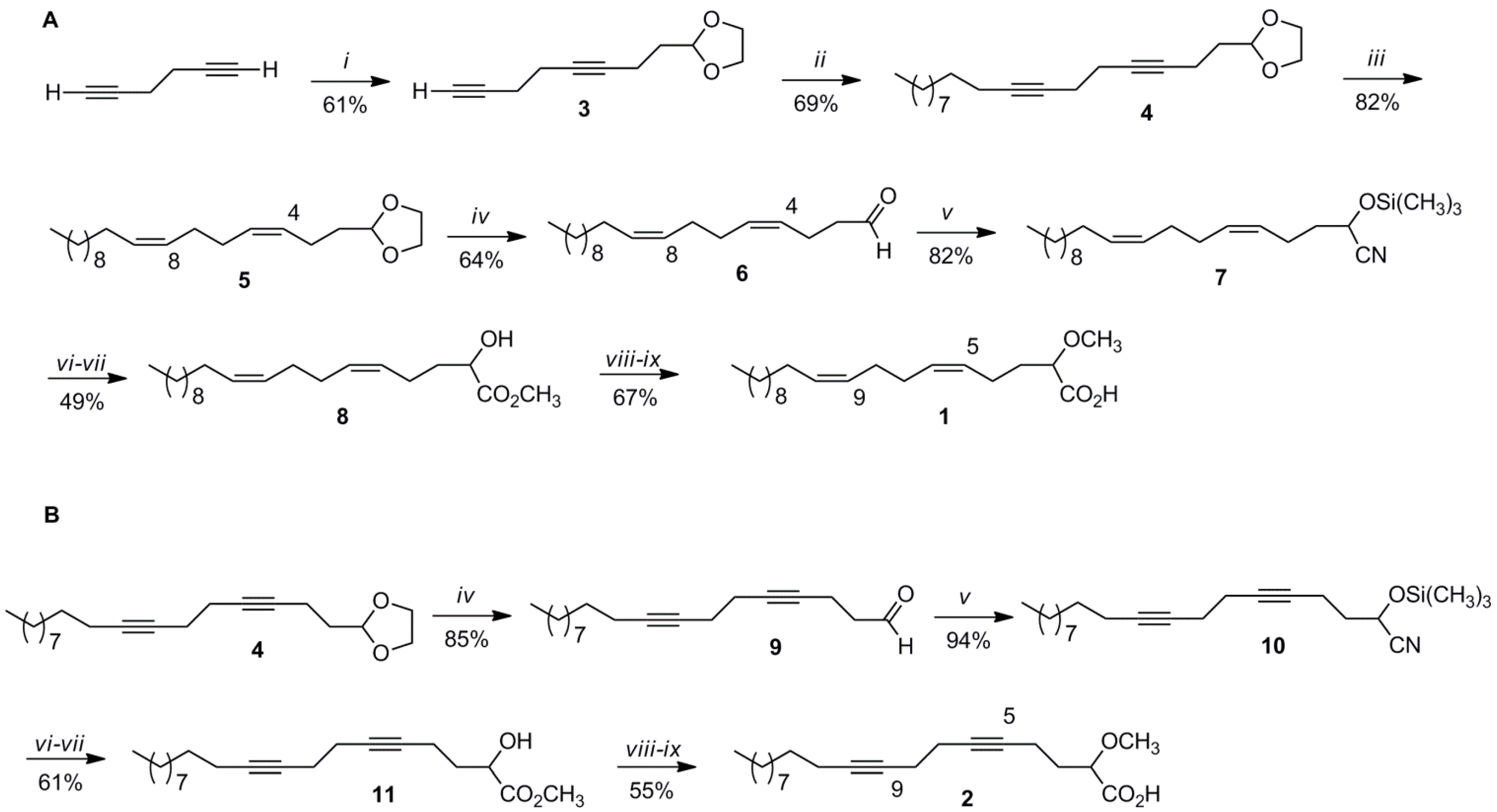
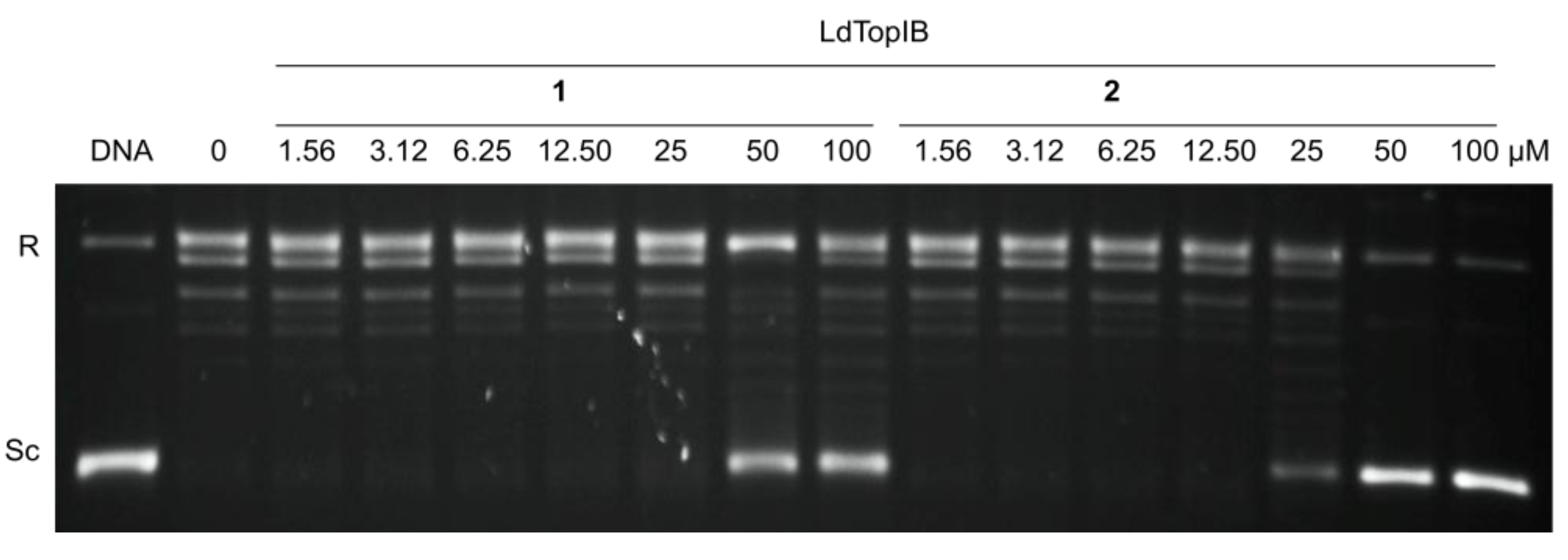
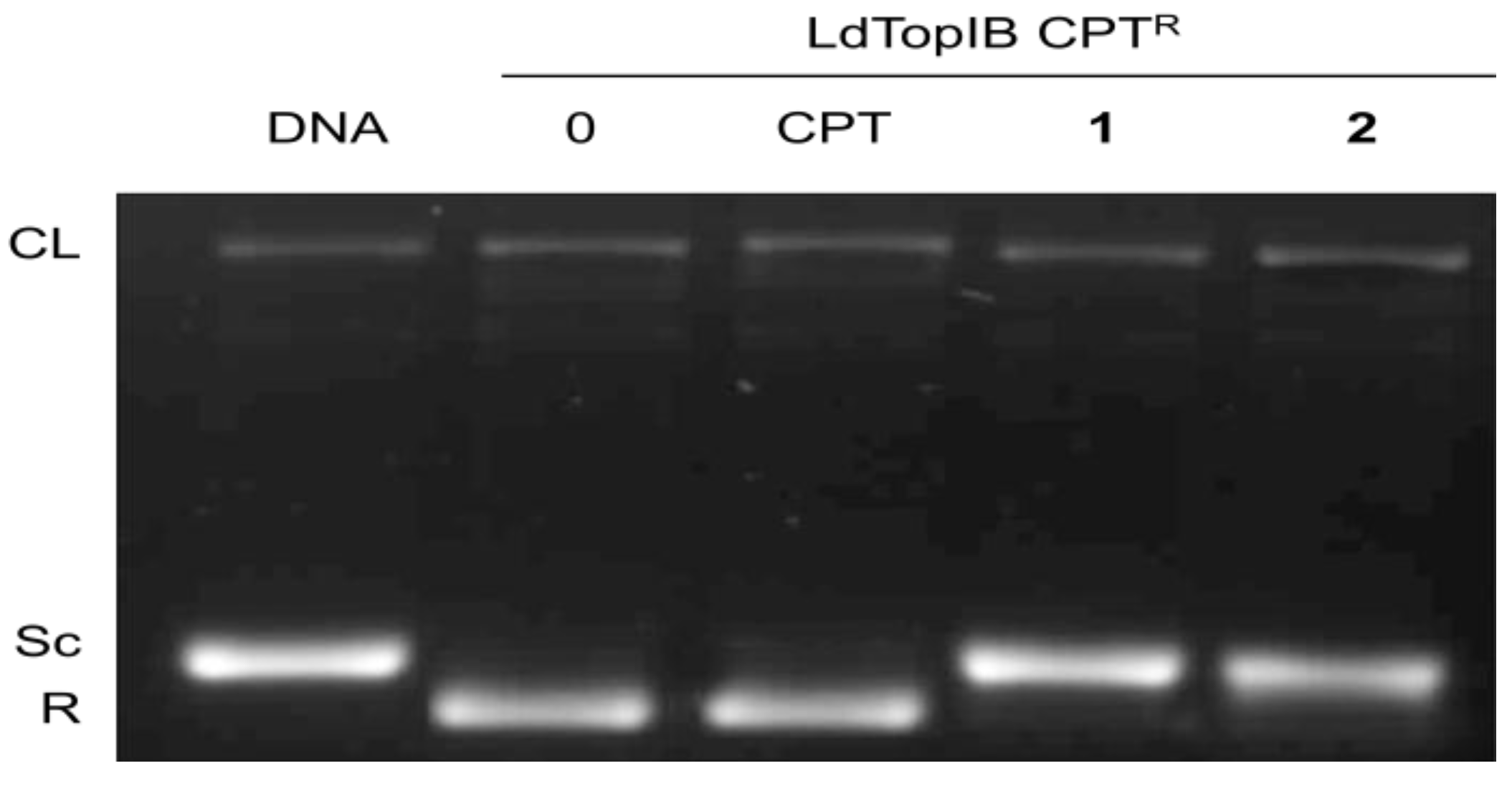
3.2. Synthesis of (5Z,9Z)-(±)-2-Methoxy-5,9-eicosadienoic Acid (1)
3.2.1. 2-(3,7-Octadiynyl)-1,3-dioxolane (3)
It was as obtained as a colorless oil in a 61% yield from the reaction of 3.7 mL (19.23 mmol) of 1,5-hexadiyne in 40 ml of dry THF with 5.1 mL (55.47 mmol) of
n-BuLi (2.5 M in hexane) at −78 °C and 1.5 mL (12.85 mmol) of 2-(2-bromoethyl)-1,3-dioxolane following the procedure already described and with identical spectral data as previously reported [
7].
3.2.2. 2-(Octadeca-3,7-diynyl)-1,3-dioxolane (4)
To a stirred solution of 3 (1.39 g, 7.81 mmol) in dry THF (13 mL) and under argon was added 2.1 mL (22.8 mmol) of n-BuLi (2.5 M) in hexanes at −10 °C followed by 45 min of stirring. After this time the temperature was lowered to −70 °C and 3.1 mL of HMPA was added to the reaction mixture followed by 2.7 mL (13.01 mmol) of 1-bromodecane, and stirring for 24 h. Then, the reaction mixture was quenched with water. The organic product was extracted with brine solution (2 × 15 mL), diethyl ether (2 × 15 mL), dried over MgSO4, filtered, and evaporated in vacuo. The product was purified using silica gel column chromatography eluting with hexane/ether (9:1). Dioxolane 4 (1.71 g, 5.37 mmol) was obtained as colorless oil for a 69% yield.
1H (CDCl3, 300 MHz) δ (ppm) 4.95 (1H, t, H-2), 3.95–3.81 (4H, m, -OCH2-), 2.30 (4H, m,), 2.25 (2H, m), 2.11 (2H, t, H-3), 1.81 (2H, m), 1.43 (2H, m), 1.26 (14H, brs, -CH2-), 0.86 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 103.3 (d, C-2), 81.2 (s), 79.8 (s), 79.1 (s), 78.6 (s), 64.9 (t), 33.2 (t), 31.9 (t), 29.7 (t), 29.5 (t), 29.3 (t), 29.1 (t), 29.0 (t), 28.8 (t), 22.7 (t), 19.4 (t), 19.3 (t), 18.8 (t), 14.1 (q, -CH3), 13.7 (t); GC/MS (70 eV) m/z (relative intensity): 318 (M+, 1), 303 (1), 290 (2), 289 (5), 273 (1), 235 (1), 191 (6), 177 (2), 163 (5), 139 (55), 105 (10), 99 (23), 86 (12), 73 (100); HRMS (APCI) Calcd for C21H35O2 [M + H]+ 319.2632, found 319.2630.
3.2.3. 2-[(3Z,7Z)-Octadeca-3,7-dien-1-yl]-1,3-dioxolane (5)
Into a 50-mL two-necked round-bottomed flask containing Lindlar’s catalyst (0.64 g) was added a solution of 4 (0.27 g, 0.86 mmol) in dry hexane (8.6 mL) and catalytic amounts of quinoline. The reaction mixture was stirred under H2 for 24 h, filtered, and the solvent removed in vacuo. The product was purified under vacuum distillation (Kugelrohr) by removing impurities and quinoline at 130 °C/3 mmHg. The dioxolane 5 (0.23 g, 0.70 mmol) was obtained as colorless oil in an 82% yield.
IR (NaCl) νmax: 3005, 2924, 2854, 1456, 1408, 1212, 1140, 1040, 944, 723 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 5.45–5.39 (m, 4H, -CH=CH-), 4.85 (1H, t, H-2), 3.97–3.85 (4H, m, -OCH2-) 2.17 (2H, m), 2.09 (2H, m), 2.00 (2H, m), 1.71 (2H, m), 1.29 (16H, brs, -CH2-), 0.87 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 130.5 (d), 130.0 (d), 129.0 (d), 128.9 (d), 104.1 (d, C-2), 64.9 (t), 33.8 (t), 31.9 (t), 29.7 (t), 29.6 (t), 29.5 (5), 29.3 (t), 29.2 (t), 27.3 (t), 22.7 (t), 21.4 (t), 14.1 (q, -CH3); GC/MS (70 eV) m/z (relative intensity): 322 (M+, 1), 321 (1), 279 (3), 265 (1), 239 (1), 195 (4), 155 (6), 141 (32), 128 (22), 119 (8), 99 (73), 86 (35), 81 (18), 79 (29), 73 (100), 69 (15), 67 (23), 55 (22); HRMS (APCI) Calcd for C21H39O2 [M + H]+ 323.2945, found 323.2943.
3.2.4. (4Z,8Z)-Nonadeca-4,8-dienal (6)
To a stirred solution of 5 (0.20 g, 0.62 mmol) in acetone/water (1.6 mL each) was added 0.6 mL of HCl (conc.) followed by reflux at 60 °C for 24 h. Then, the reaction mixture was extracted with diethyl ether (1 × 15 mL), water (1 × 15 mL), dried over MgSO4, filtered, and evaporated in vacuo affording aldehyde 6 (0.11 g, 0.40 mmol) in 64% yield as colorless oil.
IR (NaCl) νmax: 3418, 3007, 2925, 2855, 1715 (C=O), 1464, 1377, 1258, 1051, 722 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 9.77 (1H, t, CHO), 5.43-5.35 (4H, m, -CH=CH-), 2.48 (2H, m), 2.37 (2H, m), 2.09 (4H, m), 2.02 (2H, m), 1.26 (16H, brs, -CH2-), 0.87 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 202.2 (d, C-2), 131.0 (d), 130.7 (d), 128.7 (d), 127.5 (d), 43.8 (t), 31.9 (t), 29.7 (t), 29.63 (t), 29.60 (t), 29.3 (t), 27.3 (t), 27.1 (t), 22.7 (t), 20.1 (t), 14.1 (q, -CH3); GC/MS (70 eV) m/z (relative intensity): 278 (M+, 1), 260 (1), 249 (1), 137 (11), 134 (11), 123 (10), 119 (13), 111 (13), 109 (14), 97 (90), 95 (29), 83 (69), 81 (36), 79 (74), 69 (100), 67 (69), 57 (40), 55 (94); HRMS (APCI) Calcd for C19H35O [M + H]+ 279.2682, found 279.2682.
3.2.5. (5Z,9Z)-(±)-2-Trimethylsilyloxy-5,9-eicosadienonitrile (7)
To a stirred solution of aldehyde 6 (0.11 g, 0.38 mmol) in dry CH2Cl2 (5.0 mL) at 0 °C was added trimethylsilyl cyanide (TMSCN) (0.8 mL, 0.57 mmol) and catalytic amounts of triethylamine. The mixture was stirred under argon for 3 h. Then, the solvent was removed in vacuo, the crude mixture washed with water (2 × 10 mL), extracted with diethyl ether (2 × 10 mL), dried over MgSO4, filtered, and after solvent removal 7 (0.12 g, 0.31 mmol) was obtained as a yellow oil in 82% yield.
IR (NaCl) νmax: 3800, 2925, 2854, 2251 (CN), 1655, 1437, 1254, 1078, 846, 722 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 5.45–5.31 (4H, m, -CH=CH-), 4.39 (1H, t, H-2), 2.22 (2H, m), 2.11 (4H, m), 2.00 (2H, m), 1.83 (2H, m), 1.25 (12H, brs, -CH2-), 0.87 (3H, t, -CH3), 0.19 (9H, s, -OSi(CH3)3); 13C (CDCl3, 75 MHz) δ (ppm) 131.4 (d), 130.7(d), 128.7 (d), 127.5 (d), 120.0 (s, C-1), 60.8 (d, C-2), 36.1 (t), 31.9 (t), 29.7 (t), 29.6 (t), 29.5 (t), 29.3 (t), 29.2 (t), 27.4 (t), 27.3 (t), 27.1 (t), 22.7 (t), 22.3 (t), 14.1 (q, -CH3), -0.39 (q, -OSi(CH3)3); GC/MS (70 eV) m/z (relative intensity): 377 (M+, 17), 362 (16), 350 (11), 349 (30), 286 (4), 234 (10), 208 (14), 160 (12), 155 (18), 142 (29), 128 (40), 119 (25), 116 (73), 106 (33), 97 (43), 83 (53), 81 (41), 80 (77), 79 (67), 75 (36), 73 (100), 69 (59), 67 (62), 57 (36), 55 (74); HRMS (APCI) Calcd for C23H44O2NSi [M + H]+ 378.3187, found 378.3187.
3.2.6. Methyl (5Z,9Z)-(±)-2-Hydroxy-5,9-eicosadienoate (8)
To a stirred solution of 7 (0.12 g, 0.34 mmol) in 2-MeTHF (5.1 mL) was added concentrated HCl (2.0 mL). The reaction mixture was stirred at 60 °C for 24 h. After this time, the crude was washed with water (2 × 10 mL), extracted with diethyl ether (2 × 10 mL), dried over MgSO4, filtered, and evaporated in vacuo. After purification by Kugelrohr distillation at 140 °C/3 mmHg, the (5Z,9Z)-(±)-2-hydroxy-5,9-eicosadienoic acid was obtained which was esterified in 20.0 mL of methanol by adding HCl (conc.), and stirring for 3 h at 35 °C. After methanol removal in vacuo, the crude was washed with water (2 × 10 mL), ether (2 × 10 mL), dried over MgSO4, filtered, and concentrated by evaporation in vacuo. The methyl ester was purified using silica gel column chromatography eluting with hexane/ether (7:3). Methyl ester 8 (0.06 g, 0.17 mmol) was obtained as colorless oil in a 49% overall yield for the last two steps.
IR (NaCl) νmax: 3501, 2926, 2855, 1740 (C=O), 1456, 1436, 1362, 1199, 1127, 807, 722 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 5.45–5.31 (4H, m, -CH=CH-), 4.19 (1H, m, H-2), 3.79 (3H, s, -OCH3), 2.19 (2H, m), 2.09 (4H, m), 2.01 (2H, m), 1.88 (2H, m), 1.27 (12H, br s, -CH2-), 0.87 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 175.8 (s, C-1), 130.7 (d), 130.5 (d), 128.9 (d), 128.3 (d), 69.9 (d, C-2), 52.5 (q, -OCH3), 31.9 (t), 29.6 (t), 29.5 (t), 29.3 (t), 29.0 (t), 27.3 (t), 25.3 (t), 22.7 (t), 14.1 (q, -CH3); GC/MS (70 eV) m/z (relative intensity): 338 (M+, 1), 322 (1), 307 (1), 295 (1), 279 (3), 261 (6), 213 (6), 165 (11), 157 (13), 139 (13), 123 (11), 109 (20), 97 (100), 90 (74), 83 (32), 81 (40), 79 (65), 69 (48), 67 (51), 57 (21), 55 (54); HRMS (APCI) Calcd for C21H39O3 [M + H]+ 339.2894, found 339.2897.
3.2.7. (5Z,9Z)-(±)-2-Methoxy-5,9-eicosadienoic acid (1)
To a stirred solution of NaH (0.01 g, 0.50 mmol) in dry THF (3.0 mL) under argon was added a solution of 8 (0.05 g, 0.14 mmol) in dry THF (3.0 mL). The reaction mixture was stirred at rt for 10 min, and then methyl iodide (0.03 mL, 0.48 mmol) was added dropwise at 0 °C, followed by 3 h stirring. After that, HCl (conc.) was added to the solution until the pH was acidic. The crude was extracted with diethyl ether (2 × 10 mL), dried over MgSO4 and evaporated in vacuo. The product was purified using silica gel column chromatography first eluting with hexane/ether (9:1) and then with ether affording the methyl (5Z,9Z)-(±)-2-methoxy-5,9-eicosadienoate. To obtain 1, a solution of KOH/ethanol (1 M) (20.0 mL) and the methoxylated methyl ester was stirred for 2 h at 60 °C. After this time the solvent was removed in vacuo and hexane (5.0 mL) and 5.0 mL of 6M HCl was added to the solution. The crude product was washed with water (1 × 10 mL), diethyl ether (2 × 10 mL), dried over MgSO4, filtered, and the ether evaporated in vacuo. The final product was purified using Florisil® (activated magnesium silicate) column chromatography eluting with diethyl ether affording 1 (0.032 g, 0.09 mmol) as an oil for a 67% yield.
IR (NaCl) νmax: 3500–2500 (-OH), 3006, 2925, 2854, 1706 (C=O), 1463, 1378, 1200, 1125, 722 cm−1; 1H (CDCl3, 500 MHz) δ (ppm) 5.45–5.31 (4H, m, -CH=CH-), 3.81 (1H, t, H-2), 3.44 (3H, s, -OCH3), 2.21 (2H, m), 2.10 (4H, m), 2.01 (2H, m), 1.27 (16H, brs, -CH2-), 0.87 (3H, t, -CH3); 13C (CDCl3, 125 MHz) δ (ppm) 177.3 (s, C-1), 130.9 (d, C-10), 130.6 (d, C-6), 128.9 (d, C-9), 128.1 (d, C-5), 79.5 (d, C-2), 58.3 (q, -OCH3), 31.9 (t), 29.7 (t), 29.69 (t), 29.64 (t), 29.5 (t), 29.3 (t), 27.3 (t), 27.29 (t), 27.24 (t), 22.7 (t), 22.6 (t), 14.1 (q, -CH3); HRMS (APCI) Calcd for C21H37O3 [M + H]+ 337.2748, found 337.2743.
3.3. Synthesis of (±)-2-Methoxy-5,9-eicosadiynoic Acid (2)
3.3.1. Nonadeca-4,8-diynal (9)
Aldehyde
9 was obtained as a white solid (mp 57–60 °C) in an 85% yield by refluxing dioxolane
4 (0.51 g, 1.60 mmol) in acetone/water (4.0 mL each) with HCl (conc) (1.60 mL) following the procedure outlined in
Section 3.2.4.
IR (NaCl) νmax: 3311, 2954, 2924, 2852, 1691 (C=O), 1436, 1258, 1214, 917, 803, 723, 634 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 9.78 (1H, t, CHO), 2.62 (2H, dt, H-3), 2.48 (2H, t, H-11), 2.31 (4H, m), 2.13 (2H, t, H-4), 1.46 (2H, m), 1.27 (14H, brs, -CH2-), 0.89 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 201.0 (d, C-2), 81.3 (s), 80.1 (s), 78.7 (s), 78.5 (s), 42.8 (t), 31.9 (t), 29.58 (t), 29.55 (t), 29.3 (t), 29.2 (t), 29.0 (t), 28.8 (t), 22.7 (t), 19.4 (t), 19.3 (t), 18.7 (t), 14.1 (q, -CH3), 12.1 (t, C-4); GC/MS (70 eV) m/z (relative intensity): 274 (M+, 1), 273 (3), 259 (1), 217 (2), 175 (25), 161 (32), 148 (25), 147 (63), 133 (61), 131 (25), 119 (64), 106 (29), 105 (100), 95 (41), 91 (94), 81 (34), 79 (51), 67 (58), 55 (41); HRMS (APCI) Calcd for C19H31O [M + H]+ 275.2369, found 275.2369.
3.3.2. 2-(±)-Trimethylsilyloxy-5,9-eicosadiynonitrile (10)
Nitrile
10 was obtained as yellow oil and in a 94% yield from the reaction of
9 (0.31 g, 1.12 g) with TMSCN (0.22 mL, 1.65 mmol) and catalytic amounts of Et
3N in dry CH
2Cl
2 (10.0 mL) following the procedure outlined in
Section 3.2.5. The product was used as such for the next step without further purification.
IR (NaCl) νmax: 2926, 2855, 2256 (CN), 1713, 1444, 1378, 1340, 1258, 1102, 1044, 944, 878, 722, 633 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 4.62 (1H, m, H-2), 2.32 (4H, m), 2.18 (2H, t), 1.95 (2H, m), 1.45 (2H, m), 1.25 (10H, brs, -CH2-), 0.89 (3H, t), 0.21 (9H, s, -OSi(CH3)3); 13C (CDCl3, 75 MHz) δ (ppm) 119.9 (s, C-1), 81.4 (s), 80.7 (s), 78.4 (s), 78.2 (s), 65.0 (d, C-2), 35.2 (t, C-3), 31.9 (t), 29.6 (t), 29.5 (t), 29.3 (t), 29.1 (t), 29.0 (t), 28.8 (t), 28.7 (t), 28.2 (t), 22.7 (t), 19.34 (t), 19.29 (t), 18.7 (t), 14.3 (q, -CH3), 14.1 (t, C-4), -0.49 (q, OSi(CH3)3); GC/MS (70 eV) m/z (relative intensity): 373 (M+, 6), 372 (8), 358 (29), 329 (10), 316 (9), 288 (24), 284 (15), 282 (32), 274 (30), 260 (26), 247 (38), 246 (62), 232 (29), 205 (16), 198 (21), 184 (28), 171 (15), 169 (24), 157 (32), 131 (22), 128 (34), 117 (25), 91 (34), 84 (79), 75 (42), 73 (100), 57 (57), 55 (44).
3.3.3. Methyl (±)-2-Hydroxy-5,9-eicosadiynoate (11)
The methyl hydroxy ester
11 was obtained as colorless oil (61% yield for the two steps) from the reaction of
10 (0.39 g, 1.05 mmol) with concentrated HCl (6.2 mL) in 2-MeTHF (15.4 mL) followed by methanol esterification as detailed in
Section 3.2.6.
IR (NaCl) νmax: 3501, 2926, 2855, 1740 (C=O), 1440, 1339, 1258, 1219, 1104, 998, 722, 667 cm−1; 1H (CDCl3, 300 MHz) δ (ppm) 4.32 (1H, m, H-2), 3.78 (3H, s, -CO2CH3), 2.31 (4H, m), 2.12 (2H, t), 1.97 (2H, m), 1.78 (2H, m), 1.44 (2H, m), 1.24 (14H, m, -CH2-), 0.96 (3H, t, -CH3); 13C (CDCl3, 75 MHz) δ (ppm) 175.4 (s), 81.3 (s), 79.9 (s), 79.3 (s), 78.5 (s), 69.2 (d, C-2), 52.6 (q, -OCH3), 33.4 (t, C-3), 31.9 (t), 29.6 (t), 29.5 (t), 29.3 (t), 29.1 (t), 28.96 (t), 28.8 (t), 22.7 (t), 19.5 (t), 19.4 (t), 18.7 (t), 14.6 (q, -CH3), 14.1 (t, C-4); GC/MS (70 eV) m/z (relative intensity): 334 (M+, 1), 291 (2), 275 (25), 257 (1), 245 (3), 235 (2), 221 (7), 208 (17), 195 (4), 175 (6), 161 (12), 155 (51), 147 (19), 133 (33), 121 (25), 119 (53), 105 (24), 95 (65), 93 (40), 91 (100), 90 (53), 81 (40), 79 (76), 77 (46), 67 (84), 55 (95); HRMS (APCI) Calcd for C21H35O3 [M + H]+ 335.2581, found 335.2579.
3.3.4. (±)-2-Methoxy-5,9-eicosadiynoic acid (2)
The diynoic acid
2 was obtained as an oil in a 55% yield (for the two steps) in the reaction of
11 (0.05 g, 0.16 mmol), with NaH (0.01 g, 0.54 mmol) and methyl iodide (0.03 mL, 13.01 mmol) in dry THF (4.0 mL) followed by saponification with KOH/ethanol (1M) as described in
Section 3.2.7.
IR (NaCl) νmax: 3500–2500 (-OH), 3310, 2926, 2855, 1723, 1443, 1258, 1207, 1119, 722, 637 cm−1; 1H (CDCl3, 500 MHz) δ (ppm) 3.98 (1H, m, H-2), 3.47 (3H, s, -OCH3), 2.33 (4H, m), 2.12 (2H, t), 1.97 (2H, m), 1.89 (2H, m), 1.46 (2H, m), 1.26 (14H, brs, -CH2-), 0.86 (3H, t, -CH3); 13C (CDCl3, 125 MHz) δ (ppm) 176.8 (s, C-1), 81.3 (s), 80.2 (s), 79.0 (d, C-2), 78. 6 (s), 78.5 (s), 58.7 (q, -OCH3), 31.9 (t), 29.6 (t), 29.5 (t), 29.3 (t), 29.2 (t), 29.0 (t), 28.8 (t), 22.7 (t), 19.43 (t), 19.38 (t), 18.7 (t), 14.7 (q, -CH3), 14.1 (t); HRMS (APCI) Calcd for C21H33O3 [M + H]+ 333.2435 found 333.2431.