A New Composition for Co(II)-porphyrin-based Membranes Used in Thiocyanate-selective Electrodes
Abstract
:1. Introduction
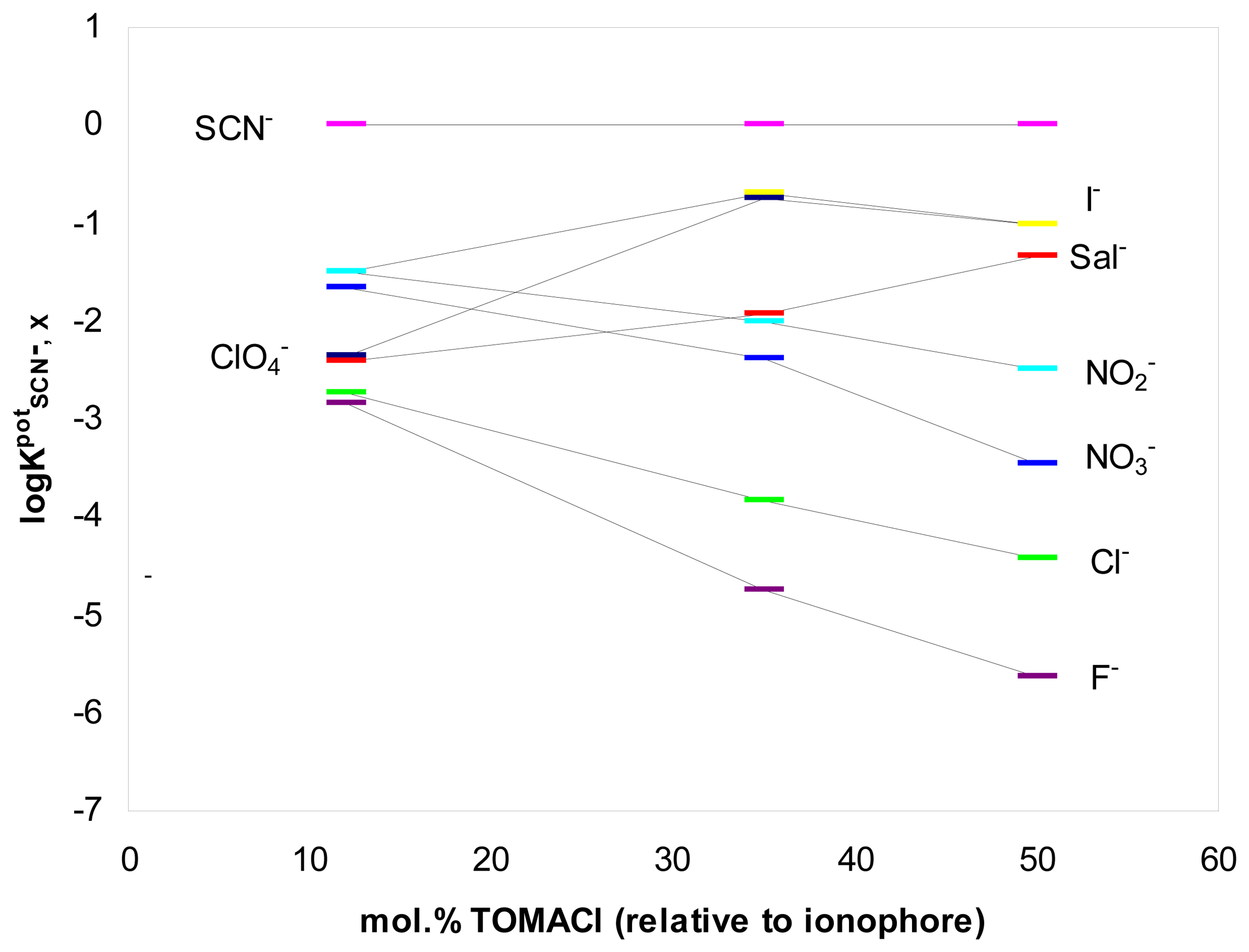
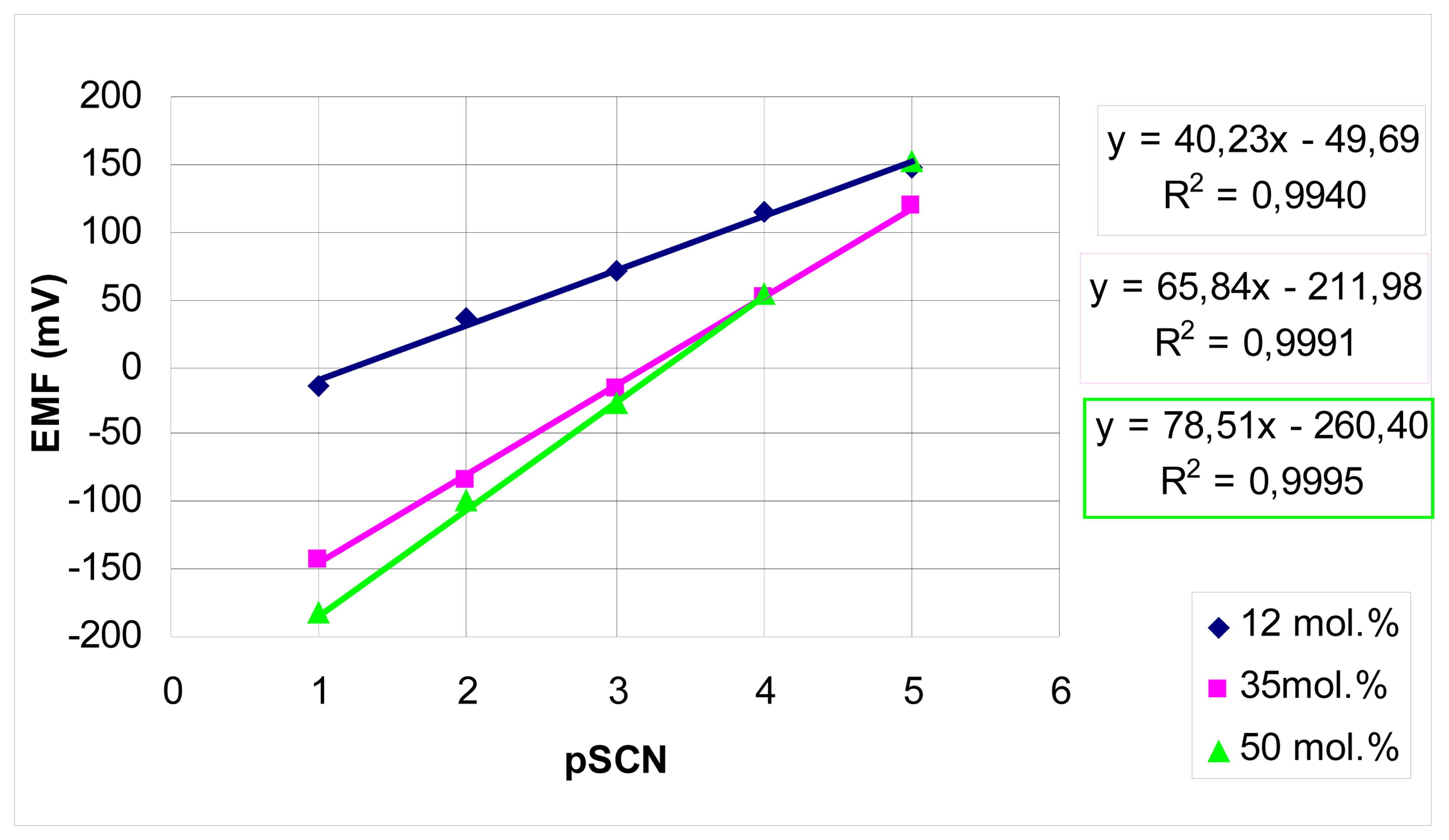
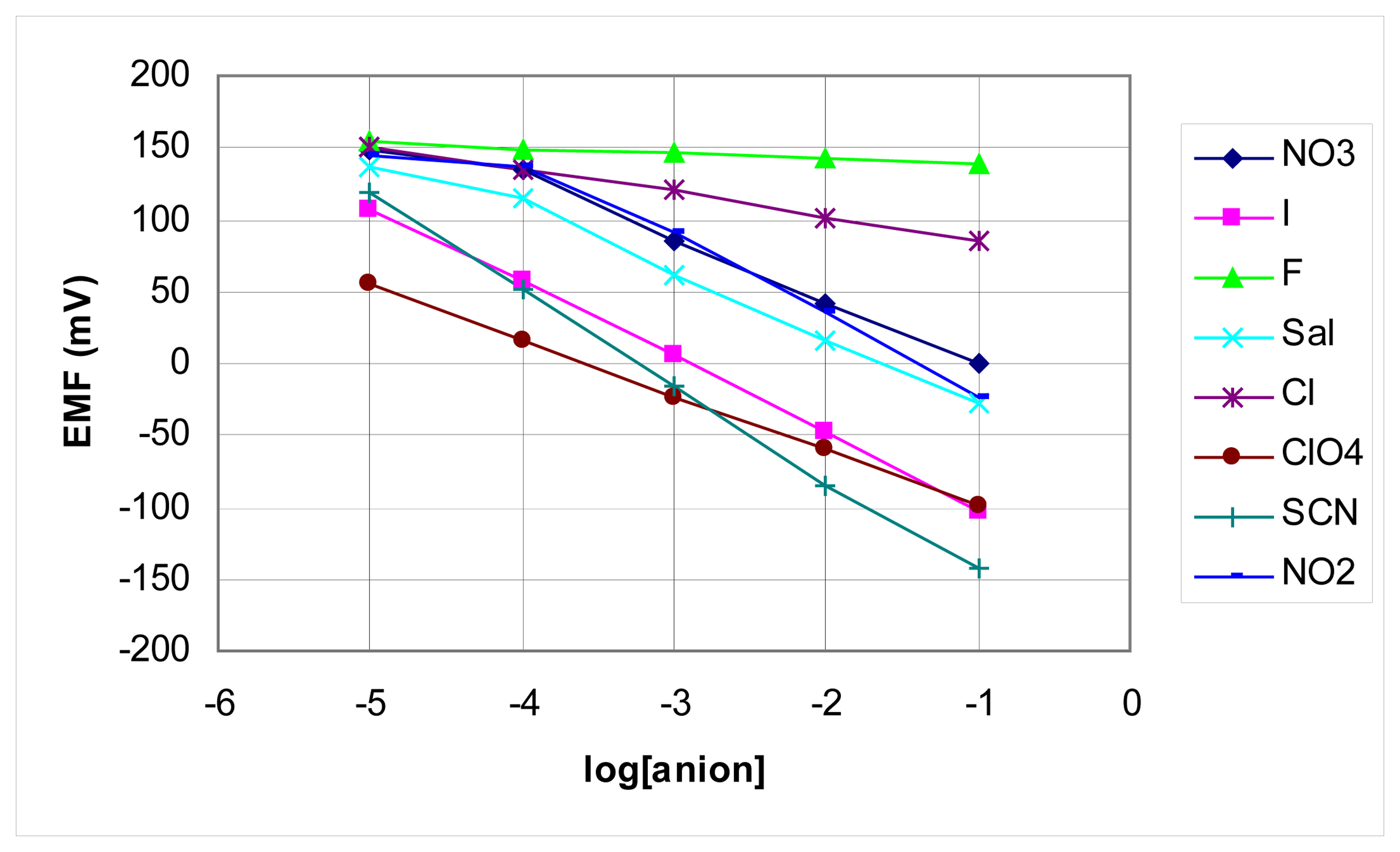
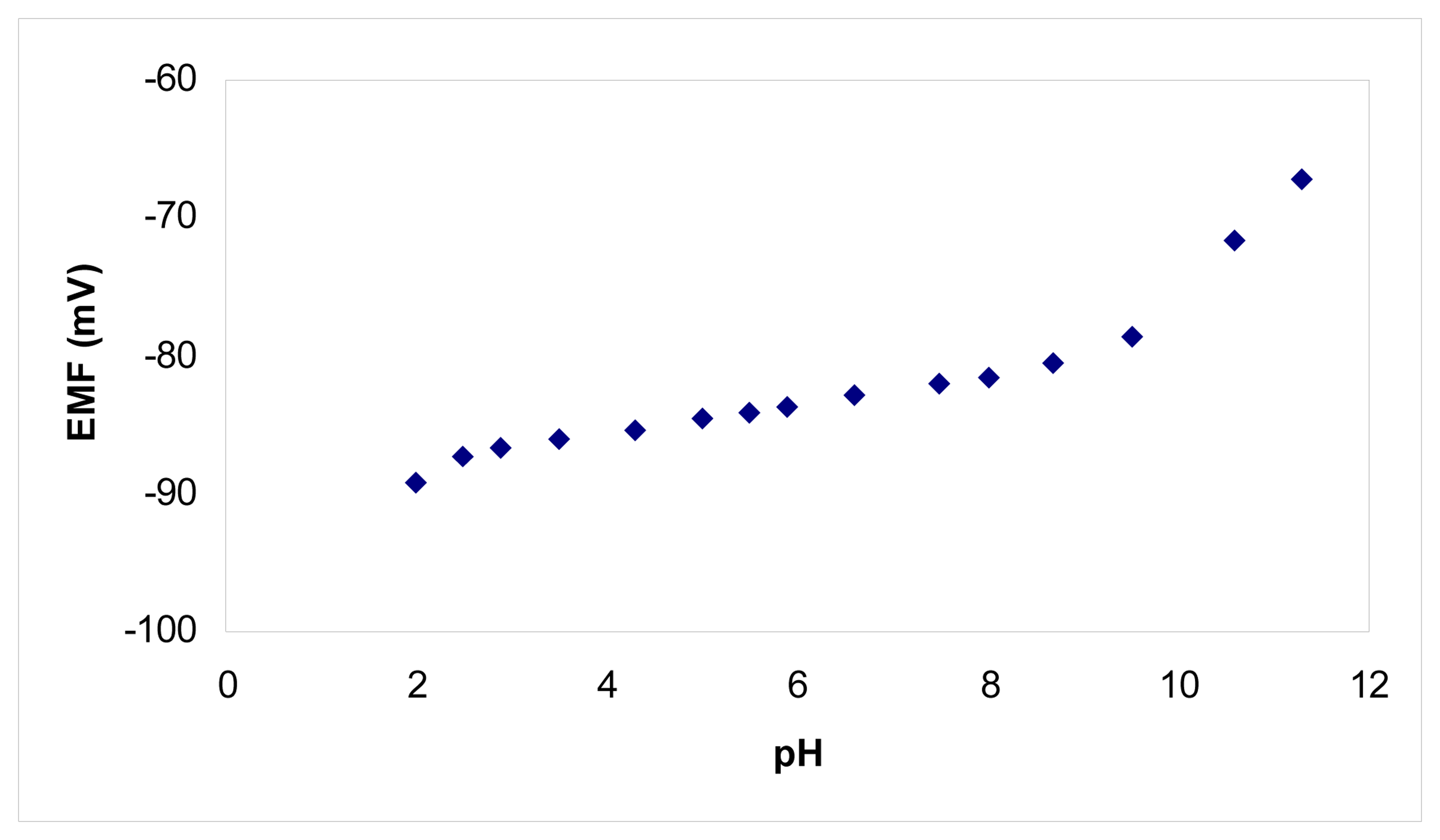
2. Results and Discussion
Analytical applications
3. Experimental Section
3.1. Reagents
3.2. ISE membrane formulation and EMF measurements. Apparatus
3.3. Method of synthesis for the meso-tetra(p-methoxyphenyl)-porphyrine
3.4. Method of synthesis for 5,10,15,20-tetrakis-(4-methoxyphenyl)-porphyrin-Co(II) (CoTMeOPP)
4. Conclusion
References and Notes
- Malinowska, E.; Meyerhoff, M.E. Role of axial ligation on potentiometric response of Co(III) tetraphenylporphyrin-doped polymeric membrane to nitrite ion. Anal. Chim. Acta. 1995, 300, 33–43. [Google Scholar]
- Bakker, E.; Pretsch, E. Lipophilicity of tetraphenylborate derivatives as anionic sites in neutral carrier-based solvent polymeric membranes of corresponding ion-selective electrochemical and optical sensors. Anal. Chim. Acta. 1995, 309, 7–17. [Google Scholar]
- Chaniotakis, N.A.; Chasser, A.M.; Meyerhoff, M.E.; Groves, J.T. Influence of porphyrin structure on anion selectivities of manganese (III) porphyrin-based membrane electrodes. Anal. Chem. 1988, 60, 185–188. [Google Scholar]
- Shamsipur, M.; Khayatian, G.; Tangestaninejad, S. Thiocyanate-selective membrane electrode based on (octabromotetraphenylporphyinato) manganese(III) chloride. Electroanalysis 1999, 11, 1340–1348. [Google Scholar]
- Khorasani, J.H.; Amini, M.K.; Mataghi, H.; Tangestaninejad, S.; Moghadam, M. Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: the influence of porphyrin substituents and additives on the response properties. Sensors and Acuators B. 2002, 87, 448–456. [Google Scholar]
- Bakker, E.; Malinowska, E.; Schiller, R.D.; Meyerhoff, M.E. Anion-selective membrane electrodes based on metalloporphyrins. The influence of lipophilic anionic and cationic sites on potentiometric selectivity. Talanta 1994, 41, 881–890. [Google Scholar]
- Gupta, Vinod K.; Chandra, S.; Chauhan, D.K.; Mangla, R. Membranes of 5,10,15,20-Tetrakis(4-Methoxyphenyl) Porphyrinatocobalt (TMOPP-Co) (I) as MoO42- - Selective. Sensors 2002, 2, 164–173. [Google Scholar]
- Heliövaara, M.; et al. Serum thiocyanate concentration and cigarette smoking in relation to overall mortality and to deaths from coronary heart disease and lung cancer. J. Chron. Dis. 1981, 34, 305–311. [Google Scholar]
- Meberg, A.; Marstein, S. Smoking During Pregnancy: Effects on the Fetal Thyroid Function. Acta Paediatrica Scandinavica 1986, 75(5), 762–766. [Google Scholar]
- Vlascici, D.; Spiridon-Bizerea, O.; F ăg ădar-Cosma, E. Thiocyanate-selective electrode based on rhodium porphyrin derivates. J. Optoelectron. Adv. Mat. 2006, 8(2), 883–887. [Google Scholar]
- Huang, X.; Chai, Y.; Yuan, R.; Wang, X.; Li, Q. Highly selective thiocyanate electrode based on bis-[N-(2hydroxyethyl)salicylaldimino]copper(II) complex as a neutral carrier. Anal. Sci. 2004, 20, 1185–1188. [Google Scholar]
- Umezawa, Y.; Buhlmann, P.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric selectivity coefficients of ion-selective electrodes. Part I. Inorganic cations. Pure Appl. Chem. 2002, 74(6), 923–994. [Google Scholar]
- Walker, F. A. An ESR Study of Coordination to the Fifth and Sixth Position of α, β, γ, δ-Tetra-(p-methoxyphenyl)porphinatocobalt(II). J. Am. Chem. Soc. 1970, 92, 4235–4244. [Google Scholar]
- Adler, A.D.; Longo, F.R.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for meso-tetraphenylporphine. J. Org. Chem. 1967, 32, 476–487. [Google Scholar]
- Dorough, G.D.; Miller, J.R.; Huennekens, F.M. Spectra of the Metallo-derivatives of α,β,γ,δ- Tetraphenylporphine. J. Am. Chem.Soc. 1951, 73, 4315–4320. [Google Scholar]
| Interfering anion | ||||
|---|---|---|---|---|
| Ref. [5] | Ref. [10] | Ref. [11] | This work | |
| F− | -3,44 | -3,80 | - | -4,80 |
| Sal− | -1,62 | -1,96 | -2,40 | -1,95 |
| Cl− | -3,88 | -3,71 | -3,00 | -3,84 |
| I− | -2,49 | -1,39 | -2,30 | -0,85 |
| CIO4− | -1,54 | -0,71 | -1,90 | -0,93 |
| NO3− | -2,90 | -2,87 | -3,20 | -2,46 |
| NO2− | -3,88 | -1,71 | -2,90 | -2,00 |
| Sample | Method | Non-smoker | Smoker |
|---|---|---|---|
| SCN− (mol/L) ± Sa | |||
| Urine | Potentiometry | (2.8 ± 0.1) ×10-4 | (8.2 ± 0.2) ×10-4 |
| Spectrophotometry | (2.7 ± 0.2) ×10-4 | (8.3 ± 0.1) ×10-4 | |
| Saliva | Potentiometry | (5.6± 0.1) ×10-4 | (1.7 ± 0.1) ×10-3 |
| Spectrophotometry | (5.7 ± 0.2) ×10-4 | (1.8 ± 0.1) ×10-3 | |
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Vlascici, D.; Fagadar-Cosma, E.; Bizerea-Spiridon, O. A New Composition for Co(II)-porphyrin-based Membranes Used in Thiocyanate-selective Electrodes. Sensors 2006, 6, 892-900. https://doi.org/10.3390/s6080892
Vlascici D, Fagadar-Cosma E, Bizerea-Spiridon O. A New Composition for Co(II)-porphyrin-based Membranes Used in Thiocyanate-selective Electrodes. Sensors. 2006; 6(8):892-900. https://doi.org/10.3390/s6080892
Chicago/Turabian StyleVlascici, Dana, Eugenia Fagadar-Cosma, and Otilia Bizerea-Spiridon. 2006. "A New Composition for Co(II)-porphyrin-based Membranes Used in Thiocyanate-selective Electrodes" Sensors 6, no. 8: 892-900. https://doi.org/10.3390/s6080892
APA StyleVlascici, D., Fagadar-Cosma, E., & Bizerea-Spiridon, O. (2006). A New Composition for Co(II)-porphyrin-based Membranes Used in Thiocyanate-selective Electrodes. Sensors, 6(8), 892-900. https://doi.org/10.3390/s6080892





