrossRef]
- Hacchou, Y.; Uematsu, T.; Ueda, O.; Usui, Y.; Uematsu, S.; Takahashi, M.; Uchihashi, T.; Kawazoe, Y.; Shiba, T.; Kurihara, S.; et al. Inorganic polyphosphate: A possible stimulant of bone formation. J. Dent. Res. 2007, 86, 893–897. [Google Scholar] [CrossRef]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of vertebrate skeletal mineralization by polyphosphates. PLoSOne 2009, 4, e5634. [Google Scholar]
- Lorenz, B.; Marmé, S.; Müller, W.E.G.; Unger, K.; Schröder, H.C. Preparation and use of polyphosphate-modified zirconia for purification of nucleic acids and proteins. Anal. Biochem. 1994, 216, 118–126. [Google Scholar] [CrossRef]
- Lorenz, B.; Müller, W.E.G.; Kulaev, I.S.; Schröder, H.C. Purification and characterization of an exopolyphosphatase activity from Saccharomyces. cerevisiae. J. Biol. Chem. 1994, 269, 22198–22204. [Google Scholar]
- Lorenz, B.; Münkner, J.; Oliveira, M.P.; Kuusksalu, A.; Leitão, J.M.; Müller, W.E.G.; Schröder, H.C. Changes in metabolism of inorganic polyphosphate in rat tissues and human cells during development and apoptosis. Biochim. Biophys. Acta 1997, 1335, 51–60. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.H.; Schröder, H.C.; Kolb, U.; Schloßmacher, U.; Ushijima, H.; Müller, W.E.G. The role of biosilica in the osteoprotegerin/RANKL ratio in human osteoblast-like cells. Biomaterials 2010, 31, 7716–7725. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.H.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Ushijima, H.; Schröder, H.C.; Müller, W.E.G. Osteogenic potential of bio-silica on human osteoblast-like (SaOS-2) cells. Calcif. TissueInt. 2010, 87, 513–524. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, X.H.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Lieberwirth, I.; Glasser, G.; Wiens, M.; Schröder, H.C. Inorganic polymeric phosphate/polyphosphate as an inducer of alkaline phosphatase and a modulator of intracellular Ca2+ level in osteoblasts (SaOS-2 cells) in vitro. Acta Biomater. 2011, 7, 2661–2671. [Google Scholar] [CrossRef]
- Tanaka, H.; Nagai, E.; Murata, H.; Tsubone, T.; Shirakura, Y.; Sugiyama, T.; Taguchi, T.; Kawai, S. Involvement of bone morphogenic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumatology 2001, 40, 1163–1168. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, J.Y.; Xi, C.X.; Xie, J.X.; Feng, X.; Wang, C.Y.; Mei, L.; Xiong, W.C. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J. BoneMiner. Res. 2008, 23, 1084–1096. [Google Scholar]
- Adkisson, H.D.; Strauss-Schoenberger, J.; Gillis, M.; Wilkins, R.; Jackson, M.; Hruska, K.A. Rapid quantitative bioassay of osteoinduction. J. Orthop. Res. 2000, 18, 503–511. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef]
- Wittrant, Y.; Theoleyre, S.; Chipoy, C.; Padrines, M.; Blanchard, F.; Heymann, D.; Redini, F. RANKL/RANK/OPG: New therapeutic targets in bone tumours and associated osteolysis. Biochim. Biophys. Acta 2004, 1704, 49–57. [Google Scholar]
- Williams, G.; Sallis, J.D. Structural factors influencing the ability of compounds to inhibit hydroxyapatite formation. Calcif. TissueInt. 1982, 34, 169–177. [Google Scholar] [CrossRef]
- Wang, X.H.; Schröder, H.C.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Wiens, M.; Müller, W.E.G. Dual effect of inorganic polymeric phosphate/polyphosphate on osteoblasts and osteoclasts in vitro. J. TissueEng. Regen. Med. 2012. [Google Scholar] [CrossRef]
- Filgueira, L. Fluorescence-based staining for tartrate-resistant acidic phosphatase (TRAP) in osteoclasts combined with other fluorescent dyes and protocols. J. Histochem. Cytochem. 2004, 52, 411–414. [Google Scholar] [CrossRef]
- Lee, Z.H.; Kim, H.H. Signal transduction by receptor activatior of nuclear factor kappa B in osteoclasts. Biochem. Biophys. Res. Commun. 2003, 305, 211–214. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wang, X.H.; Cui, F.Z.; Jochum, K.P.; Tremel, W.; Bill, J.; Schröder, H.C.; Natalio, F.; Schloßmacher, U.; Wiens, M. Sponge spicules as blueprints for the biofabrication of inorganic-organic composites and biomaterials. Appl. Microbiol. Biotechnol. 2009, 83, 397–413. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast–osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Moussian, B. Recent advances in understanding mechanisms of insect cuticle differentiation. InsectBiochem. Molec. Biol. 2010, 40, 363–375. [Google Scholar]
- Cha, J.N.; Shimizu, K.; Zhou, Y.; Christianssen, S.C.; Chmelka, B.F.; Stucky, G.D.; Morse, D.E. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 361–365. [Google Scholar]
- Müller, W.E.G.; Wang, X.H.; Kropf, K.; Boreiko, A.; Schloßmacher, U.; Brandt, D.; Schröder, H.C.; Wiens, M. Silicatein expression in the hexactinellid Crateromorpha meyeri: The lead marker gene restricted to siliceous sponges. Cell TissueRes. 2008, 333, 339–351. [Google Scholar]
- Greenberg, S.A.; Sinclair, D. The polymerization of silicic acid. J. Phys. Chem. 1955, 59, 435–440. [Google Scholar] [CrossRef]
- Wang, X.H.; Schloßmacher, U.; Wiens, M.; Batel, R.; Schröder, H.C.; Müller, W.E.G. Silicateins, silicatein interactors, and cellular interplay in sponge skeletogenesis: Formation of glass fiber-like spicules. FEBS J. 2012, 279, 1721–1736. [Google Scholar]
- Wang, X.H.; Schröder, H.C.; Wang, K.; Kaandorp, J.A.; Müller, W.E.G. Genetic, biological and structural hierarchies during sponge spicule formation: From soft sol-gels to solid 3D silica composite structures. SoftMatter 2012, 5, 3657–3662. [Google Scholar]
- Shimizu, K.; Cha, J.; Stucky, G.D.; Morse, D.E. Silicatein alpha: Cathepsin L-like protein in sponge biosilica. Proc. Natl. Acad. Sci. USA 1998, 95, 6234–6238. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schloßmacher, U.; Wang, X.H.; Boreiko, A.; Brandt, D.; Wolf, S.E.; Tremel, W.; Schröder, H.C. Poly(silicate)-metabolizing silicatein in siliceous spicules and silicasomes of demosponges comprises dual enzymatic activities (silica-polymerase and silicaesterase). FEBSJ. 2008, 275, 362–370. [Google Scholar]
- Krasko, A.; Gamulin, V.; Seack, J.; Steffen, R.; Schröder, H.C.; Müller, W.E.G. Cathepsin, a major protease of the marine sponge Geodia cydonium: Purification of the enzyme and molecular cloning of cDNA. Mol. Mar. Biol. Biotechnol. 1997, 6, 296–307. [Google Scholar]
- Struyf, E.; Conley, D.J. Silica: An essential nutrient in wetland biogeochemistry. Front. Ecol. Environ. View 2009, 7, 88–94. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon as an Essential Trace Element in Animal Nutrition. In Silicon Biochemistry, CIBA Foundation Symposium 121; Evered, D., O’Connor, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1986; pp. 123–136. [Google Scholar]
- Van Dyck, K.; Robberecht, H.; van Cauwenbergh, R.; van Vlaslaer, V.; Deelstra, H. Indication of silicon essentiality in humans. Biol. TraceElem. Res. 2000, 77, 25–32. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: An essential element for the chick. Science 1972, 178, 619–621. [Google Scholar]
- Baud’huin, M.; Lamoureux, F.; Duplomb, L.; Rédini, F.; Heymann, D. RANKL, RANK, osteoprotegerin: Key partners of osteoimmunology and vascular diseases. Cell. Mol. LifeSci. 2007, 64, 2334–2350. [Google Scholar] [CrossRef]
- Feige, U. Osteoprotegerin. Ann. Rheum. Dis. 2001, 60, iii81–iii84. [Google Scholar]
- Suda, T.; Takahashi, N.; Udagawa, N.; Jimi, E.; Gillespie, M.T.; Martin, T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr. Rev. 1999, 20, 345–357. [Google Scholar] [CrossRef]
- Martin, T.J.; Sims, N.A. Osteoclast-derived activity in the coupling of bone formation to resorption. TrendsMol. Med. 2005, 11, 76–81. [Google Scholar] [CrossRef]
- Nagase, Y.; Iwasawa, M.; Akiyama, T.; Kadono, Y.; Nakamura, M.; Oshima, Y.; Yasui, T.; Matsumoto, T.; Hirose, J.; Nakamura, H.; et al. Anti-apoptotic molecule Bcl-2 regulates the differentiation, activation, and survival of both osteoblasts and osteoclasts. J. Biol. Chem. 2009, 284, 36659–36669. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Wiens, M.; Adell, T.; Gamulin, V.; Schröder, H.C.; Müller, I.M. Bauplan of urmetazoa: basis for genetic complexity of Metazoa. Intern. Rev. Cytol. 2004, 235, 53–92. [Google Scholar] [CrossRef]
- Müller, W.E.G. Spatial and Temporal Expression Patterns in Animals. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Meyers, R.A., Ed.; WILEY-VCH Press: Weinheim, Germany, 2005; Volume 13, pp. 269–309. [Google Scholar]
- Schwarz, K.; Milne, D.B. Growth-promoting effects of silicon in rats. Nature 1972, 239, 333–334. [Google Scholar]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr. HealthAging 2007, 11, 99–110. [Google Scholar]
- Schröder, H.C.; Borejko, A.; Krasko, A.; Reiber, A.; Schwertner, H.; Müller, W.E.G. Mineralization of SaOS-2 cells on enzymatically (Silicatein) modified bioactive osteoblast-stimulating surfaces. J. Biomed. Mat. Res. Part B 2005, 75B, 387–392. [Google Scholar] [CrossRef]
- Schröder, H.C.; Wang, X.H.; Wiens, M.; Diehl-Seifert, B.; Kropf, K.; Schloßmacher, U.; Müller, W.E.G. Silicate modulates the cross-talk between osteoblasts (SaOS-2) and osteoclasts (RAW 264.7 cells): Inhibition of osteoclast growth and differentiation. J. Cell. Biochem. 2012, 113, 3197–3206. [Google Scholar] [CrossRef]
- Wiens, M.; Wang, X.H.; Natalio, F.; Schröder, H.C.; Schloßmacher, U.; Wang, S.; Korzhev, M.; Geurtsen, W.; Müller, W.E.G. Bioinspired fabrication of bio-silica-based bone substitution materials. Adv. Eng. Mater. 2010, 12, B438–B450. [Google Scholar] [CrossRef]
- Warren, S.M.; Steinbrech, D.S.; Mehrara, B.J.; Saadeh, P.B.; Greenwald, J.A.; Spector, J.A.; Bouletreau, P.J.; Longaker, M.T. Hypoxia regulates osteoblast gene expression. J. Surg. Res. 2001, 99, 147–155. [Google Scholar] [CrossRef]
- Unger, R.E.; Wolf, M.; Peters, K.; Motta, A.; Migliaresi, C.; Kirkpatrick, J.C. Growth of human cells on a nonwoven silk fibroin net: A potential for use in tissue engineering. Biomaterials 2004, 25, 1069–1075. [Google Scholar] [CrossRef]
- Seol, Y.J.; Lee, J.Y.; Park, Y.J.; Lee, Y.M.; Ku, Young; Rhyu, I.C.; Lee, S.J.; Han, S.B.; Chung, C.P. Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnol. Lett. 2004, 26, 1037–1041. [Google Scholar] [CrossRef]
- Tuzlakoglu, K.; Pashkuleva, I.; Rodrigues, M.R.; Gomes, V.L.; Müller, R.; Reis, R.L. A new route to produce starch-based fiber mesh scaffolds by wet spinning and the improvement in cell attachment and proliferation by tailoring their surface properties. J. Biomed. Mater. Res. PartA 2010, 92, 369–377. [Google Scholar]
- Sombatmankhong, K.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Bone scaffolds from electrospun fiber mats of poly (3-hydroxybutyrate), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and their blend. Polymer 2007, 48, 1419–1427. [Google Scholar] [CrossRef]
- Gugala, Z.; Gogolewski, S. The in vitro growth and activity of sheep osteoblasts on three-dimensional scaffolds from poly(L/DL-lactide) 80/20%. J. Biomed. Mater. Res. Part A 2005, 75A, 702–709. [Google Scholar] [CrossRef]
- Yoon, C.H.; Hur, J.; Park, K.W.; Kim, J.H.; Lee, C.S.; Oh, I.Y.; Kim, T.Y.; Cho, H.J.; Kang, H.J.; Chae, I.H.; et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation 2005, 112, 1618–1627. [Google Scholar] [CrossRef]
- Li, T.; Yu, Y.T.; Wang, J.; Tang, T.S. 1,25-Dihydroxyvitamin D(3) stimulates bone neovascularization by enhancing the interactions of osteoblasts-like cells and endothelial cells. J. Biomed. Mater. Res. Part A 2008, 86, 583–588. [Google Scholar]
- Wang, J.C.; Alanay, A.; Mark, D.; Kanim, L.E.; Campbell, P.A.; Dawson, E.G.; Lieberman, J.R. A comparison of commercially available demineralized bone matrix for spinal fusion. Eur. Spine J. 2007, 16, 1233–1240. [Google Scholar]
- Bae, H.; Zhao, L.; Zhu, D.; Kanim, L.E.; Wang, J.C.; Delamarter, R.B. Variability across ten production lots of a single demineralized bone matrix product. J. BoneJointSurg. Am. 2010, 92, 427–435. [Google Scholar]
- Chan, B.P.; Hui, T.Y.; Wong, M.Y.; Yip, K.H.; Chan, G.C. Mesenchymal stem cell-encapsulated collagen microspheres for bone tissue engineering. TissueEng. PartC 2010, 16, 225–235. [Google Scholar]
- Park, Y.J.; Kim, K.H.; Lee, J.Y.; Ku, Y.; Lee, S.J.; Min, B.M.; Chung, C.P. Immobilization of bone morphogenetic protein-2 on a nanofibrous chitosan membrane for enhanced guided bone regeneration. Biotechnol. Appl. Biochem. 2006, 43, 17–24. [Google Scholar] [CrossRef]
- Brydone, A.S.; Meek, D.; Maclaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Hao, Y.L.; Li, S.J.; Sun, S.Y.; Yang, R. Effect of Zr and Sn on Young’s modulus and superelasticity of Ti–Nb-based alloys. Mater. Sci. Eng. A 2006, 441, 112–118. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Oates, T.W.; Maller, S.C.; West, J.; Steffensen, B. Human gingival fibroblast integrin subunit expression on titanium implant surfaces. J. Periodontol. 2005, 76, 1743–1750. [Google Scholar] [CrossRef]
- Sambrook, P.; Cooper, C. Osteoporosis. Lancet 2006, 367, 2010–2018. [Google Scholar] [CrossRef]
- Lane, N.E.; Yao, W. Developments in the scientific understanding of osteoporosis. ArthritisRes. Ther. 2009, 11, 228. [Google Scholar] [CrossRef]
- Wang, Y.; Grainger, D.W. siRNA knock-down of RANK signaling to control osteoclast-mediated bone resorption. Pharm. Res. 2010, 27, 1273–1284. [Google Scholar] [CrossRef]
- Hu, R.; Liu, W.; Li, H.; Yang, L.; Chen, C.; Xia, Z.Y.; Guo, L.J.; Xie, H.; Zhou, H.D.; Wu, X.P. A RUNX2/MIR-3960/MIR-2861 regulatory feedback loop during mouse osteoblast differentiation. J. Biol. Chem. 2011, 286, 12328–12339. [Google Scholar]
- Lee, S.J.; Kang, S.W.; Do, H.J.; Han, I.; Shin, D.A.; Kim, J.H.; Lee, S.H. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 2010, 31, 5652–5659. [Google Scholar]
- Bellido, T.; Plotkin, L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone 2011, 49, 50–55. [Google Scholar] [CrossRef]
- Agholme, F.; Li, X.; Isaksson, H.; Ke, H.Z.; Aspenberg, P. Sclerostin antibody treatment enhances metaphyseal bone healing in rats. J. BoneMiner. Res. 2010, 25, 2412–2418. [Google Scholar]
- Hayase, Y.; Muguruma, Y.; Lee, M.Y. Osteoclast development from hematopoietic stem cells: Apparent divergence of the osteoclast lineage prior to macrophage commitment. Exp. Hematol. 1997, 25, 19–25. [Google Scholar]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Cerri, P.S.; Boabaid, F.; Katchburian, E. Combined TUNEL and TRAP methods suggest that apoptotic bone cells are inside vacuoles of alveolar bone osteoclasts in young rats. J. Periodont. Res. 2003, 38, 223–226. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Santini, D.; Schiavon, G.; Vincenzi, B.; Gaeta, L.; Pantano, F.; Russo, A.; Ortega, C.; Porta, C.; Galluzzo, S.; Armento, G.; et al. Receptor activator of NF-κB (RANK) expression in primary tumors associates with bone metastasis occurrence in breast cancer patients. PLoS One 2011, 6, e19234. [Google Scholar]
- Zhang, S.; Liu, C.; Huang, P.; Zhou, S.; Ren, J.; Kitamura, Y.; Tang, P.; Bi, Z.; Gao, B. The affinity of human RANK binding to its ligand RANKL. Arch. Biochem. Biophys. 2009, 487, 49–53. [Google Scholar] [CrossRef]
- Fuller, K.; Ross, J.L.; Szewczyk, K.A.; Moss, R.; Chambers, T.J. Bone is not essential for osteoclast activation. PLoS One 2010, 5, e12837. [Google Scholar] [CrossRef]
- Jansen, I.D.C.; Mardones, P.; Lecanda, F.; de Vries, T.J.; Recalde, S.; Hoeben, K.A.; Schoenmaker, T.; Ravesloot, J.H.; van Borren, M.M.G.J.; van Eijden, T.M. Ae2a,b-deficient mice exhibit osteopetrosis of long bones but not of calvaria. FASEBJ. 2009, 23, 3470–3481. [Google Scholar] [CrossRef]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor activator of nuclear factor κB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar]
- Jabbar, S.; Drury, J.; Fordham, J.N.; Datta, H.K.; Francis, R.M.; Tuck, S.P. Osteoprotegerin, RANKL and bone turnover in postmenopausal osteoporosis. J. Clin. Pathol. 2011, 64, 354–357. [Google Scholar] [CrossRef]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell.Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar]
- Weiner, S.; Wagner, H.D. The material bone: Structure mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Mayer, G. Rigid biological systems as models for synthetic composites. Science 2005, 310, 1144–1147. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kamitakahara, M.; Miyazaki, T. Bioactive ceramic-based materials with designed reactivity for bone tissue regeneration. J. R. Soc. Interf. 2009, 6, S349–S360. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissueengineering scaffolds. TissueEng. 2005, 11, 101–109. [Google Scholar]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. DrugDeliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. DrugDeliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. TissueEng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef]
- Sill, T.J.; Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of pourous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. PartB 2005, 74B, 782–788. [Google Scholar] [CrossRef]
- Fierz, F.C.; Beckmann, F.; Huser, M.; Irsen, S.H.; Leukers, B.; Witte, F.; Degistirici, O.; Andronache, A.; Thie, M.; Müller, B. The morphology of anisotropic 3D-printed hydroxyapatite scaffolds. Biomaterials 2008, 29, 3799–3806. [Google Scholar] [CrossRef]
- Detsch, R.; Schaefer, S.; Deisinger, U.; Ziegler, G.; Seitz, H.; Leukers, B. In vitro: Osteoclastic activity studies on surfaces of 3D printed calcium phosphate scaffolds. J. Biomater. Appl. 2011, 26, 359–380. [Google Scholar]
- Bergmann, C.; Lindner, M.; Zhang, W.; Koczur, K.; Kirsten, A.; Telle, R.; Fischer, H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010, 30, 2563–2567. [Google Scholar]
- Gregor, A.; Hošek, J. 3D Printing Methods of Biological Scaffolds Used in Tissue Engineering. In Proceedings of International Conference on Innovations, Recent Trends and Challenges in Mechatronics, Mechanical Engineering and New High-Tech Products Development—MECAHITECH’11, Bucharest, Romania, 22–23 September 2011; 3, pp. 88–95.
- Fielding, G.A.; Bandyopadhyay, A.; Bose, S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent. Mater. 2012, 28, 113–122. [Google Scholar] [CrossRef]
- Lee, K.W.; Wang, S.; Dadsetan, M.; Yaszemski, M.J.; Lu, L. Enhanced cell ingrowth and proliferation through three-dimensional nanocomposite scaffolds with controlled pore structures. Biomacromolecules 2010, 11, 682–689. [Google Scholar] [CrossRef]
- Liu, C.Z.; Xia, Z.D.; Han, Z.W.; Hulley, P.A.; Triffitt, J.T.; Czernuszka, J.T. Novel 3D collagen scaffolds fabricated by indirect printing technique for tissue engineering. J. Biomed. Mater. Res. Part B 2008, 85, 519–528. [Google Scholar]
- Liu, C.Z.; Sachlos, E.; Wahl, D.A.; Han, Z.; Czernuszka, J.T. On the manufacturability of scaffold mould using a 3D printing technology. RapidPrototyp. J. 2007, 13, 163–174. [Google Scholar]
- Sachlos, E.; Reis, N.; Ainsley, C.; Derby, B.; Czernuszka, J.T. A process to make collagen scaffolds with an artificial circulatory system using rapid prototyping. Mater. Res. Soc. Symp. Proc. 2003, 758, LL5.3.1–LL5.3.6. [Google Scholar]
- Belton, D.J.; Deschaume, O.; Perry, C.C. An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J. 2012, 279, 1710–1720. [Google Scholar] [CrossRef]
- Schlossmacher, U.; Wiens, M.; Schröder, H.C.; Wang, X.H.; Jochum, K.P.; Müller, W.E.G. Silintaphin-1: Interaction with silicatein during structureguiding biosilica formation. FEBSJ. 2011, 278, 1145–1155. [Google Scholar]
- Schröder, H.C.; Wang, X.H.; Manfrin, A.; Yu, S.H.; Grebenjuk, V.A.; Korzhev, M.; Wiens, M.; Schloßmacher, U.; Müller, W.E.G. Silicatein: Acquisition of structure-guiding and structure-forming properties during maturation from the pro-silicatein to the silicatein form. J. Biol. Chem. 2012, 287, 22196–22205. [Google Scholar] [CrossRef]
- Wiens, M.; Bausen, M.; Natalio, F.; Link, T.; Schlossmacher, U.; Müller, W.E.G. The role of the silicatein-α interactor silintaphin-1 in biomimetic biomineralization. Biomaterials 2009, 30, 1648–1656. [Google Scholar] [CrossRef]
- Lam, C.X.F.; Mo, X.M.; Teoh, S.H.; Hutmacher, D.W. Scaffold development using 3D printing with a starch-based polymer. Mater. Sci. Eng. C 2002, 20, 49–56. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Schröder, H.C.; Feng, Q.; Draenert, F.; Müller, W.E.G. The Deep-Sea Natural Products, Biogenic Polyphosphate (Bio-PolyP) and Biogenic Silica (Bio-Silica), as Biomimetic Scaffolds for Bone Tissue Engineering: Fabrication of a Morphogenetically-Active Polymer. Mar. Drugs 2013, 11, 718-746. https://doi.org/10.3390/md11030718
Wang X, Schröder HC, Feng Q, Draenert F, Müller WEG. The Deep-Sea Natural Products, Biogenic Polyphosphate (Bio-PolyP) and Biogenic Silica (Bio-Silica), as Biomimetic Scaffolds for Bone Tissue Engineering: Fabrication of a Morphogenetically-Active Polymer. Marine Drugs. 2013; 11(3):718-746. https://doi.org/10.3390/md11030718
Chicago/Turabian StyleWang, Xiaohong, Heinz C. Schröder, Qingling Feng, Florian Draenert, and Werner E. G. Müller. 2013. "The Deep-Sea Natural Products, Biogenic Polyphosphate (Bio-PolyP) and Biogenic Silica (Bio-Silica), as Biomimetic Scaffolds for Bone Tissue Engineering: Fabrication of a Morphogenetically-Active Polymer" Marine Drugs 11, no. 3: 718-746. https://doi.org/10.3390/md11030718