Study of Copper and Purine-Copper Complexes on Modified Carbon Electrodes by Cyclic and Elimination Voltammetry
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2 Instrumentation
2.3 Preparation of graphite electrodes
2.4. Methods
2.4.1 Elimination voltammetry with linear scan
- a)
- A total voltammetric current I can be given as a sum of particular currents Ij:where Id, Ic, and Ik are the diffusion, charging, and kinetic currents, respectively;
- b)
- A particular current Ij can be expressed as a product of two functions—potential function Yj(E) and scan rate function Wj(v):Based on the dependence of partial current on scan rate, Equation (2) can be rewritten:
2.4.2 Confidence ellipse
2.4.3 Medusa
3. Results and Discussion
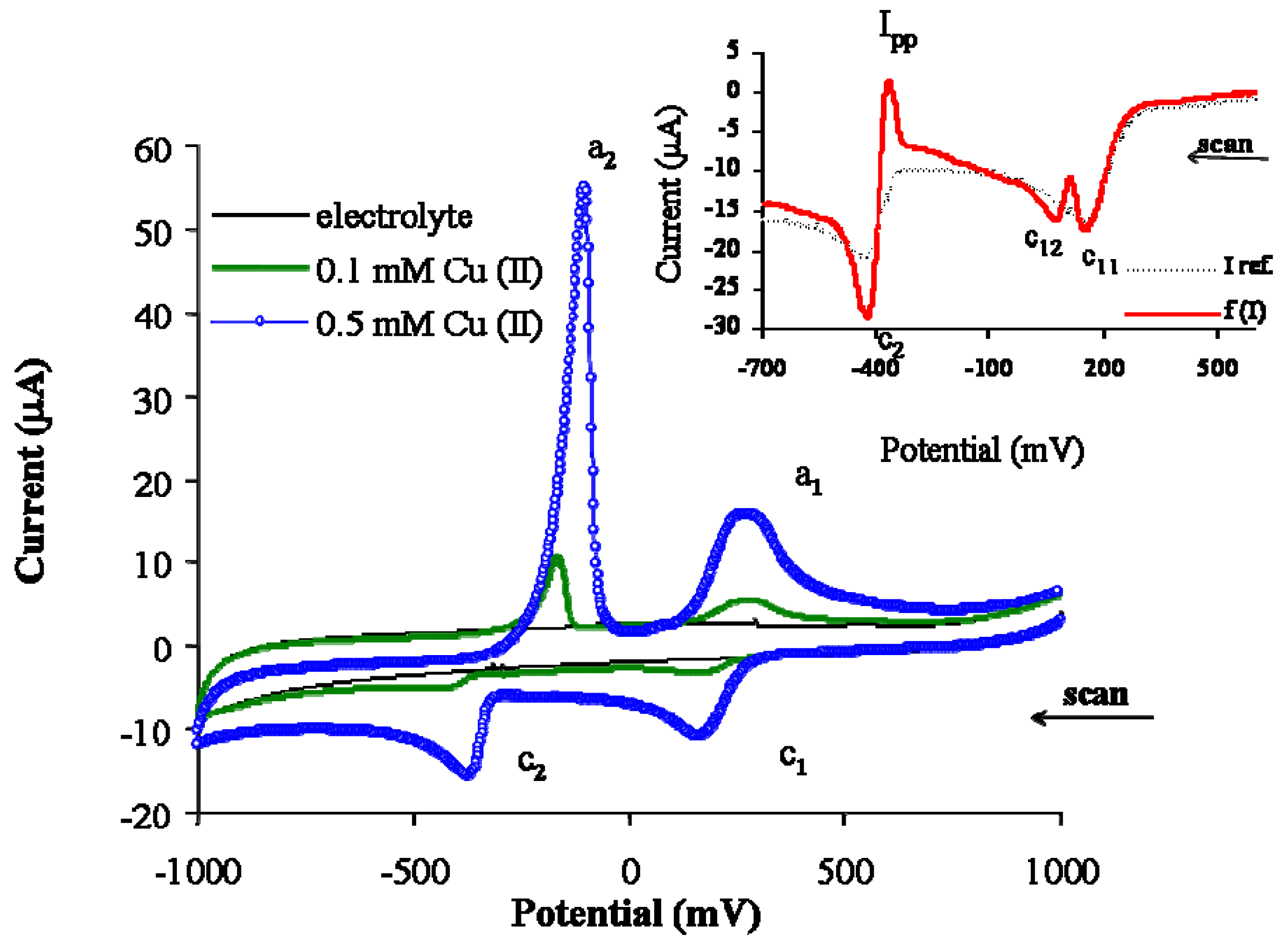
3.1. Electrode process of copper ions on PIGE
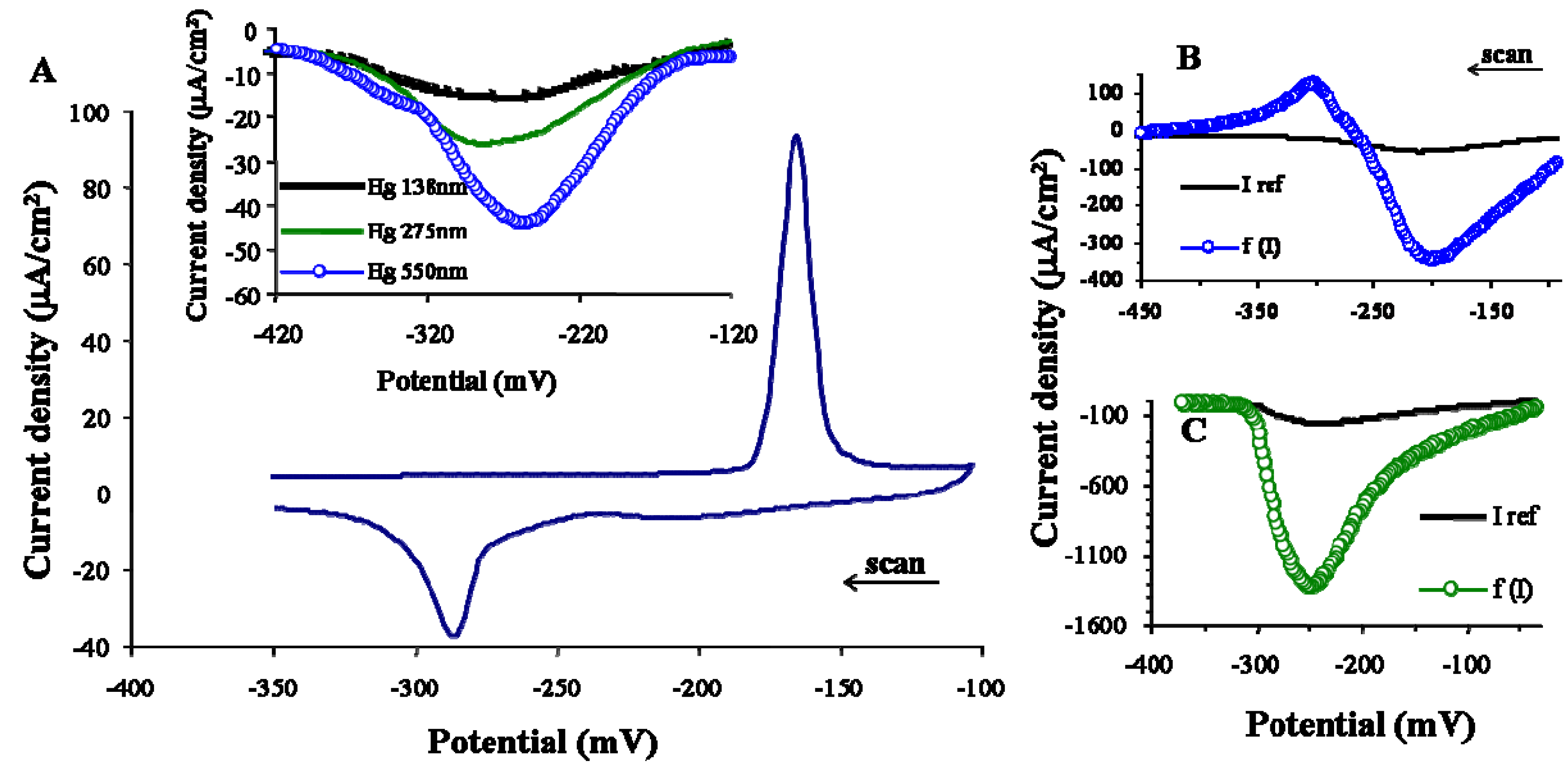
3.2. Electrode process of copper ions on Hg-PGEb
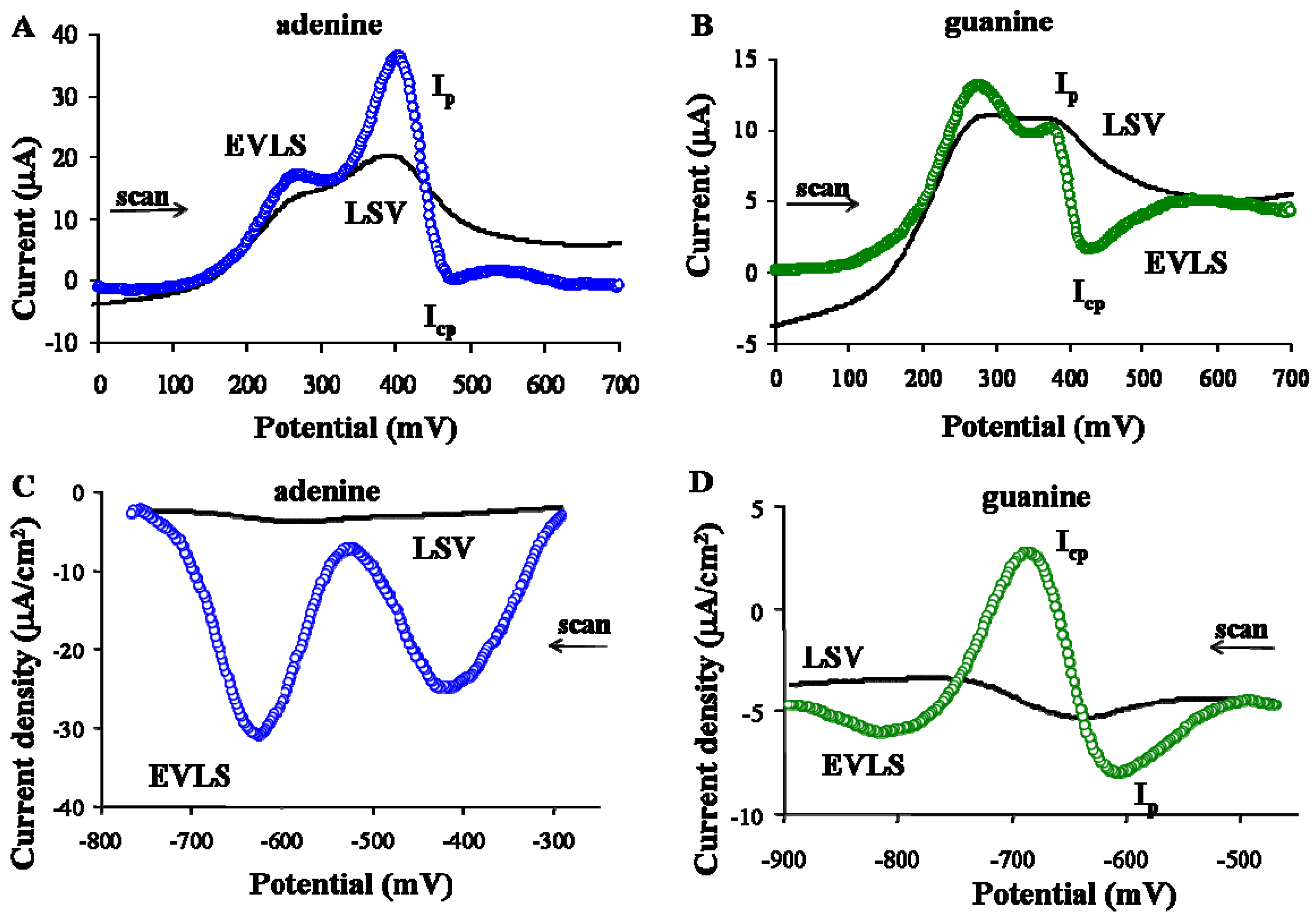
3.3. Detection of purine-copper complexes on PIGE and Hg-PGEb
3.4. Statistical evaluation of experiments on PIGE and Hg-PGEb
4. Conclusion
Acknowledgments
References
- Palecek, E.; Jelen, F. Electrochemistry of Nucleic Acids; Elsevier: Amsterdam, 2005; pp. 74–173. [Google Scholar]
- Fadrna, R.; Yosypchuk, B.; Fojta, M.; Navratil, T.; Novotny, L. Voltammetric determination of adenine, guanine, and DNA using liquid mercury free polished silver solid amalgam electrode. Anal. Lett. 2004, 37, 399–413. [Google Scholar]
- Farias, P.A.M.; Wagener, A.D.; Castro, A.A. Ultratrace determination of adenine in the presence of copper by adsorptive stripping voltammetry. Talanta 2001, 55, 281–290. [Google Scholar]
- Hason, S.; Jelen, F.; Fojt, L.; Vetterl, V. Determination of picogram quantities of oligodeoxynucleotides by stripping voltammetry at mercury modified graphite electrode surfaces. J. Electroanal. Chem. 2005, 577, 263–272. [Google Scholar]
- Jelen, F.; Kourilova, A.; Pecinka, P.; Palecek, E. Microanalysis of DNA by stripping transfer voltammetry. Bioelectrochemistry 2004, 63, 249–252. [Google Scholar]
- Wang, J.; Kawde, A.B. Amplified label-free electrical detection of DNA hybridization. Analyst 2002, 127, 383–386. [Google Scholar]
- dosSantos, M.M.C.; Lopes, C.M.L.F.; Goncalves, M.L.S. Voltammetric studies of purine bases and purine nucleosides with copper. Bioelectrochem. Bioenerg. 1996, 39, 55–60. [Google Scholar]
- Glodowski, S.; Bilewicz, R.; Kublik, Z. Determination of traces of purine by cathodic stripping voltammetry at the hanging copper amalgam drop. Anal. Chim. Acta 1986, 186, 39–47. [Google Scholar]
- McCreery, R.L.; K., C.K. Carbon Electrodes; Marcel Dekker, 1996; pp. 293–332. [Google Scholar]
- Cui, H.; Zou, G.Z.; Lin, X.Q. Electrochemiluminescence of luminol in alkaline solution at a paraffin-impregnated graphite electrode. Anal. Chem. 2003, 75, 324–331. [Google Scholar]
- Gong, J.M.; Lin, X.G. Facilitated electron transfer of hemoglobin embedded in nanosized Fe3O4 matrix based on paraffin impregnated graphite electrode and electrochemical catalysis for trichloroacetic acid. Microchem. J. 2003, 75, 51–57. [Google Scholar]
- Komorsky-Lovric, S. Redox kinetics of adriamycin adsorbed on the surface of graphite and mercury electrodes. Bioelectrochemistry 2006, 69, 82–87. [Google Scholar]
- Walcarius, A. Zeolite-modified paraffin-impregnated graphite electrode. J. Solid State Electrochem. 2006, 10, 469–478. [Google Scholar]
- Orinakova, R.; Streckova, M.; Trnkova, L.; Rozik, R.; Galova, M. Comparison of chloride and sulphate electrolytes in nickel electrodeposition on a paraffin impregnated graphite electrode. J. Electroanal. Chem. 2006, 594, 152–159. [Google Scholar]
- Orinakova, R.; Trnkova, L.; Galova, M.; Supicova, M. Application of elimination voltammetry in the study of electroplating processes on the graphite electrode. Electrochim. Acta 2004, 49, 3587–3594. [Google Scholar]
- Rozik, R.; Trnkova, L. Cadmium reduction process on paraffin impregnated graphite electrode studied by elimination voltammetry with linear scan. J. Electroanal. Chem. 2006, 593, 247–257. [Google Scholar]
- Streckova, M.; Orinakova, R.; Rozik, R.; Trnkova, L.; Galova, M. A study of nickel electrodeposition on paraffin-impregnated graphite electrode. Helvetica Chim. Acta 2006, 89, 622–634. [Google Scholar]
- McCreery, R.L. Electron Transfer Kinetics at Carbon Electrodes; Marcel Dekker, 1991; pp. 221–374. [Google Scholar]
- Dryhurst, G.; Elving, P.J. Electrochemical Oxidation of Adenine - Reaction Products and Mechanisms. J. Electrochem. Soc. 1968, 115, 1014–1020. [Google Scholar]
- Goyal, R.N.; Brajtertoth, A.; Dryhurst, G. Further Insights into the Electrochemical Oxidation of Uric-Acid. J. Electroanal. Chem. 1982, 131, 181–202. [Google Scholar]
- Goyal, R.N.; Rajeshwari, I.; Mathur, N.C. Electrochemical Oxidation of Uric-Acid and 6-Thiouric Acid. Bioelectrochem. Bioenerg. 1990, 24, 355–360. [Google Scholar]
- Ibrahim, M.S.; Temerk, Y.M.; Kamal, M.M.; Ahmed, G.A.W.; Ibrahim, H.S.M. Ultra-sensitive anodic stripping voltammetry for the determination of xanthine at a glassy carbon electrode. Microchim. Acta 2004, 144, 249–256. [Google Scholar]
- Kachoosangi, R.T.; Banks, C.E.; Compton, R.G. Simultaneous determination of uric acid and ascorbic acid using edge plane pyrolytic graphite electrodes. Electroanalysis 2006, 18, 741–747. [Google Scholar]
- Shiraishi, H.; Takahashi, R. Accumulation of Adenine and Guanine as Cu+ Compounds at Glassy-Carbon Electrodes Followed by Anodic-Stripping Voltammetry. Bioelectrochem. Bioenerg. 1993, 31, 203–213. [Google Scholar]
- Safavi, A.; Maleki, N.; Shams, E.; Shahbaazi, H.R. Determinaton of copper by adsorptive stripping voltammetry of its complex with adenine. Electroanalysis 2002, 14, 929–934. [Google Scholar]
- Farias, P.A.M.; Wagener, A.D.R.; Bastos, M.B.R.; da Silva, A.T.; Castro, A.A. Cathodic adsorptive stripping voltammetric behaviour of guanine in the presence of copper at the static mercury drop electrode. Talanta 2003, 61, 829–835. [Google Scholar]
- Farias, P.A.M.; Castro, A.A.; Wagener, A.D.R.; Junqueira, A.A. DNA determination in the presence of copper in diluted alkaline electrolyte by adsorptive stripping voltammetry at the mercury film electrode. Electroanalysis 2007, 19, 1207–1212. [Google Scholar]
- Jelen, F.; Yosypchuk, B.; Kourilova, A.; Novotny, L.; Palecek, E. Label-Free Determination of Picogram Quantities of DNA by Stripping Voltammetry with Solid Copper Amalgam or Mercury Electrodes in the Presence of Copper. Anal. Chem. 2002, 74, 4788–4793. [Google Scholar]
- Trnkova, L.; Dracka, O. Elimination voltammetry. Experimental verification and extension of theoretical results. J. Electroanal. Chem. 1996, 413, 123–129. [Google Scholar]
- Palecek, E. Oszillographische Polarographie der Nucleinsauren und ihrer Bestandteile. Naturwiss. 1958, 45, 186–187. [Google Scholar]
- Trnkova, L.; Jelen, F.; Postbieglova, I. Application of elimination voltammetry to the resolution of adenine and cytosine signals in oligonucleotides. I. Homooligodeoxynucleotides dA(9) and dC(9). Electroanalysis 2003, 15, 1529–1535. [Google Scholar]
- Trnkova, L.; Jelen, F.; Postbieglova, I. Application of elimination voltammetry to the resolution of adenine and cytosine signals in oligonucleotides II. Hetero-oligodeoxynucleotides with different sequences of adenine and cytosine nucleotides. Electroanalysis 2006, 18, 662–669. [Google Scholar]
- Galus, Z. Fundamentals of Electrochemical analysis; Polish Scientific Publisher PWN, 1994. [Google Scholar]
- Scholz, F. Electroanalytical Methods, Guide to Experiment and Applications; Springer: Heidelberg, Germany, 2002. [Google Scholar]
- Scholz, F.; Meyer, B. Electrochemical solid state analysis: state of the art. Chem. Soc. Rev. 1994, 23, 341–347. [Google Scholar]
- Dracka, O. Theory of current elimination in linear scan voltammetry. J. Electroanal. Chem. 1996, 402, 19–28. [Google Scholar]
- Supicova, M.; Rozik, R.; Trnkova, L.; Orinakova, R.; Galova, M. Influence of boric acid on the electrochemical deposition of Ni. J. Solid State Electrochem. 2005, 10, 61–68. [Google Scholar]
- Trnkova, L. Elektrochemické eliminační metody. Chem. Listy 2001, 95, 518–527. [Google Scholar]
- Trnkova, L. Identification of current nature by elimination voltammetry with linear scan. J. Electroanal. Chem. 2005, 582, 258–266. [Google Scholar]
- Trnkova, L.; Friml, J.; Dracka, O. Elimination voltammetry of adenine and cytosine mixtures. Bioelectrochem. 2001, 54, 131–136. [Google Scholar]
- Trnkova, L.; Kizek, R.; Dracka, O. Application of elimination voltammetry to adsorptive stripping of DNA. Electroanalysis 2000, 12, 905–911. [Google Scholar]
- Trnkova, L.; Kizek, R.; Dracka, O. Appplication of elimination methods in electrochemical study of DNA. J. Biomol. Struct. Dynam. 2000, 17, 1169–70. [Google Scholar]
- Trnkova, L.; Kizek, R.; Dracka, O. Elimination voltammetry of nucleic acids on silver electrodes. Bioelectrochem. 2002, 55, 131–133. [Google Scholar]
- Trnkova, L.; Postbieglova, I.; Holik, M. Electroanalytical determination of d(GCGAAGC) hairpin. Bioelectrochem. 2004, 63, 25–30. [Google Scholar]
- Mikelova, R.; Trnkova, L.; Jelen, F.; Adam, V.; Kizek, R. Resolution of overlapped reduction signals in short hetero-oligonucleotides by elimination voltammetry. Electroanalysis 2007, 19, 348–355. [Google Scholar]
- Holik, M.; Halamek, J. Transformation of a Free-Wilson Matrix into Fourier Coefficients. Quant. Struct.-Act. Relat. 2002, 20, 422–428. [Google Scholar]
- Souto, R.M.; Saakes, M.; Sluyters-Rehbach, M.; Sluyters, J.H. The catalysis of the electrochemical reduction of camium ions by chloride ions. J. Electroanal. Chem. 1988, 245, 167–189. [Google Scholar]
- Grujicic, D.; Pesic, B. Reaction and nucleation mechanisms of copper electrodeposition from ammoniacal solutions on vitreous carbon. Electrochim. Acta. 2005, 50, 4426–4443. [Google Scholar]
- Jagner, D.; Sahlin, E.; Renman, L. Experimental and Computational Study of Species Formed During Electrochemical Stripping Oxidation of Copper in Chloride Media - Determination of Copper(Ii) in the Ng 1(-1) Range by Stripping Potentiometry. Talanta 1995, 42, 1447–1455. [Google Scholar]
- Hason, S.; Vetterl, V. Amplified oligonucleotide sensing in microliter volumes containing copper ions by solution streaming. Anal. Chem. 2006, 78, 5179–5183. [Google Scholar]
- Hason, S.; Vetterl, V. Microanalysis of oligodeoxynucleotides by cathodic stripping voltammetry at amalgam-alloy surfaces in the presence of copper ions. Talanta 2006, 69, 572–580. [Google Scholar]
- Jelen, F.; Hason, S.; Trnkova, L. Microanalysis of nucleic acids bases and oligonucleotides by electrochemical stripping techniques; Research Signpost, 2007; pp. 153–171. [Google Scholar]
- Holik, M.; Halamek, J. Calculation of the tilts of curved lines. Chemometrics Intell. Lab. Syst. 2003, 1382, 1–9. [Google Scholar]
- Bilewicz, R.; Glodowski, S.; Kublik, Z. The influence of adenine on the electrochemical behaviour of the Cu/II/ / Cu/0/ system. J. Electroanal. Chem. 1989, 274, 201–212. [Google Scholar]
- Bilewicz, R.; Muszalska, E. Voltammetric behaviour of copper complexes with the antitumour drug 6-mercaptopurine. J. Electroanal. Chem. 1991, 300, 147–157. [Google Scholar]
- Palecek, E.; Fojta, M. Detecting DNA hybridization and damage. Anal. Chem. 2001, 73, 74A–83A. [Google Scholar]
- Thorp, H.H. Cutting out the middleman: DNA biosensors based on electrochemical oxidation. Trends Biotechnol. 1998, 16, 117–121. [Google Scholar]
- Thorp, H.H. Reagentless detection of DNA sequences on chemically modified electrodes. Trends Biotechnol. 2003, 21, 522–524. [Google Scholar]
- Wang, J. Towards genoelectronics: Electrochemical biosensing of DNA hybridization. Chem.- Eur. J. 1999, 5, 1681–1685. [Google Scholar]


© 2008 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Trnkova, L.; Zerzankova, L.; Dycka, F.; Mikelova, R.; Jelen, F. Study of Copper and Purine-Copper Complexes on Modified Carbon Electrodes by Cyclic and Elimination Voltammetry. Sensors 2008, 8, 429-444. https://doi.org/10.3390/s8010429
Trnkova L, Zerzankova L, Dycka F, Mikelova R, Jelen F. Study of Copper and Purine-Copper Complexes on Modified Carbon Electrodes by Cyclic and Elimination Voltammetry. Sensors. 2008; 8(1):429-444. https://doi.org/10.3390/s8010429
Chicago/Turabian StyleTrnkova, Libuse, Lenka Zerzankova, Filip Dycka, Radka Mikelova, and Frantisek Jelen. 2008. "Study of Copper and Purine-Copper Complexes on Modified Carbon Electrodes by Cyclic and Elimination Voltammetry" Sensors 8, no. 1: 429-444. https://doi.org/10.3390/s8010429



