Microcystin-LR and Cylindrospermopsin Induced Alterations in Chromatin Organization of Plant Cells
Abstract
:1. Introduction
2. Cyanotoxins-Microcystins and Cylindrospermopsins
2.1. Microcystins
2.2. Cylindrospermopsins
3. Particularities of Plant Chromatin and Microtubule Organization
4. An Overview of the Effects of MCY and CYN in Plants
| Cyanotoxins used in the experiments | Type of the cyanotoxin induced alterations | Plant taxon | References |
|---|---|---|---|
| I. Growth alterations | |||
| MCY-LR, -RR, -LF, -FR, -LW, -YR in purified, -LR, -RR, -FR, -YR in extract, M. aeruginosa bloom samples | Inhibited seed germination, growth/elongation of shoot, primary root, leaves, inhibited increase of frond number, fresh/dry weight | Brassica napus, Ceratophyllum demersum, Lemna minor, L. gibba, L. japonica, Lens esculenta, Lepidium sativum, Lolium perenne, Malus pumila, Medicago sativa, Myriophyllum variifolium, Oryza sativa, Phragmites australis, Pisum sativum, Sinapis alba, Spirodela oligorrhiza, Triticum durum, Vallisneria natans, Vicia faba, Vicia faba inoculated with rhizobial strains, Wolffia arrhiza, Zea mays | [38,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69] |
| MCY-RR | Decreased cell viability | Tobacco BY-2 cells | [70] |
| CYN (purified, crude extract) | Inhibited seed germination, growth/elongation of whole plant, shoot and mainroot, inhibited increase of fresh weight of leaves | Brassica oleracea var. sabellica, Brassica juncea, Lactuca sativa, Lemna minor, callus-derived Phragmites australis plantlets, Sinapis alba, Wolffia arrhiza | [52,71,72,73,74,75] |
| CYN in crude extracts | Concentration- and exposure time/exposed organ dependent stimulation or inhibition of growth | Hydrilla verticillata, Lactuca sativa, Oryza sativa, Phaseolus vulgaris, Pisum sativum, Solanum lycopersicum, Spirodela oligorrhiza | [74,76,77,78] |
| CYN | Inhibited pollen germination | Nicotiana tabacum | [79] |
| II. Morphological/Developmental alterations | |||
| MCY-LR, -RR purified and in crude extract | Inhibited root elongation and altered primary root/lateral root formation, missing root hairs/crown root formation, radial expansion in roots, root coalescence | Lens esculenta, Oryza sativa, Phaseolus vulgaris, Phragmites australis, Pisum sativum, Sinapis alba, Triticum durum, Vallisneria natans, Zea mays | [43,46,51,52,56,58,60,69,80,81] |
| MCY-LR, -RR, -YR purified and in crude extract | Inhibited photomorphogenesis of cotyledons: Chlorotic and smaller cotyledons, missing trichomes of petioles, malformed, chlorotic fronds/leaves, inhibited shoot elongation, the seedlings lying horizontally on the paper bed. Stimulation of flowering | Brassica napus, Lemna minor, Sinapis alba, Spinacia oleracea variants | [45,51,52,53,82] |
| MCY-LR | Inhibited shoot and root formation, decrease of somatic embryo number | Phragmites australis, Solanum tuberosum tissue cultures | [46,58] |
| CYN | Increased root number, inhibited elongation, radial expansion of roots | Hydrilla verticillata, callus-derived Phragmites australis plantlets, Sinapis alba | [69,72,77] |
| CYN | Inhibited photomorphogenesis of cotyledons: The chlorotic, smaller cotyledons were violet colored in consequence of high level of anthocyanins | Sinapis alba seedlings | [52] |
| CYN in crude extract | Prolonged (9 days) exposure induced decrease of water content, browning, green color lost, shrunk leaves | Oryza sativa | [78] |
| III. Histological and cytological alterations | |||
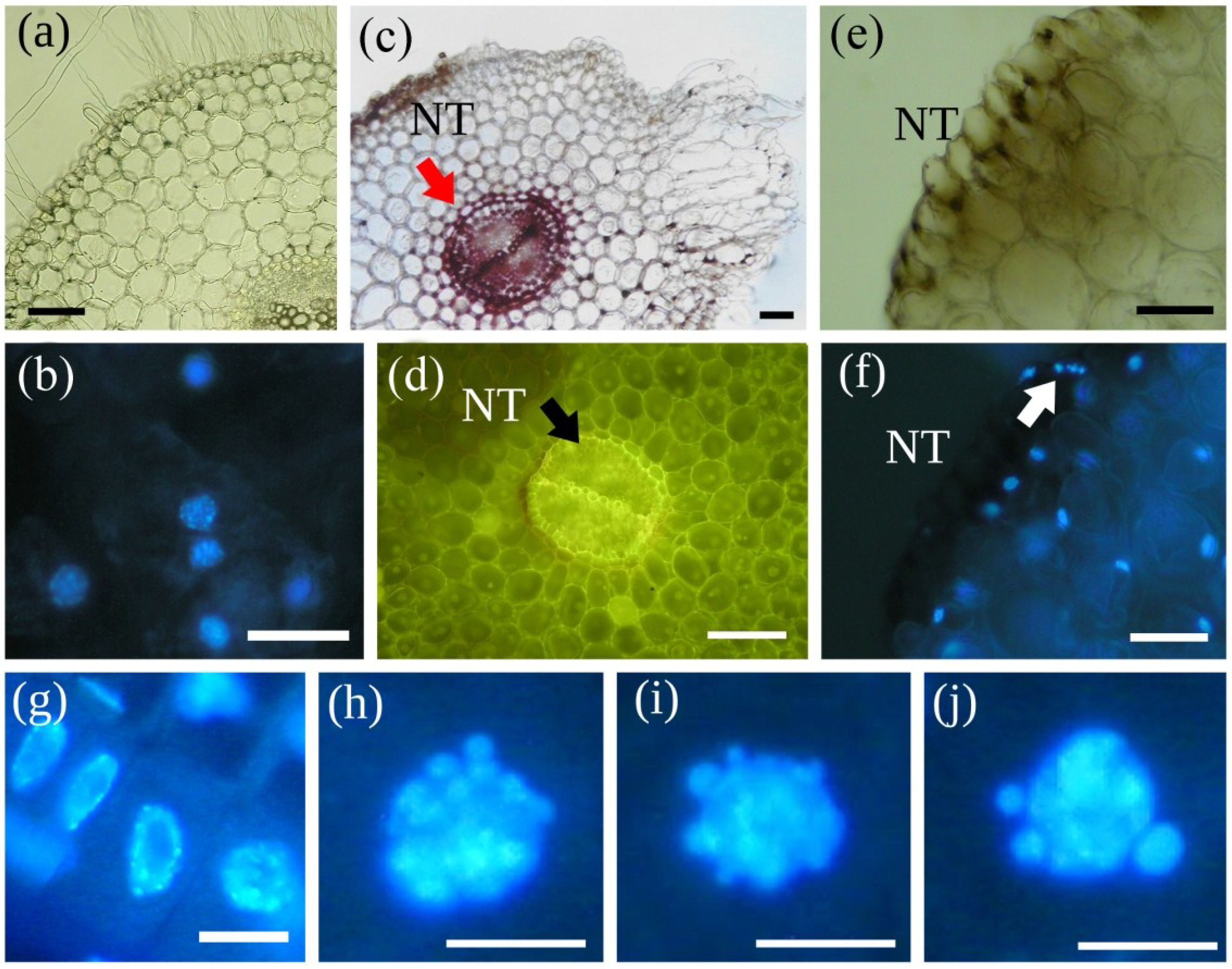
| MCY-LR | Lignification in cell walls (in cortical parenchyma, endodermis and pericycle, with increased autofluorescence) | Phragmites australis plantlets, Sinapis alba seedlings | Section 5.2; [58,69,80] |
| MCY-LR | Swelling of cells and formation of a callus-like tissue (in main roots and at the transit between main root and hypocotyls in mustard, in rhizome and roots of reed) | Phragmites australis plantlets, Sinapis alba seedlings | Section 5.2; [58,69,80] |
| MCY-LR | Early aerenchyma formation | Phragmites australis plantlets | [58] |
| MCY-LR | Inhibition of formation of vascular cylinder, xylem differentiation (xylem area and number of vessel elements) | Phaseolus vulgaris, Sinapis alba seedlings | Section 5.2; [60,69] |
| MCY-LR, -RR, -YR purified and in crude extract | Cell death by necrosis in cotyledon, shoot and root tissues | Brassica napus, Lemna minor, Phaseolus vulgaris seedlings, Phragmites australis, Solanum tuberosum tissue culture, Sinapis alba seedlings | Section 5.2; [43,46,51,52,53,55,58,69,80,83] |
| CYN | Lignification in cell walls was detected in some endodermis and pericycle cells at high CYN concentration | Sinapis alba seedlings | [69] |
| CYN | Formation of callus-like tissue and necrosis in reed root cortex, cell swelling in pith tissue without necrosis in mustard | Phragmites australis plantlets, Sinapis alba seedlings | [69,72] |
| CYN | Inhibition of xylem differentiation | Sinapis alba seedlings | [69] |
| IV. Physiology | |||
| MCY-LR purified and MCY-RR, -LR, -YR, -(H4)YR, -WR, and -FR in crude extract | Inhibition/alteration of photosynthesis, decreased chlorophyll, carotenoid content, altered chl a/chl b ratio, alterations in chlorophyll fluorescence parameters | Ceratophyllum demersum, Elodea canadensis, Lemna minor, L. gibba, Lens esculenta, Lolium perenne, Myriophyllum spicatum, Phaseolus vulgaris, Phragmites australis, Pisum sativum, Potamogeton sps., Sinapis alba, Solanum tuberosum tissue culture, Spinacia oleracea variants, Spirodela oligorrhiza, Triticum durum, Vicia faba inoculated with rhizobial strains, Zea mays | [45,46,47,48,52,59,61,62,63,64,68,82,83,84,85] |
| MCY-LR | After transient induction, inhibited anthocyanin accumulation in the cotyledons | Sinapis alba | [51,52] |
| MCY-LR, M. aeruginosa toxic culture | Inhibition of medium, nutrient uptake/absorbtion rates of phosphorus and nitrogen, nitrogen assimilation; Increase of mineral nutrients content in roots per fresh weight | Lens esculenta, Phaseolus vulgaris, Pisum sativum, Potamogeton sps., Triticum durum, Vicia faba inoculated withrhizobial strains, Zea mays | [46,61,67,68,85] |
| MCY-LR | Decreased water and protein content | Ceratophyllum demersum | [63] |
| CYN in crude extract | Decreased chlorophyll content or/and changes in the chl- a/chl-b ratio | Hydrilla verticillata, Sinapis alba | [52,77] |
| CYN purified and in crude extract | Soluble protein content per unit fresh weight showed mild increases, especially in W. arrhiza, increases of tubulin content in reed roots | Lemna minor, Phragmites australis plantlets, Wolffia arrhiza | [72,73] |
| V. Enzymology | |||
| MCY-LR, MCY-RR, -LW, -LR, -LR in crude extract | Inhibition of protein-phosphatases PP1 and PP2A: in vitro inhibition of active forms of PP1 and PP2A in diluted seed extract (both PP1 and PP2A IC50: ~0.1 nM); in vivo inhibition of PP1 and PP2A | Brassica napus seed extract, Medicago sativa, Phragmites australis, Sinapis alba | [9,43,69,80,86] |
| MCY-LR | Inhibition of PP1 and PP2A, blocking of sucrose-inducible gene expression (mRNAs of β-amylase, sporamin, AGPase) | Ipomoea batatas, transgenic Nicotiana tabacum | [87] |
| MCY-LR | Inhibition of PP2A, the major sucrose-phosphate synthase (SPS) phosphatase blocking of the light-induced activation of SPS and decreasing sucrose biosynthesis and CO2 fixation | Spinacia oleracea | [88] |
| MCY-LR | Disturbance of jasmonic acid (JA) signal transduction; abrogation of the response to JA (both the increase in the specific activity of acid phosphatase (AP) and the reduction in overall protein content shows opposite tendency) | Nicotiana tabacum | [89] |
| MCY-LR (purified and in extract) | Alterations in activities of hydrolase enzymes: Changes in activity of constitutive acid phosphatase and RNase; induction of ssDNase activities; PCD associated changes of ssDNase and dsDNase activities in plant cells | Spirodela oligorrhiza Sinapis alba Phragmites australis | [48,51,90] |
| MCY-LR, -LF, -LR in extract | Lipid peroxidation, increased α- and β-tocopherol concentration (as a lipid antioxidant) | Arabidopsis thaliana cell suspension, Medicago sativa, Triticum aestivum | [91,92,93] |
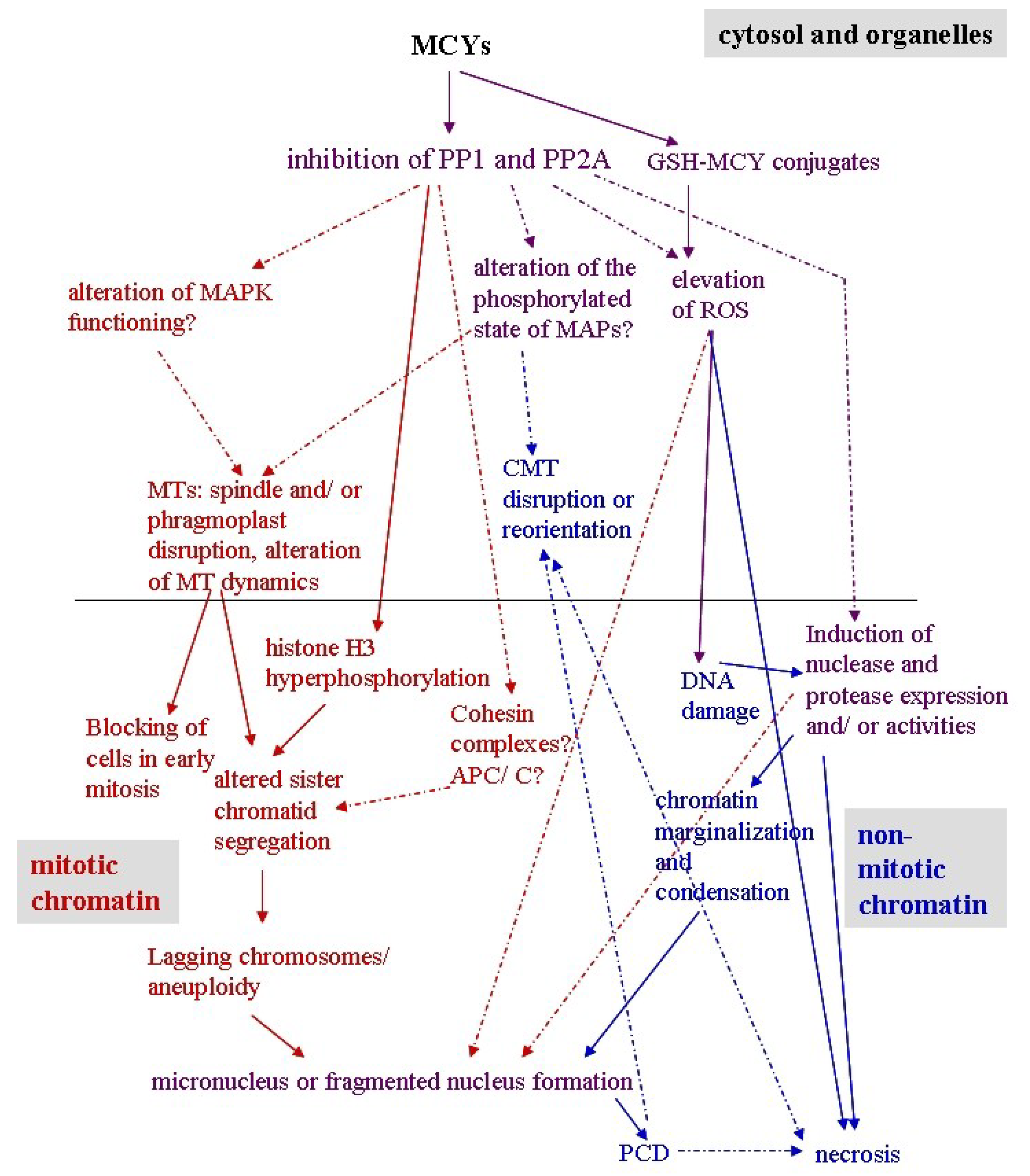
| MCY-LR, -RR, -LF, -LW, -WR both in purified and in crude cyanobacterial extracts | Phenomena induced by oxidative stress: in vitro reaction of MCY-LR and extracted plant GST producing GSH-MCY-LR conjugate, identification of in vivo formed GSH-MCY-LR conjugate; formation of H2O2, other ROS, increase in phenolic compounds, phenylalanine ammonia lyase (PAL), polyphenol oxidase (PPO) activities, concentration of endogenous nitric oxide (NO); decrease/alterations in glutathione pool; reduced glutathione (GSH) and glutathione disulfide concentration; induction/alterations of oxidative stress enzyme activities: microsomal and cytosolic/soluble glutathione-S-transferase (mGST and sGST), -peroxidases (GPx), -glutathione reductase (GR), ascorbate peroxidase (APX, POD), superoxide dismutase (SOD), catalase (CAT) | Arabidopsis thaliana cell suspension, Brassica napus, Brassica rapa, Ceratophyllum demersum, Elodea canadensis, Lemna gibba, L. minor, Lepidium sativum, Medicago sativa, Myriophyllum spicatum, Oryza sativa, Phaseolus vulgaris, Phragmites australis , Sinapis alba, Spinacia oleracea variants, tobacco BY-2 cell suspension, Triticum aestivum, Vicia faba inoculated with rhizobial strains, Vigna unguiculata species variants | [38,50,52,53,55,59,62,65,68,70,82,91,92,94,95,9697,98,99,100] |
| MCY-LR in extract | Inhibited production of nitric oxide (NO), decreased auxin (IAA) concentration in roots | Oryza sativa | [81,101] |
| CYN | Alteration in protein synthesis: CYN inhibited the eukaryotic protein synthesis apparatus with similar potency in plant and mammalian cell extracts, partial inhibition of protein production in germinating pollen tubes | wheat germ extract, Nicotiana tabacum | [79,102] |
| CYN | Significantly decreased PP1 and PP2A activities in extracts of CYN treated plants (CYN did not cause significant decrease in PP1 activity in vitro) | Sinapis alba | [69] |
| CYN purified and in crude extract | Protease isoenzyme activity gels showed significant alterations in protease enzyme pattern and activities; crude extract induced an increase of total protease activity at pH 5 and pH 8, while purified CYN increased the activity only at lower concentration regimes (0.01—1 μg mL−1) | Lemna minor, Wolffia arrhiza | [73] |
| CYN purified and in crude extract | Induction of oxidative stress enzyme activities: Increased GST, GPx activities; increased POD activity only at low (0.05 μg mL−1) concentration—transient effect | Oryza sativa, Sinapis alba | [52,78] |
5. Effects of MCY on Plant Mitotic and Non-Mitotic Chromatin
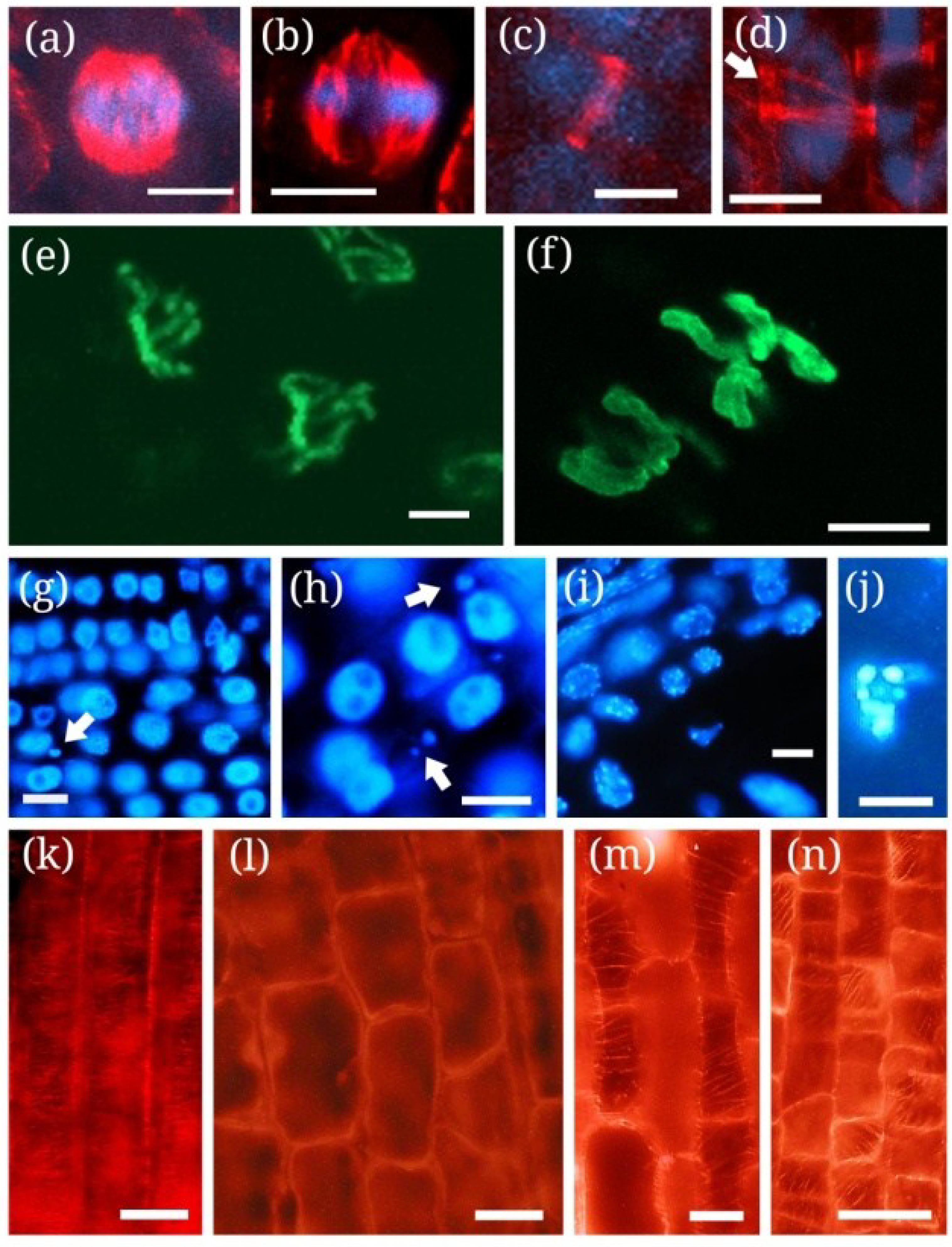
5.1. Mitotic Chromatin
| Cyanotoxin | Plant material | Effect | Mechanisms elucidated or probably involved | References |
|---|---|---|---|---|
| 1.Mitotic chromatin | ||||
| MCY-LR, MCY-RR | Tradescantia virginiana stamen hair cells | Increase of metaphase transit time, temporary delay of sister chromatid segregation | Inhibition of serine-threonine protein phosphatases (type 1 and 2A) | [106] |
| MCY-LR | Root tip meristems of Phragmites australis, Sinapis alba | Dualistic response: Mitotic activity increases at low, decreases at high cyanotoxin concentrations | Inhibition of serine-threonine protein phosphatases (type 1 and 2A) | [69,80] |
| MCY-LR | Root tip meristems of P. australis, Vicia faba | Transient increase of early and late mitotic activity | Inhibition of serine-threonine protein phosphatases (type 1 and 2A) | [80,105] |
| MCY-LR | Shoot tip meristems of Ceratophyllum demersum | Arrest of mitosis in prophase/prometaphase | Blocking of MT dynamics at early mitosis; inhibition of serine-threonine protein phosphatases (type 1 and 2A) | [63] |
| MCY-LR | Root tip meristems of V. faba | Acceleration of cell cycle at exposure to high (10 μg mL−1) toxin concentration | Inhibition of serine- threonine protein phosphatases (type 1 and 2A) | [105] |
| MCY-LR, MCY-XR | Root tip meristems of P. australis, S. alba, V. faba, Allium cepa | Delay of metaphase/anaphase transition, incomplete sister chromatid segregation, the formation of micronuclei | Disruption of mitotic MT structures; inhibition of serine-threonine protein phosphatases (type 1 and 2A); * Hyperphosphorylation of histone H3 at Ser10 | [69,80,105,114] |
| CYN | Root tip meristems of P. australis | Alteration of early mitotic activity (increase of prophase/prometaphase, decrease of metaphase indices) | * Alteration of PPB development, probably due to protein synthesis inhibition | [72] |
| CYN | Root tip meristems of P. australis, S. alba | Alteration of sister chromatid segregation | Disruption of mitotic MT structures | [72] |
| 2. Non-mitotic chromatin | ||||
| MCY-RR | Tobacco BY-2 cells | Perinuclear chromatin marginalization | Oxidative stress (ROS generation) | [115,116] |
| MCY-LR, MCY-RR | Tobacco BY-2 cells, Vallisneria natans mesophyll cells, P. australis root tips | Chromatin condensation | Oxidative stress, induction of SSP nuclease activities | [90,115,117118] |
| MCY-RR, MCY-LR | Tobacco BY-2 cells, S. alba roots | Nuclear fragmentation | Oxidative stress | Figure 1j and Figure 2g-j, [115] |
| MCYs (cyanobacterial extract) | Oryza sativa seedlings | DNA fragmentation (smearing) | Probably oxidative stress | [119] |
5.2. Non-Mitotic Chromatin
6. Effects of CYN on Plant Mitotic and Non-Mitotic Chromatin
6.1. Mitotic Chromatin
6.2. Non-Mitotic Chromatin
7. Conclusions
Acknowledgements
Conflicts of Interest
References
- Nigrelli, R.F.; Stempien, M.F.; Ruggirei, G.D.; Liguori, V.R.; Cecil, J.T. Substances of potential biomedical importance from marine organisms. Fed. Proc. 1967, 26, 1197–1205. [Google Scholar]
- Bhatnagar, I.; Kim, S.-K. Immense essence of excellence: Marine microbial bioactive compounds. Mar. Drugs 2010, 8, 2673–2701. [Google Scholar] [CrossRef]
- Vasas, G.; Borbely, G.; Nanasi, P.; Nanasi, P.P. Alkaloids from Cyanobacteria with Diverse Powerful Bioactivities. Mini. Rev. Med. Chem. 2010, 10, 946–955. [Google Scholar] [CrossRef]
- De Pauw, N.; Persoone, G. Microalgae for Aquaculture. In Microalgal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University: Cambridge, UK, 1988; pp. 197–221. [Google Scholar]
- Carmichael, W.W. Cyanobacteria secondary metabolites—The cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef]
- Sivonen, K.K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water; Chorus, I., Bartram, J., Eds.; Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Moreira, C.; Azevedo, J.; Antunes, A.; Vasconcelos, V. Cylindrospermopsin: Occurrence, methods of detection and toxicology. J. Appl. Microbiol. 2012, 114, 605–620. [Google Scholar]
- Botes, D.P.; Wessels, P.L.; Kruger, H.; Runnegar, M.T.C.; Santikarn, S.; Smith, R.J.; Barna, J.C.J.; Williams, D.H. Structural studies on cyanoginosins-LR, YR, YA, and YM, peptide toxins from Microcystis aeruginosa. J. Chem. Soc. Perkin Trans. I 1985, 1, 2747–2748. [Google Scholar]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, S.; Deans Zirattu, S.; Lee, E.Y.C. Mutagenesis of the catalytic subunit of rabbit muscle protein phosphatase-1. Mol. Cell. Biochem. 1993, 127, 113–119. [Google Scholar]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.W.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar] [CrossRef]
- Goldberg, J.; Huang, H.; Kwon, Y.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar] [CrossRef]
- Abdel-Rahman, S.; El Ayouty, Y.M.; Kamael, H.A. Characterization of heptapeptide toxins extracted from Microcystis aeruginosa (Egyptian isolate)—Comparison with some synthesized analogs. Int. J. Pept. Protein Res. 1993, 41, 1–7. [Google Scholar] [CrossRef]
- Bourne, D.G.; Jones, G.J.; Blakeley, R.L.; Jones, A.; Negri, A.P.; Riddles, P. Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide toxin Microcystin-LR. Appl. Environ. Microbiol. 1996, 62, 4086–4094. [Google Scholar]
- Wiegand, C.; Pflugmacher, S. Ecotoxicological effects of selected cyanobacterial secondary metabolites: A short review. Toxicol. Appl. Pharmacol. 2005, 203, 201–218. [Google Scholar] [CrossRef]
- Banker, R.; Carmeli, S.; Werman, M.; Teltsch, B.; Porat, R.; Sukenik, A. Uracil moiety is required for toxicity of the cyanobacterial hepatotoxin cylindrospermopsin. J. Toxicol. Environ. Health Part A 2001, 62, 281–288. [Google Scholar] [CrossRef]
- Kinnear, S. Cylindrospermopsin: A decade of progress on bioaccumulation research. Mar. Drugs 2010, 8, 542–564. [Google Scholar] [CrossRef]
- Harada, K.I.; Ohtani, I.; Iwamoto, K.; Suzuki, M.; Watanabe, M.F.; Watanabe, M.; Terao, K. Isolation of cylindrospermopsin from a cyanobacterium Umezakia natans and its screening method. Toxicon 1994, 32, 73–84. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Hawkins, P.R.; Runnegar, M.T.C.; Jackson, A.R.B.; Falconer, I.R. Severe hepatotoxicity caused by the tropical cyanobacterium (blue-green alga) Cylindrospermopsis raciborskii (Woloszynska) Seenaya and Subba Raju isolated from a domestic water supply reservoir. Appl. Environ. Microbiol. 1985, 50, 1292–1295. [Google Scholar]
- Runnegar, M.T.; Kong, S.M.; Zhong, Y.Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fenech, M.; Thomas, P.; Falconer, I.R. Micronucleus induction and chromosome loss in transformed human white cells indicate clastogenic and aneugenic action of the cyanobacterial toxin, cylindrospermopsin. Mutat. Res. 2000, 472, 155–161. [Google Scholar] [CrossRef]
- Humpage, A.R.; Fontaine, F.; Froscio, S.; Burcham, P.; Falconer, I.R. Cylindrospermopsin genotoxicity and cytotoxicity, role of cytochrome P-450 and oxidative stress. J. Toxicol. Environ. Health A 2005, 68, 739–753. [Google Scholar] [CrossRef]
- Mathur, J. The illuminated plant cell. Trends Plant Sci. 2007, 12, 506–513. [Google Scholar] [CrossRef]
- Goddard, R.H.; Wick, S.M.; Silflow, C.D.; Snustad, D.P. Microtubule components of the plant cell cytoskeleton. Plant Physiol. 1994, 104, 1–6. [Google Scholar]
- Baskin, T.I. The Cytoskeleton. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; The American Society of Plant Biologists: Rockville, MD, USA, 2000; pp. 202–258. [Google Scholar]
- Mathur, J. Cell shape development in plants. Trends Plant Sci. 2004, 9, 583–590. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Mathur, J.; Hülskamp, M.; Gadella, T.W.J. Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biol. 2005, 3. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.J.; Liu, B. Cytoskeletal Motor Proteins in Plant Cell Division. In Cell Division Control in Plants, Plant Cell Monographs 9; Verma, D.P.S., Hong, Z., Eds.; Springer: Berlin, Heidelberg, Germany, 2008; pp. 169–193. [Google Scholar]
- Mineyuki, T. The preprophase band of microtubules: Its function as a cytokinetic apparatus in higher plants. Int. Rev. Cytol. 1999, 187, 1–49. [Google Scholar] [CrossRef]
- Yokota, E.; Imamichi, N.; Tominaga, M.; Shimmen, T. Actin cytoskeleton is responsible for the change of cytoplasmic organization in root hair cells induced by a protein phosphatase inhibitor, calyculin A. Protoplasma 2000, 213, 184–193. [Google Scholar] [CrossRef]
- Smertenko, A.P.; Chang, H.-Y.; Sonobe, S.; Fenyk, S.I.; Weingartner, M.; Bögre, L.; Hussey, P.J. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. J. Cell Sci. 2006, 119, 3227–3237. [Google Scholar] [CrossRef]
- Hamada, T. Microtubule associated proteins in higher plants. J. Plant Res. 2007, 120, 79–98. [Google Scholar] [CrossRef]
- Jones, A.M.; Dangl, J.L. Logjam at the Styx: Programmed cell death in plants. Trends Plant Sci. 1996, 1, 114–119. [Google Scholar] [CrossRef]
- Levine, A.; Pennell, R.I.; Alvarez, M.E.; Palmer, R.; Lamb, C. Calcium mediated apoptosis in a plant hypersensitive disease resistance response. Curr. Biol. 1996, 6, 427–437. [Google Scholar] [CrossRef]
- Kosslak, R.M.; Chamberlin, M.A.; Palmer, R.G.; Bowen, B.A. Programmed cell death in the root cortex of soybean root necrosis mutants. Plant J. 1997, 11, 729–745. [Google Scholar]
- White, S.H.; Duivenvoorden, L.J.; Fabbro, L.D. A decision-making framework for ecological impacts associated with the accumulation of cyanotoxins (cylindrospermopsin and microcystin). Lakes Reserv. Res. Manag. 2005, 10, 25–37. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Jung, K.; Lundvall, L.; Neumann, S.; Peuthert, A. Effects of cyanobacterial toxins and cyanobacterial cell-free crude extract on germination of alfalfa (Medicago sativa) and induction of oxidative stress. Environ. Toxicol. Chem. 2006, 25, 2381–2387. [Google Scholar] [CrossRef]
- Babica, P.; Bláha, L.; Maršálek, B. Exploringthe natural role of microcystins—A reviewof effects on photoautotrophic organisms. J. Phycol. 2006, 42, 9–20. [Google Scholar] [CrossRef]
- Saqrane, S.; Oudra, B. CyanoHAB occurrence and water irrigation cyanotoxin contamination: Ecological impacts and potential health risks. Toxins 2009, 1, 113–122. [Google Scholar] [CrossRef]
- Yamasaki, S. Probable effects of algal bloom on the growth of Phragmites australis (Cav.) Trin. ex. Steud. J. Plant Res. 1993, 106, 113–120. [Google Scholar] [CrossRef]
- Kós, P.; Gorzó, G.; Surányi, G.; Borbély, G. Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal. Biochem. 1995, 225, 49–53. [Google Scholar] [CrossRef]
- Kurki-Helasmo, K.; Meriluoto, J. Microcystin uptake inhibits growth and protein phosphatase activity in mustard (Sinapis alba L.) seedlings. Toxicon 1998, 36, 1921–1926. [Google Scholar] [CrossRef]
- Casanova, T.M.; Burch, D.M.; Brock, A.M.; Bond, M.P. Does toxic Microcystis aeruginosa affect aquatic plant establishment? Environ. Toxicol. 1999, 14, 97–109. [Google Scholar] [CrossRef]
- Weiss, J.; Liebert, H.P.; Braune, W. Influence of microcystin-RR on growth and photosynthetic capacity of the duckweed Lemna minor L. J. Appl. Bot. Angew. Bot. 2000, 74, 100–105. [Google Scholar]
- McElhiney, J.; Lawton, L.A.; Leifert, C. Investigations into the inhibitory effects of microcystins on plant growth, and the toxicity of plant tissues following exposure. Toxicon 2001, 39, 1411–1420. [Google Scholar] [CrossRef]
- Pflugmacher, S. Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ. Toxicol. 2002, 17, 407–413. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Tarczynska, M. The influance of microcystin-LR and hepatotoxic extract on the water plant Spirodela oligorrhiza. Environ. Toxicol. 2002, 17, 434–440. [Google Scholar] [CrossRef]
- Wiegand, C.; Peuthert, A.; Pflugmacher, S.; Carmeli, S. Effects of microcin SF608 and microcystin-LR, two cyanobacterial compounds produced by Microcystis sp., on aquatic organisms. Environ. Toxicol. 2002, 17, 400–406. [Google Scholar] [CrossRef]
- Gehringer, M.M.; Kewada, V.; Coates, N.; Downing, T.G. The use of Lepidium sativum in a plant bioassay system for the detection of microcystin-LR. Toxicon 2003, 41, 871–876. [Google Scholar] [CrossRef]
- M-Hamvas, M.; Máthé, C.; Papp, M.; Grigorszky, I.; Molnár, E.; Vasas, G.; Borbély, G. Microcystin-LR alters growth, anthocyanin content and single-stranded DNase enzyme activities in Sinapis alba L. seedlings. Aquat. Toxicol. 2003, 62, 1–9. [Google Scholar] [CrossRef]
- M.-Hamvas, M.; Máthé, C.; Vasas, G.; Jámbrik, K.; Papp, M.; Beyer, D.; Mészáros, I.; Borbély, G. Cylindrospermopsin and microcystin-LR alter the growth, development and peroxidase enzyme activity of white mustard (Sinapis alba L.) seedlings, a comparative analysis. Acta Biol. Hung. 2010, 61, 35–48. [Google Scholar] [CrossRef]
- Chen, J.; Song, L.; Dai, J.; Gan, N.; Liu, Z. Effects of microcystins on the growth and the activity of superoxide dismutase and peroxidase of rape (Brassica napus L.) and rice (Oryza sativa L.). Toxicon 2004, 43, 393–400. [Google Scholar] [CrossRef]
- Chen, J.; Dai, J.; Zhang, H.; Wang, C.; Zhou, G.; Han, Z.; Liu, Z. Bioaccumulation of microcystin and its oxidative stress in the apple (Malus pumila). Ecotoxicology 2010, 19, 796–803. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Allis, O.; Furey, A.; James, K.J. Bioaccumulation and harmful effects of microcystin-LR in the aquatic plants Lemna minor and Wolffia arrhiza and the filamentous alga Chladophora fracta. Ecotoxicol. Environ. Saf. 2005, 61, 345–352. [Google Scholar] [CrossRef]
- Yin, L.; Huang, J.; Li, D.; Liu, Y. Microcystin-RR uptake and its effects on the growth of submerged macrophyte Vallisneria natans (lour.) hara. Environ. Toxicol. 2005, 20, 308–313. [Google Scholar] [CrossRef]
- Jang, H.M.; Ha, K.; Takamura, N. Reciprocal allelopathic responses between toxic cyanobacteria (Microcystis aeruginosa) and duckweed (Lemna japonica). Toxicon 2007, 49, 727–733. [Google Scholar] [CrossRef]
- Máthé, C.; M-Hamvas, M.; Vasas, G.; Surányi, G.; Bácsi, I.; Beyer, D.; Tóth, S.; Tímár, M.; Borbély, G. Microcystin-LR, a cyanobacterial toxin, induces growth inhibition and histological alterations in common reed (Phragmites australis) plants regenerated from embryogenic calli. New Phytol. 2007, 176, 824–835. [Google Scholar] [CrossRef]
- Saqrane, S.; El ghazali, I.; Ouahid, Y.; El Hassni, M.; El Hadrami, I.; Bouarab, L.; del Campo, F.F.; Oudra, B.; Vasconcelos, V. Phytotoxic effects of cyanobacteria extract on the aquatic plant Lemna gibba: Microcystin accumulation, detoxication and oxidative stress induction. Aquat. Toxicol. 2007, 83, 284–294. [Google Scholar] [CrossRef]
- Saqrane, S.; El Ghazali, I.; Oudra, B.; Bouarab, L.; Vasconcelos, V. Effects of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J. Environ. Sci. Health Part B 2008, 43, 443–451. [Google Scholar]
- Saqrane, S.; Ouahid, Y.; El Ghazali, I.; Oudra, B.; Bouarab, L.; del Campo, F.F. Physiological changes in Triticum durum, Zea mays, Pisum sativum and Lens esculenta cultivars, caused by irrigation with water contaminated with microcystins: A laboratory experimental approach. Toxicon 2009, 53, 786–796. [Google Scholar] [CrossRef]
- Yi, D.; Yijun, Z.; Xue, B.; Zhihui, F.; Kai, C. Phytotoxic effects of cyanobacteria extract on Lemna minor and Myriophyllum spicatum phyto-tolerance and superoxide dismutase activity. Environ. Toxicol. 2009, 24, 304–308. [Google Scholar] [CrossRef]
- Szigeti, Z.M.; Jámbrik, K.; Roszik, J.; M-Hamvas, M.; Tándor, I.; Beyer, D.; Vasas, G.; Vereb, G.; Surányi, G.; Máthé, C. Cytoskeletal and developmental alterations in Ceratophyllum demersum induced by microcystin-LR, a cyanobacterial toxin. Aquat. Bot. 2010, 92, 179–184. [Google Scholar] [CrossRef]
- Bácsi, I.; Surányi, G.; Gonda, S.; Gyémánt, G.; Vasas, G. Observation of sward destruction caused by irrigation with toxic Microcystis morphospecies containing water in Southern Hungary. Bull. Environ. Contam. Toxicol. 2011, 86, 232–237. [Google Scholar] [CrossRef]
- El Khalloufi, F.; Oufdou, K.; Lahrouni, M.; El Ghazali, I.; Saqrane, S.; Vasconcelos, V.; Oudra, B. Allelopatic effects of cyanobacteria extracts containing microcystins on Medicago sativa-Rhizobia symbiosis. Ecotoxicol. Environ. Saf. 2011, 74, 431–438. [Google Scholar] [CrossRef]
- Kováts, N.; Ács, A.; Paulovits, G.; Vasas, G. Response of Lemna minor clones to microcystis toxicity. Appl. Ecol. Environ. Res. 2011, 9, 17–26. [Google Scholar]
- Lahrouni, M.; Oufdou, K.; Faghire, M.; Peix, A.; El Khalloufi, F.; Vasconcelos, V.; Oudra, B. Cyanobacterial extracts containing microcystins affect the growth, nodulation process and nitrogen uptake of faba bean (Vicia faba L., Fabaceae). Ecotoxicology 2012, 21, 681–687. [Google Scholar] [CrossRef]
- Lahrouni, M.; Oufdou, K.; El Khalloufi, F.; Baz, M.; Lafuente, A.; Dary, M.; Pajuelo, E.; Oudra, B. Physiological and biochemical defense reactions of Vicia faba L.-Rhizobium symbiosis face to chronic exposure to cyanobacterial bloom extract containing microcystins. Environ. Sci. Pollut. Res. 2013, 20, 5405–5415. [Google Scholar] [CrossRef]
- Máthé, C.; Vasas, G.; Borbély, G.; Erdődi, F.; Beyer, D.; Kiss, A.; Surányi, G.; Gonda, S.; Jámbrik, K.; M-Hamvas, M. Histological, cytological and biochemical alterations induced by microcystin-LR and cylindrospermopsin in white mustard (Sinapis alba L.) seedlings. Acta Biol. Hung. 2013, 64, 75–89. [Google Scholar]
- Yin, L.Y.; Huang, J.Q.; Huang, W.M.; Li, D.H.; Wang, G.H.; Liu, Y.D. Microcystin-RR-induced accumulation of reactive oxygen species and alteration of antioxidant systems in tobacco BY-2 cells. Toxicon 2005, 46, 507–512. [Google Scholar] [CrossRef]
- Vasas, G.; Gásprár, A.; Surányi, G.; Batta, G.; Gyémánt, G.; M-Hamvas, M.; Máthé, C.; Grigorszky, I.; Molnár, E.; Borbely, G. Capillary Electrophoretic assay and purification of cylindrospermopsin, a cyanobacterial toxin from Aphanizomenon ovalisporum by plant test (Blue-Green Sinapis Test). Anal. Biochem. 2002, 302, 95–103. [Google Scholar] [CrossRef]
- Beyer, D.; Surányi, G.; Vasas, G.; Roszik, J.; Erdődi, F.; M-Hamvas, M.; Bácsi, I.; Bátori, R.; Serfőző, Z.; Szigeti, Z.M.; Vereb, G.; Demeter, Z.; Gonda, S.; Máthé, C. Cylindrospermopsin induces alterations of root histology and microtubule organization in common reed (Phragmites australis) plantlets cultured in vitro. Toxicon 2009, 54, 440–449. [Google Scholar] [CrossRef]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Bácsi, I.; Surányi, G.; Gonda, S.; Borbély, G.; M-Hamvas, M. Cylindrospermopsin inhibits growth and modulates protease activity in the aquatic plants Lemna minor L. and Wolffia arrhiza (L.) Horkel. Acta Biol. Hung. 2010, 61, 77–94. [Google Scholar] [CrossRef]
- Silva, P.; Vasconcelos, V. Allelopathic effect of Cylindrospermopsis raciborskii extracts on the germination and growth of several plant species. Chem. Ecol. 2010, 26, 263–271. [Google Scholar] [CrossRef]
- Kittler, K.; Schreiner, M.; Krumbein, A.; Manzei, S.; Koch, M.; Rohn, S.; Maul, R. Uptake of the cyanobacterial toxin cylindrospermopsin in Brassica vegetables. Food Chem. 2012, 133, 875–879. [Google Scholar] [CrossRef]
- Kinnear, S.H.W.; Duivenvoorden, L.J.; Fabbro, L.D. Growth and bioconcentration in Spirodella oligorrhiza following exposure to Cylindrospermopsis raciborskii whole cell extracts. Austr. J. Ecotoxicol. 2007, 13, 19–31. [Google Scholar]
- Kinnear, S.H.W.; Fabbro, L.D.; Duivenvoorden, L.J. Variable growth responses of water thyme (Hydrilla verticillata) to whole-cell extracts of Cylindrospermopsis raciborskii. Arch. Environ. Contam. Toxicol. 2008, 54, 187–194. [Google Scholar] [CrossRef]
- Prieto, A.; Campos, A.; Cameán, A.; Vasconcelos, V. Effects on growth and oxidative stress status of rice plants (Oryza sativa) exposed to two extracts of toxin-producing cyanobacteria (Aphanizomenon ovalisporum and Microcystis aeruginosa). Ecotoxicol. Environ. Saf. 2011, 74, 1973–1980. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Barakate, A.; Codd, G.A. Inhibition of plant protein synthesis by the cyanobacterialhepatotoxin, cylindrospermopsin. FEMS Microbiol. Lett. 2004, 235, 125–129. [Google Scholar] [CrossRef]
- Máthé, C.; Beyer, D.; Erdődi, F.; Serfőző, Z.; Székvölgyi, L.; Vasas, G.; M-Hamvas, M.; Jámbrik, K.; Gonda, S.; Kiss, A.; Szigeti, Z.M.; Surányi, G. Microcystin-LR induces abnormal root development by altering microtubule organization in tissue-cultured common reed (Phragmites australis) plantlets. Aquat. Toxicol. 2009, 92, 122–130. [Google Scholar] [CrossRef]
- Chen, J.; Han, F.X.; Wang, F.; Zhang, H.Q.; Shi, Z.Q. Accumulation and phytotoxicity of microcystin-LR in rice (Oryza sativa). Ecotoxicol. Environ. Saf. 2012, 76, 193–199. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Aulhorn, M.; Grimm, B. Influence of a cyanobacterial crude extract containingmicrocystin-LR on the physiology and antioxidative defence systems of different spinach variants. New Phytol. 2007, 175, 482–489. [Google Scholar] [CrossRef]
- Abe, T.; Lawson, T.; Weyers, J.D.B.; Codd, G.A. Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: Implications for current spray irrigation practice. New Phytol. 1996, 133, 651–658. [Google Scholar] [CrossRef]
- Saqrane, S.; El Ghazali, I.; Oudra, B.; Bouarab, L.; Dekayir, S.; Mandi, L.; Ouazzani, N.; Vasconcelos, V.M. Detection of microcystin contamination by the measurement of the variability of the in vivo chlorophyll fluorescence in aquatic plant Lemna gibba. Toxicon 2009, 53, 9–14. [Google Scholar] [CrossRef]
- Zhang, S.H.; Cheng, S.P.; Wang, H.Q.; He, F.; Wu, Z.B. Allelopathic interactions between the Potamogeton spp. and toxic cyanobacteria (Microcystis aeruginosa). Allelopath. J. 2009, 23, 379–390. [Google Scholar]
- Peuthert, A.; Lawton, L.; Pflugmacher, S. In vivo influence of cyanobacterial toxins on enzyme activity and gene expression of protein phosphatases in alfalfa (Medicago sativa). Toxicon 2008, 52, 84–90. [Google Scholar] [CrossRef]
- Takeda, S.; Mano, S.; Ohto, M.; Nakamura, K. Inhibitors of protein phosphatases 1 and 2A block sugar-inducible gene expression in plants. Plant Physiol. 1994, 106, 567–574. [Google Scholar]
- Siegl, G.; MacKintosh, C.; Stittl, M. Sucrose-phosphate synthase is dephosphorylated by protein phosphatase 2A in spinach leaves. Evidence from the effects of okadaic acid and microcystin. FEBS Lett. 1990, 270, 198–202. [Google Scholar] [CrossRef]
- Kenton, P.; Mur, L.A.J.; Draper, J. A requirement for calcium and protein phosphatase in the jasmonate-induced increase in tobacco leaf acid phosphatase specific activity. J. Exp. Bot. 1999, 50, 1331–1341. [Google Scholar]
- Jámbrik, K.; Máthé, C.; Vasas, G.; Beyer, D.; Molnár, E.; Borbély, G.; M-Hamvas, M. Microcystin-LR induces chromatin alterations and modulates neutral single-strand-preferring nuclease activity in Phragmites australis. J. Plant Physiol. 2011, 168, 678–686. [Google Scholar] [CrossRef]
- Yin, L.; Huang, J.; Huang, W.; Li, D.; Liu, Y. Responses of antioxidant system in Arabidopsis thaliana suspension cells to the toxicity of microcystin-RR. Toxicon 2005, 46, 859–864. [Google Scholar] [CrossRef]
- Peuthert, A.; Chakrabarti, S.; Pflugmacher, S. Uptake of microcystins-LR and -LF (cyanobacterial toxins) in seedlings of several important agricultural plant species and the correlation with cellular damage (lipid peroxidation). Environ. Toxicol. 2007, 22, 436–442. [Google Scholar] [CrossRef]
- Peuthert, A.; Pflugmacher, S. In fluence of the cyanotoxin microcystin-LR on tocopherol in alfalfa seedlings (Medicago sativa). Toxicon 2010, 56, 411–417. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Oberemm, A.; Beattie, K.A.; Krause, E.; Codd, G.A.; Steinberg, C.E.W. Identification of enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR: The first step of detoxification. Biochim. Biophys. Acta 1998, 1425, 527–533. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Codd, G.A.; Steinberg, C.E.W. Effects of the cyanobacterial toxin microcystin-LR on detoxication emzymes in aquatic plants. Environ. Toxicol. 1999, 14, 111–115. [Google Scholar] [CrossRef]
- Pflugmacher, S.; Wiegand, C.; Beattie, K.A.; Krause, E.; Steinberg, C.E.W.; Codd, G.A. Uptake, effects, and metabolism of cyanobacterial toxins in the emergent reed plant Phragmites australis (CAV.) Trin. ex Steud. Environ. Toxicol. Chem. 2001, 20, 846–852. [Google Scholar]
- Pietsch, C.; Wiegand, C.; Amé, M.V.; Nicklisch, A.; Wunderlin, D.; Pflugmacher, S. The effects of a cyanobacterial crude extract on different aquatic organisms: Evidence for cyanobacterial toxin modulating factors. Environ. Toxicol. 2001, 16, 535–542. [Google Scholar] [CrossRef]
- Pflugmacher, S. Promotion of oxidative stress in the aquatic macrophyte Ceratophyllum demersum during biotransformation of the cyanobacterial toxin microcystin-LR. Aquat. Toxicol. 2004, 70, 169–178. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y.M.; Zhang, H.Q.; Shi, Z.Q. Nitrate reductase-dependent nitric oxide production is involved in microcystin-LR-induced oxidative stress in Brassica rapa. Water Air Soil Pollut. 2012, 223, 4141–4152. [Google Scholar] [CrossRef]
- Pichardo, S.; Pflugmacher, S. Study of the antioxidant response of several bean variants to irrigation with water containing MC-LR and cyanobacterial crude extract. Environ. Toxicol. 2011, 26, 300–306. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.Q.; Hu, L.B.; Shi, Z.Q. Microcystin-LR-induced phytotoxicity in rice crown root is associated with the cross-talk between auxin and nitric oxide. Chemosphere 2013, 93, 283–293. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Wickramasinghe, W.; Shaw, G.; Falconer, I.R. Interaction of the cyanobacterial toxin cylindrospermopsin with the eukaryotic protein synthesis system. Toxicon 2008, 51, 191–198. [Google Scholar] [CrossRef]
- Lankoff, A.; Banasik, A.; Obe, G.; Deperas, M.; Kuzminski, K.; Tarczynska, M.; Jurczak, T.; Wojcik, A. Effect of microcystin-LR and cyanobacterial extract from Polish reservoir of drinking water on cell cycle progression, mitotic spindle, and apoptosis in CHO-K1 cells. Toxicol. Appl. Pharmacol. 2003, 189, 204–213. [Google Scholar] [CrossRef]
- Gehringer, M.M. Microcystin-LR and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2004, 557, 1–8. [Google Scholar] [CrossRef]
- Beyer, D.; Tándor, I.; Kónya, Z.; Bátori, R.; Roszik, J.; Vereb, G.; Erdődi, F.; Vasas, G.; M-Hamvas, M.; Jambrovics, K.; Máthé, C. Microcystin-LR, a protein phosphatase inhibitor induces alterations in mitotic chromatin and microtubule organization leading to the formation of micronuclei in Vicia faba. Ann. Bot. 2012, 110, 797–808. [Google Scholar] [CrossRef]
- Wolniak, S.M.; Larsen, P.M. Changes in the metaphase transit times and the pattern of sister chromatid separation in stamen hair cells of Tradescantia after treatment with protein phosphatase inhibitors. J. Cell Sci. 1992, 102, 691–705. [Google Scholar]
- Toivola, D.M.; Eriksson, J.E. Toxins affecting cell signalling and alteration of cytoskeletal structure. Toxicol. Vitr. 1999, 13, 521–530. [Google Scholar] [CrossRef]
- Venoux, M.; Basbous, J.; Berthenet, C.; Prigent, C.; Fernandez, A.; Lamb, N.J.; Rouquier, S. ASAP is a novel substrate of the oncogenic mitotic kinase Aurora-A: Phosphorylation on Ser625 is essential to spindle formation and mitosis. Hum. Mol. Genet. 2008, 17, 215–224. [Google Scholar]
- Gácsi, M.; Antal, O.; Vasas, G.; Máthé, C.; Borbély, G.; Saker, M.L.; Győri, J.; Farkas, A.; Vehovszky, Á.; Bánfalvi, G. Comparative study of cyanotoxins affectiong cytoskeletal and chromatin structures in CHO-K1 cells. Toxicol. Vitr. 2009, 23, 710–718. [Google Scholar] [CrossRef]
- Ayaydin, F.; Vissi, E.; Mészáros, T.; Miskolczi, P.; Kovács, I.; Fehér, A.; Dombrádi, V.; Erdődi, F.; Gergely, P.; Dudits, D. Inhibition of serine/threonine-specific protein phosphatases causes premature activation of cdc2MsF kinase at G2/M transition and early microtubule organisation in alfalfa. Plant J. 2000, 23, 85–96. [Google Scholar] [CrossRef]
- Holy, J.M. Curcumin disrupts mitotic spindle structure and induces micronucleation in MCF-7 breast cancer cells. Mutat. Res. 2002, 518, 71–84. [Google Scholar] [CrossRef]
- Sasabe, M.; Soyano, T.; Takahashi, Y.; Sonobe, S.; Igarashi, H.; Itoh, T.J.; Hidaka, M.; Machida, Y. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes Dev. 2006, 20, 1004–1014. [Google Scholar] [CrossRef]
- Smertenko, A.P.; Chang, H.Y.; Wagner, V.; Kaloriti, D.; Fenyk, S.; Sonobe, S.; Lloyd, C.; Hauser, M.T.; Hussey, P.J. The Arabidopsis microtubule-associated protein AtMAP65-1: Molecular analysis of its microtubule bundling activity. Plant Cell 2004, 16, 2035–2047. [Google Scholar] [CrossRef]
- Laughinghouse, H.D., IV; Prá, D.; Silva-Stenico, M.E.; Rieger, A.; Dal-Souto Frescura, V.; Fiore, M.F.; Tedesco, S.B. Biomonitoring genotoxicity and cytotoxicity of Microcystis aeruginosa (Chroococcales, Cyanobacteria) using the Allium cepa test. 2012, 432, 180–188. [Google Scholar]
- Huang, W.; Xin, W.; Li, D.; Liu, Y. Microcystin-RR induced apoptosis in tobacco BY-2 suspension cells is mediated by reactive oxygen species and mitochondrial permeability transition pore status. Toxicol. Vitr. 2008, 22, 328–337. [Google Scholar] [CrossRef]
- Yin, L.Y.; Huang, J.Q.; Li, W.; Liu, Y.D. Microcystin-RR-induced apoptosis in tobacco BY-2 cells. Toxicon 2006, 48, 204–210. [Google Scholar] [CrossRef]
- Huang, W.; Xing, W.; Li, D.; Liu, D. Morphological and ultrastructural changes in tobacco BY-2 cells exposed to microcystin-RR. Chemosphere 2009, 76, 1006–1012. [Google Scholar] [CrossRef]
- Jiang, J.; Gu, X.; Song, R.; Wang, X.; Yang, L. Microcystin-LR induced oxidative stress and ultrastructural alterations in mesophyll cells of submerged macrophyte Vallisneria natans (Lour.) Hara. J. Hazard. Mater. 2011, 190, 188–196. [Google Scholar] [CrossRef]
- Chen, J.Z.; Ye, J.Y.; Zhang, H.Y.; Jiang, X.J.; Zhang, Y.X.; Liu, Z.L. Freshwater toxic cyanobacteria induced DNA damage in apple (Malus pumila), rape (Brassica napus) and rice (Oryza sativa). J. Hazard. Mater. 2011, 190, 240–244. [Google Scholar] [CrossRef]
- Kaszás, E.; Cande, W.Z. Phosphorylation of histone H3 is correlated with changes in the maintenance of sister chromatid cohesion during meiosis in maize, rather than the condensation of the chromatin. J. Cell Sci. 2000, 113, 3217–3226. [Google Scholar]
- Schroeder-Reiter, E.; Houben, A.; Wanner, G. Immunogold labeling of chromosomes for scanning electron microscopy: A closer look at phosphorylated histone H3 in mitotic metaphase chromosomes of Hordeum vulgare. Chromosome Res. 2003, 11, 585–596. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Marshall, J.B.; Zhong, C.X.; Dawe, R.K. Phosphoserine on maize CENTROMERIC HISTONE H3 and histone H3 demarcate the centromere and pericentromere during chromosome segregation. Plant Cell 2005, 17, 572–583. [Google Scholar] [CrossRef]
- Manzanero, S.; Rutten, T.; Kotseruba, V.; Houben, A. Alterations in the distribution of histone H3 phosphorylation in mitotic plant chromosomes in response to cold treatment and the protein phosphatase inhibitor cantharidin. Chromosome Res. 2002, 10, 467–476. [Google Scholar] [CrossRef]
- Pérez-Cadahía, B.; Drobic, B.; Davie, J.R. H3 phosphorylation: Dual role in mitosis and interphase. Biochem. Cell Biol. 2009, 87, 695–709. [Google Scholar] [CrossRef]
- Eyers, P.A.; Maller, J.L. Regulation of Xenopus Aurora A activation by TPX2. J. Biol. Chem. 2004, 279, 9008–9015. [Google Scholar] [CrossRef]
- Demidov, D.; VanDamme, D.; Geelen, D.; Blattner, F.R.; Houben, A. Identification and dynamics of two classes of Aurora-like kinases in Arabidopsis and other plants. Plant Cell 2005, 17, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Kawabe, A.; Matsunaga, S.; Nakagawa, K.; Kurihara, D.; Yoneta, A.; Hasezawa, S.; Uchiyama, S.; Fukui, K. Characterization of plant Aurora kinases during mitosis. Plant Mol. Biol. 2005, 58, 1–13. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, M.; Ren, H. Visualization of actin cytoskeletal dynamics during the cell cycle in tobacco (Nicotiana tabacum L. cv. Bright Yellow) cells. Biol. Cell 2006, 98, 295–306. [Google Scholar] [CrossRef]
- Hwang, J.U.; Lee, Y. Abscisic acid-induced actin reorganization in guard cells of dayflower is mediated by cytosooic calcium levels and by protein kinase and protein phosphatase activities. Plant Physiol. 2001, 125, 2120–2128. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.; Lee, Y. Phosphatidic acid induces actin polymerization by activating protein kinases in soybean cells. Mol. Cells 2003, 15, 313–319. [Google Scholar]
- Falconer, I.R.; Yeung, D.S.K. Cytoskeletal changes in hepatocytes induced by Microcystis toxins and their relation to hyperphosphorylation of cell proteins. Chem. Biol. Interact. 1992, 81, 181–196. [Google Scholar] [CrossRef]
- Fladmark, K.E.; Serres, M.H.; Larsen, N.L.; Yasumoto, T.; Aune, T.; Døskeland, S.O. Sensitive detection of apoptogenic toxins in suspension cultures of rat and salmon hepatocytes. Toxicon 1998, 36, 1101–1114. [Google Scholar] [CrossRef]
- Khan, S.A.; Ghosh, S.; Wickstrom, M.; Miller, L.A.; Hess, R.; Haschek, W.M.; Beasley, V.R. Comparative pathology of microcystin-LR in cultured hepatocytes, fibroblasts, and renal epithelial cells. Nat. Toxins 1995, 3, 119–128. [Google Scholar] [CrossRef]
- Abramsson-Zetterberg, L.; Beckman Sundh, U.; Mattsson, R. Cyanobacterial extracts and microcystin-LR are inactive in the micronucleus assay in vivo and in vitro. Mutat. Res. 2010, 699, 5–10. [Google Scholar] [CrossRef]
- Michalakis, J.; Georgatos, D.; Romanos, J.; Koutala, H.; Georgoulias, V.; Tsifitis, D.; Theodoropoulos, P.A. Micromolar taxol, with or without hyperthermia, induces mitotic catastrophe and cell necrosis in HeLa cells. Cancer Chemother Pharm. 2005, 56, 615–622. [Google Scholar] [CrossRef]
- Lytvyn, D.I.; Venets, A.I.; Blume, Y.B. UV-B overexposure induces programmed cell death in a BY-2 tobacco cell line. Environ. Exp. Bot. 2010, 68, 51–57. [Google Scholar] [CrossRef]
- Mankiewicz, J.; Tarczynska, M.; Fladmark, K.E.; Døskeland, S.O.; Walter, Z.; Zalewski, M. Apoptotic effect of cyanobacterial extract on rat hepatocytes and human lymphocytes. Environ. Toxicol. 2001, 16, 225–233. [Google Scholar] [CrossRef]
- McDermott, C.M.; Nho, C.W.; Howard, W.; Holton, B. The cyanobacterial toxin, microcystin-LR, can induce apoptosis in a variety of cell types. Toxicon 1998, 36, 1981–1996. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G.; Li, X.; Gorczyca, W.; Murakami, T.; Traganos, F. Cytometry in cell necrobiology: Analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997, 27, 1–20. [Google Scholar] [CrossRef]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes. Hepatology 2000, 32, 547–555. [Google Scholar] [CrossRef]
- Dangl, J.L.; Dietrich, R.A.; Thomas, H. Senescence and Programmed Cell Death. In Biochemistry and Molecular Biology of Plants; Buchanan, B., Gruissem, W., Jones, R., Eds.; The American Society of Plant Biologists: Rockville, MD, USA, 2000; pp. 1044–1100. [Google Scholar]
- Kusaka, K.; Tada, Y.; Shigemi, T.; Sakamoto, M.; Nakayashiki, H.; Tosa, Y.; Shigeyuki, M. Coordinate involvement of cysteine protease and nuclease in the executive phase of plant apoptosis. FEBS Lett. 2004, 578, 363–367. [Google Scholar] [CrossRef]
- Žegura, B.; Lah, T.T.; Filipič, M. The role of reactive oxygen species in microcystin-LR-induced DNA damage. Toxicology 2004, 200, 59–68. [Google Scholar] [CrossRef]
- Valério, E.; Chaves, S.; Tenreiro, R. Diversity and impact of prokaryotic toxins on aquatic environments: A review. Toxins 2010, 2, 2359–2410. [Google Scholar] [CrossRef]
- Ros Barceló, A. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH-oxidase-like enzyme. Planta 1998, 207, 207–216. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Review. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A. The root microtubule cytoskeleton and cell cycle analysis through desiccation of Brassica napus seedlings. Protoplasma 2008, 233, 177–185. [Google Scholar] [CrossRef]
- Lankoff, A.; Wojcik, A.; Lisowska, H.; Bialczyk, J.; Dziga, D.; Carmichael, W.W. No induction of structural chromosomal aberrations in cylindrospermopsin-treated CHO-K1 cells without and with metabolic activation. Toxicon 2007, 50, 1105–1115. [Google Scholar] [CrossRef]
- Bazin, E.; Mourot, A.; Humpage, A.R.; Fessard, V. Genotoxicity of a freshwater cyanotoxin, cylindrospermopsin, in two human cell lines: Caco-2 and HepaRG. Environ. Mol. Mutagen. 2010, 51, 251–259. [Google Scholar]
- Chong, M.W.K.; Wong, B.S.F.; Lam, P.K.S.; Shaw, G.R.; Seawright, A.A. Toxicity and uptake mechanism of cylindrospermopsin and lophyrotomin in primary rat hepatocytes. Toxicon 2002, 40, 205–211. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Žegura, B. Genotoxic effects of the cyanobacterial hepatotoxin cylindrospermopsin on the HepG2 cell line. Arch. Toxicol. 2011, 85, 1617–1626. [Google Scholar] [CrossRef]
- Shen, X.; Lam, P.K.S.; Shaw, G.R.; Wickramasinghe, W. Genotoxicity investigation of a cyanobacterial toxin, cylindrospermopsin. Toxicon 2002, 40, 1499–1501. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Jos, Á.; Pichardo, S.; Cameán, A.M. Oxidative stress responses in tilapia (Oreochromis niloticus) exposed to a single dose of pure cylindrospermopsin under laboratory conditions: Influence of exposure route and time of sacrifice. Aquat. Toxicol. 2011, 105, 100–106. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Pichardo, S.; Jos, Á.; Moreno, F.J.; Cameán, A.M. Biochemical and pathological toxic effects induced by the cyanotoxin Cylindrospermopsin on the human cell line Caco-2. Water Res. 2012, 46, 1566–1575. [Google Scholar] [CrossRef]
- Štraser, A.; Filipič, M.; Gorenc, I.; Žegura, B. The influence of cylindrospermopsin on oxidative DNA damage and apoptosis induction in HepG2 cells. Chemosphere 2013, 92, 24–30. [Google Scholar] [CrossRef]
- Fessard, V.; Bernard, C. Cell alterations but no DNA strand breaks induced in vitro by cylindrospermopsin in CHO K1 cells. Environ. Toxicol. 2003, 18, 353–359. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Máthé, C.; M-Hamvas, M.; Vasas, G. Microcystin-LR and Cylindrospermopsin Induced Alterations in Chromatin Organization of Plant Cells. Mar. Drugs 2013, 11, 3689-3717. https://doi.org/10.3390/md11103689
Máthé C, M-Hamvas M, Vasas G. Microcystin-LR and Cylindrospermopsin Induced Alterations in Chromatin Organization of Plant Cells. Marine Drugs. 2013; 11(10):3689-3717. https://doi.org/10.3390/md11103689
Chicago/Turabian StyleMáthé, Csaba, Márta M-Hamvas, and Gábor Vasas. 2013. "Microcystin-LR and Cylindrospermopsin Induced Alterations in Chromatin Organization of Plant Cells" Marine Drugs 11, no. 10: 3689-3717. https://doi.org/10.3390/md11103689




