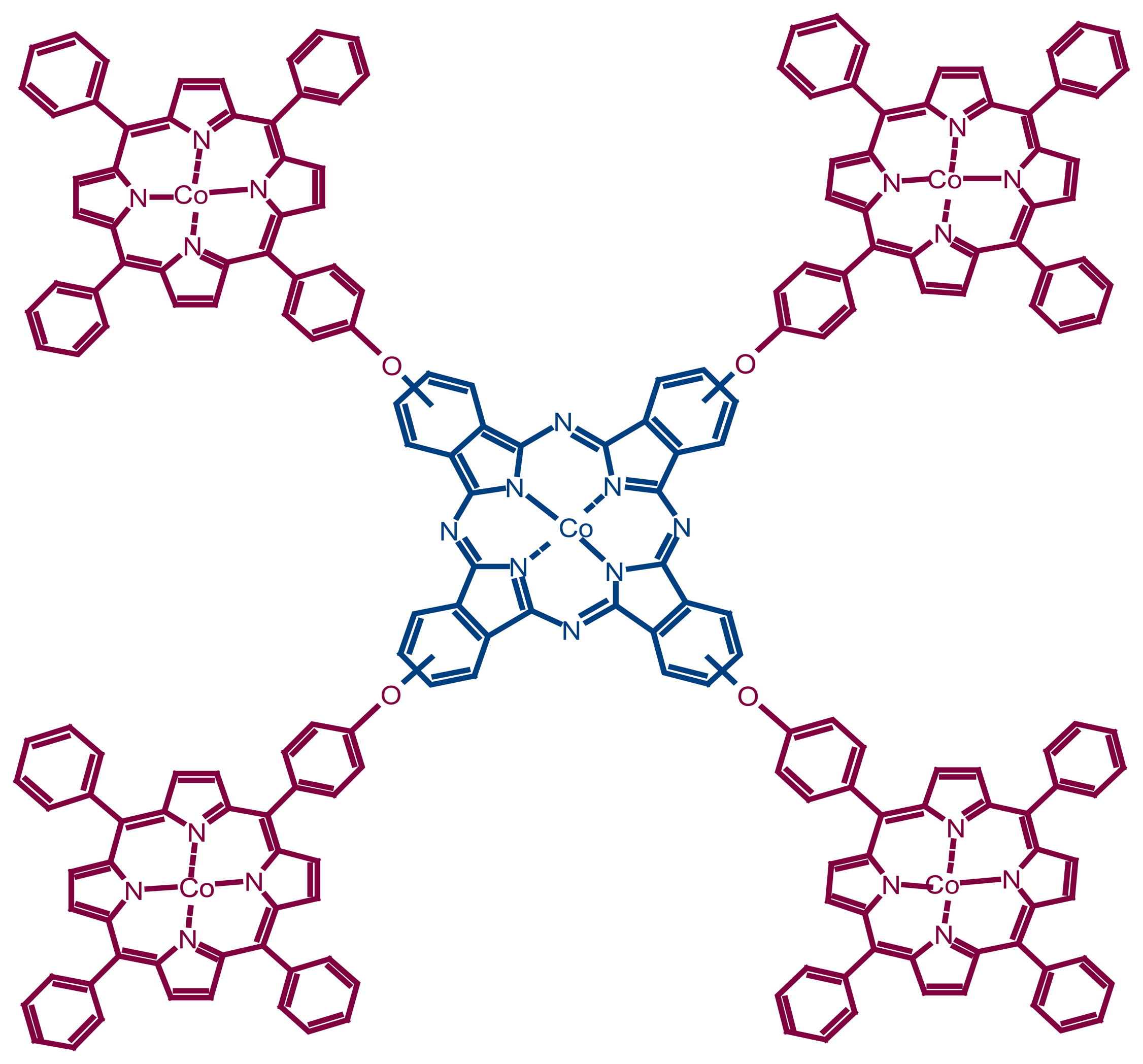
Anodic Oxidation and Amperometric Sensing of Hydrazine at a Glassy Carbon Electrode Modified with Cobalt (II) Phthalocyanine–cobalt (II) Tetraphenylporphyrin (CoPc- (CoTPP)4) Supramolecular Complex
Abstract
:1. Introduction
2. Results and Discussion
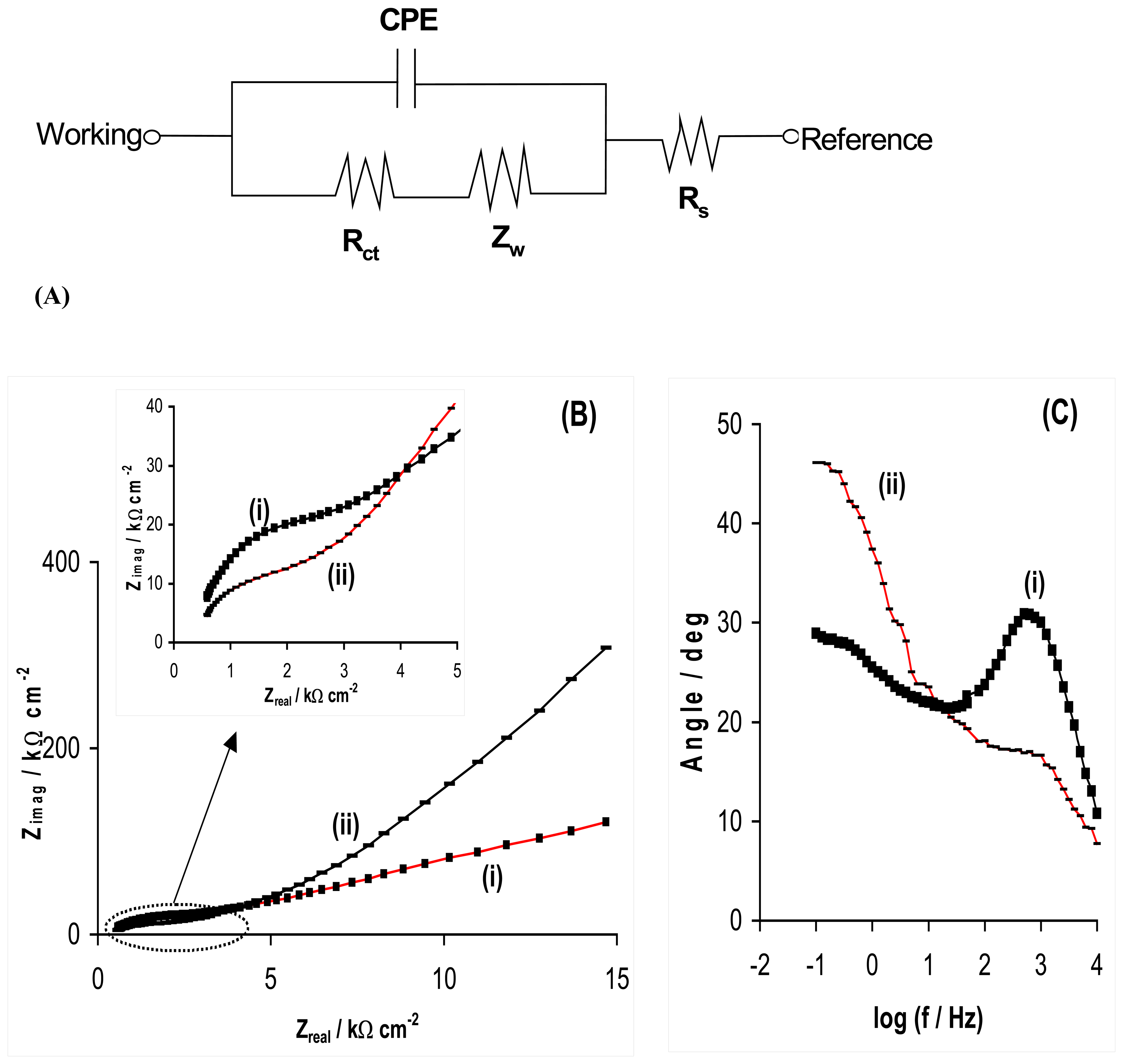
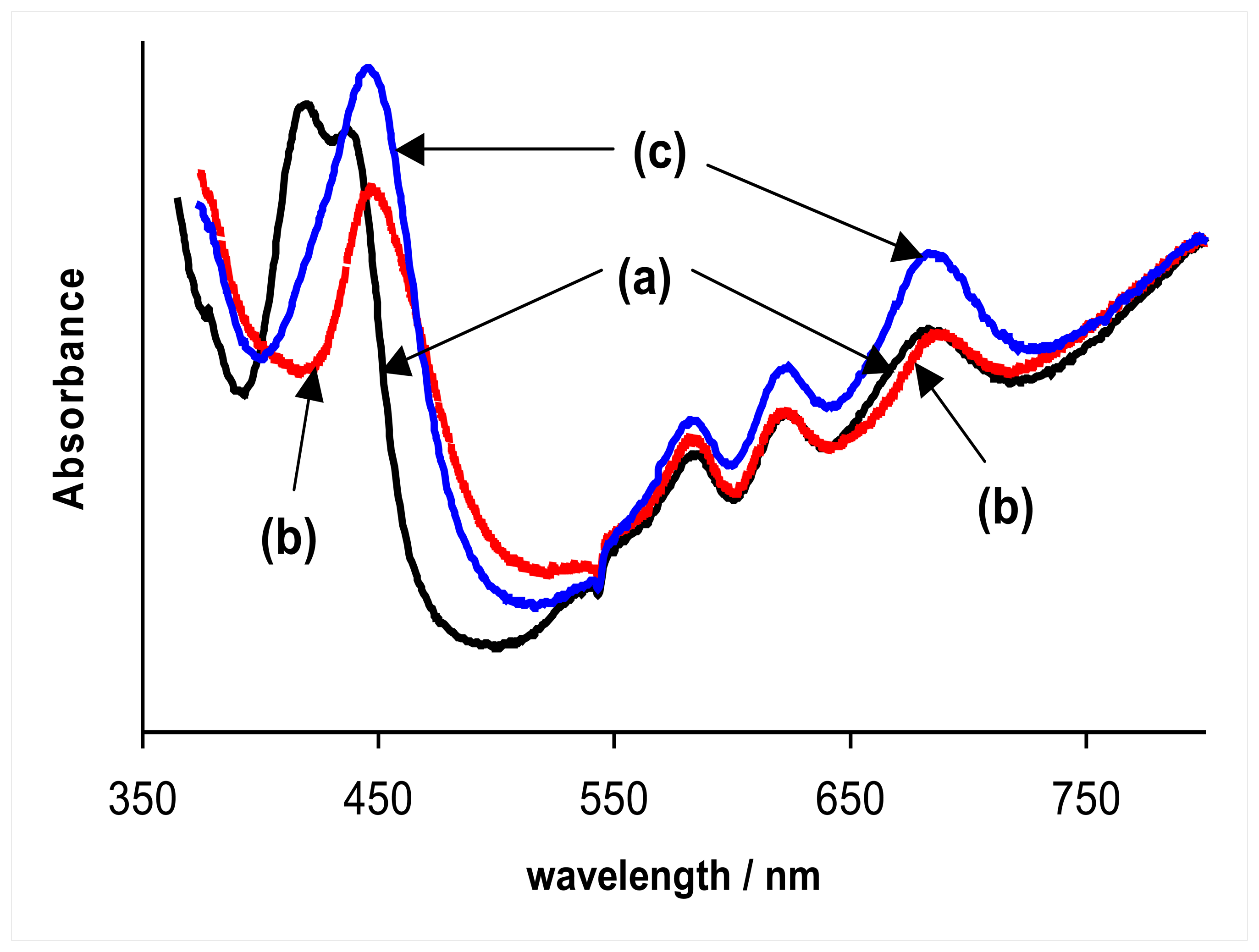
2.1. Electrochemical impedance spectroscopy studies
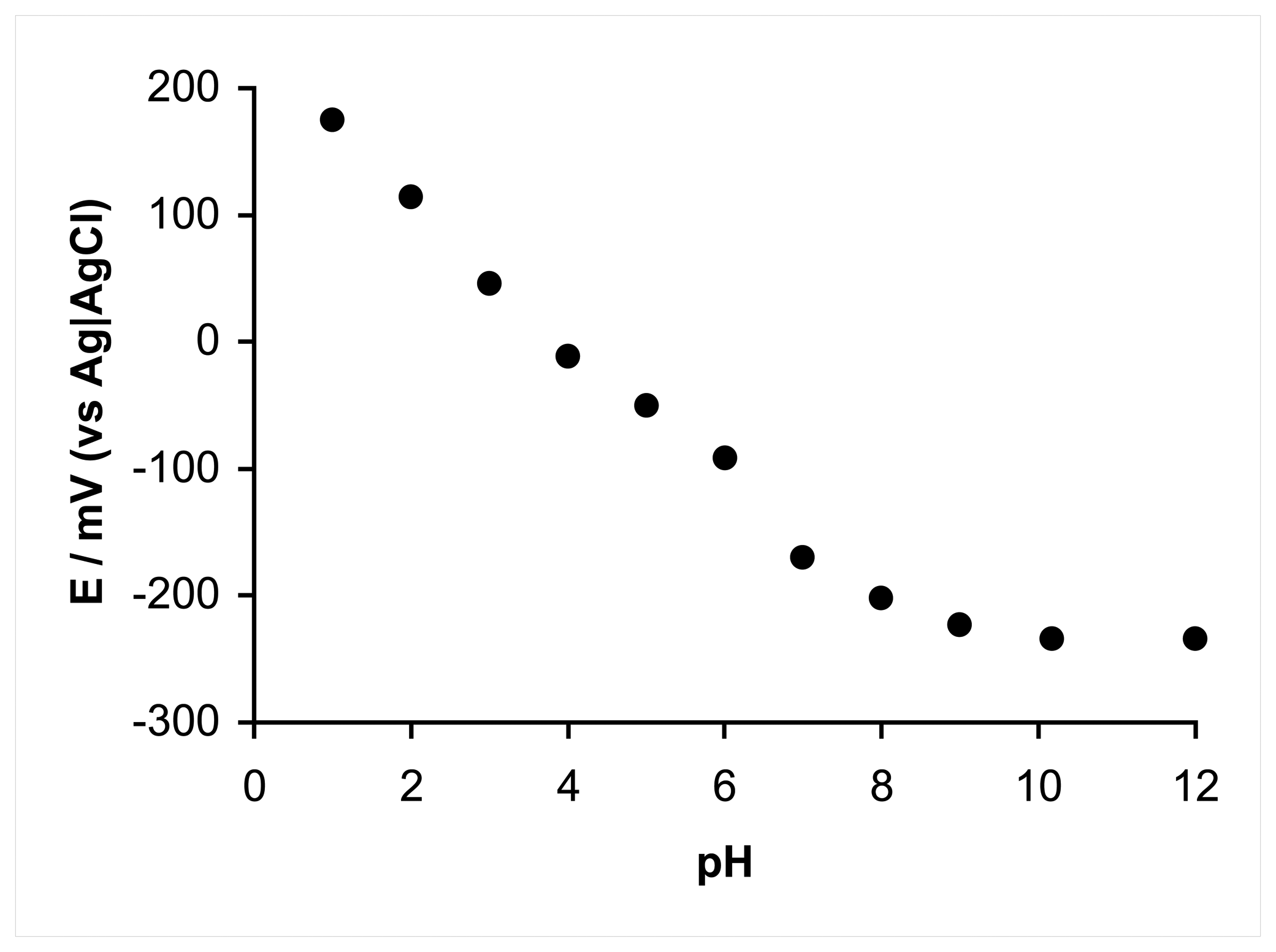
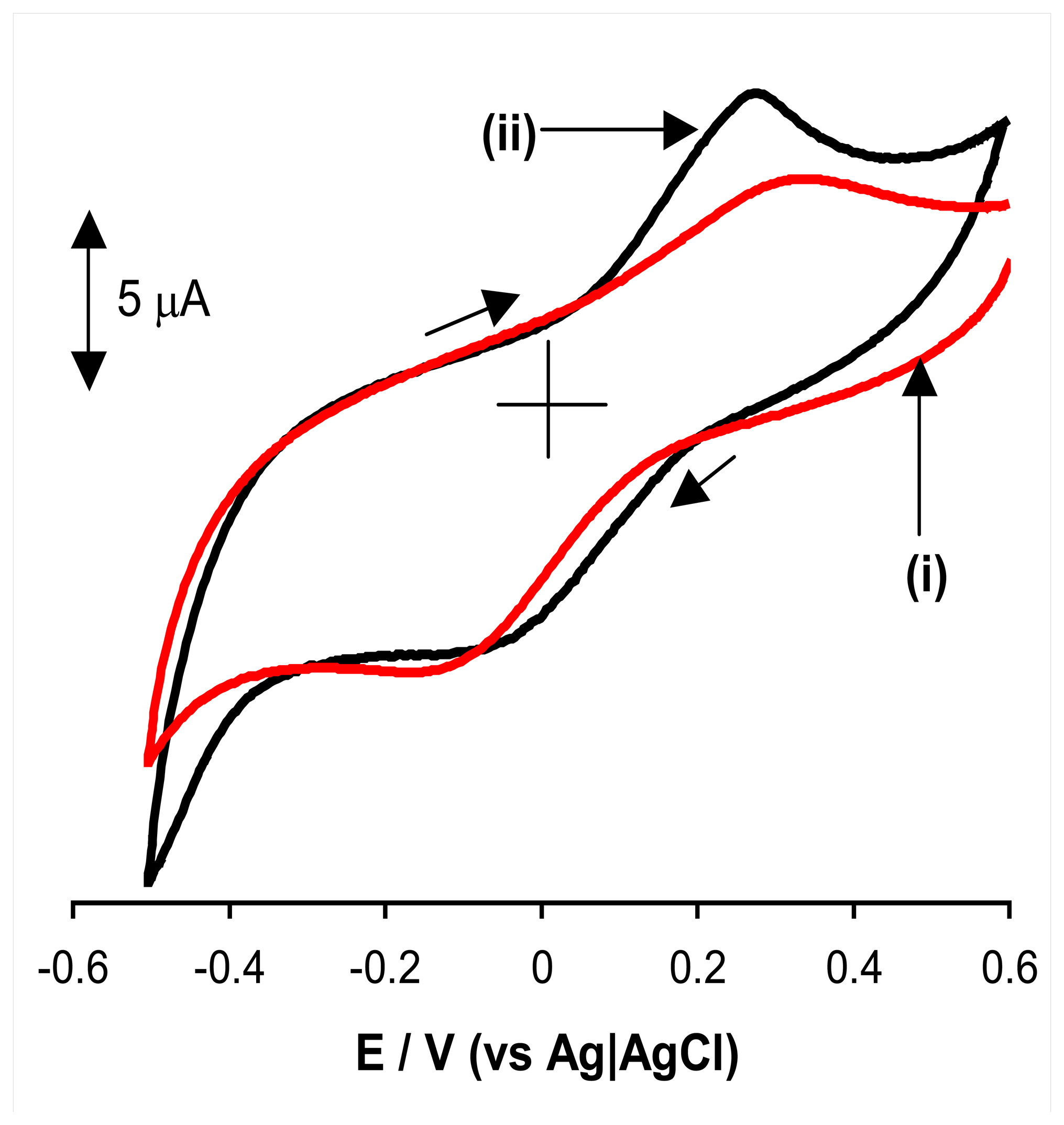
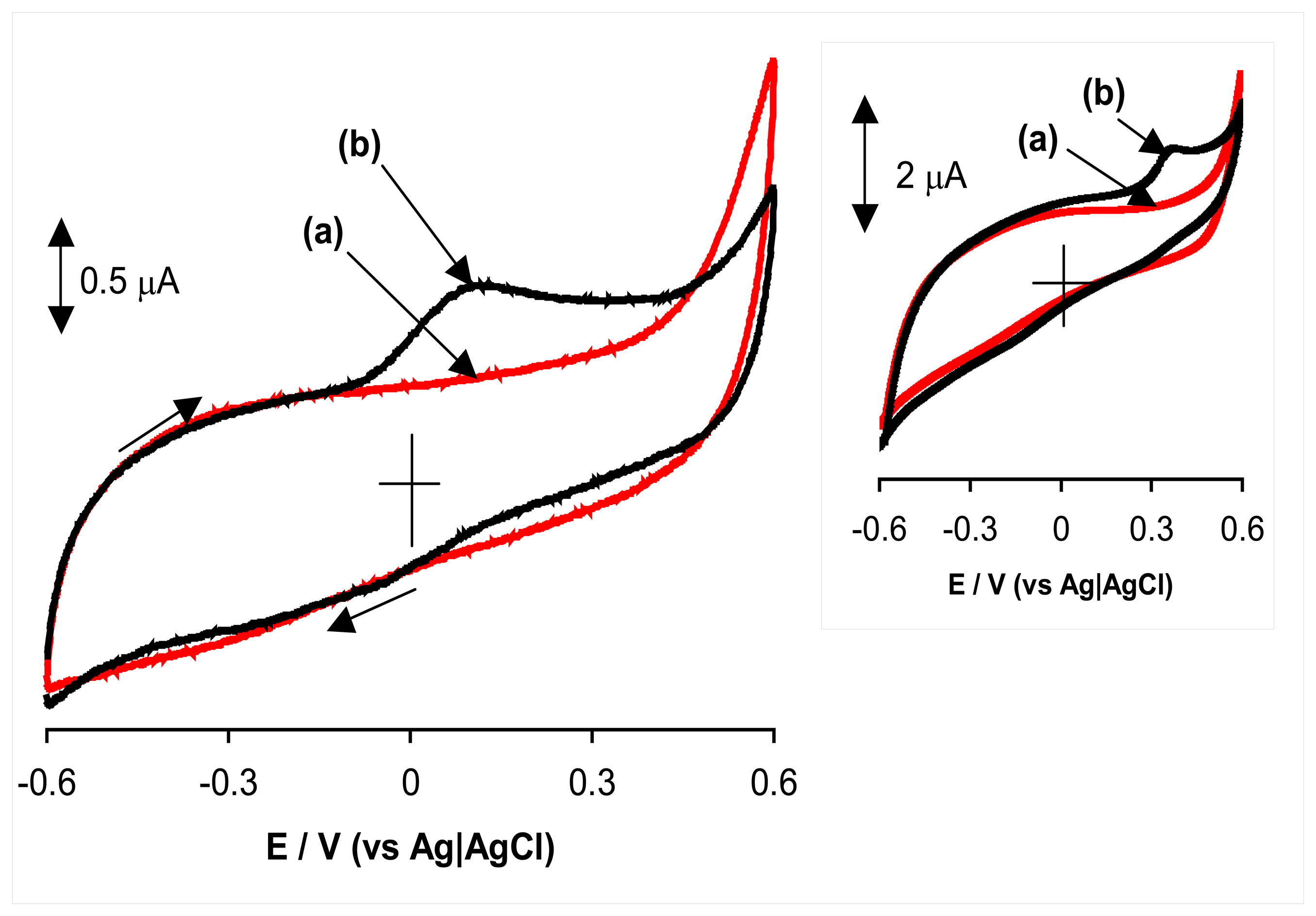
2.2. Anodic oxidation of hydrazine
2.3. Proposed mechanism for anodic oxidation of hydrazine
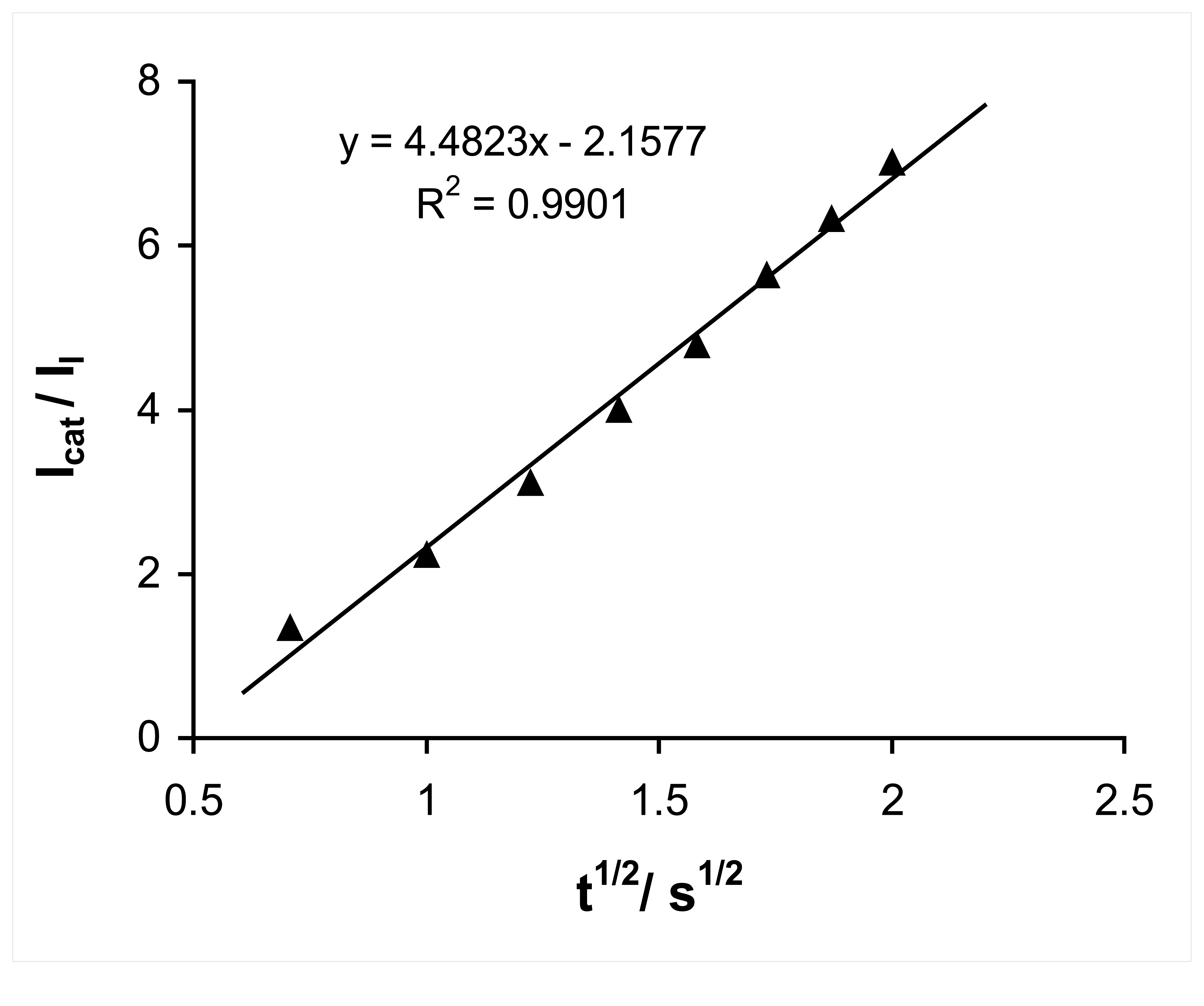
2.4. Chronoamperometric studies
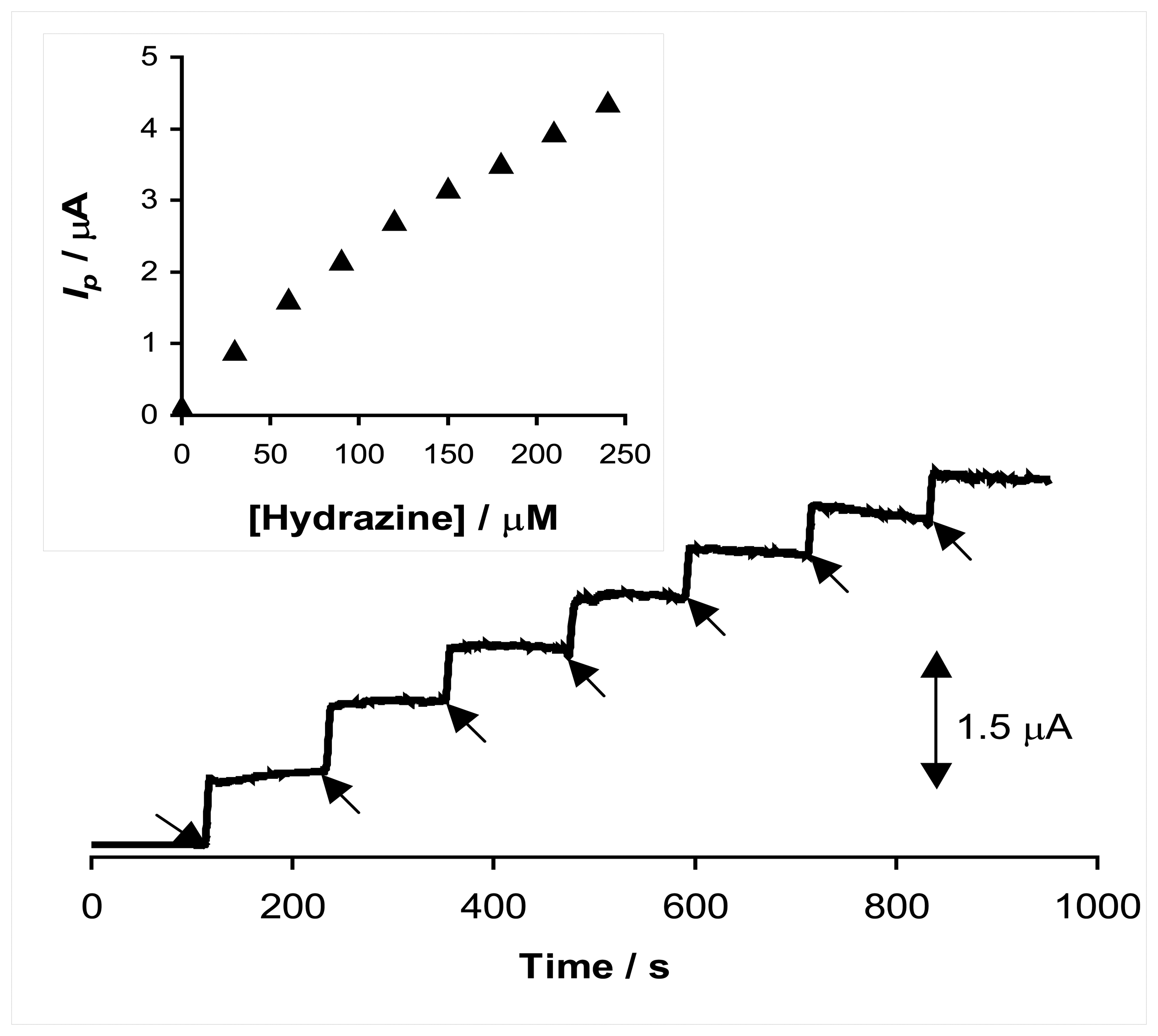
2.5. Chronoamperometric determination of hydrazine
2.6. Conclusion
3. Experimental Section
3.1. Reagents and Materials
3.2. Electrode fabrication and pretreatment
Acknowledgments
References
- Yamada, K.; Yasuda, K.; Fujiwara, N.; Siroma, Z.; Tanaka, H.; Miyazaki, Y.; Kobayashi, T. Potential application of anion-exchange membrane for hydrazine fuel cell electrolyte. Electrochem. Commun. 2003, 5, 892–896. [Google Scholar]
- Poso, A.; von Wright, A.; Gynther, J. An empirical and theoretical study on mechanisms of mutagenic activity of hydrazine compounds. Mutation Res. 1995, 332, 63–71. [Google Scholar]
- Huamin, J.; Weiying, H.; Erkany, W. Amperometric flow-injection analysis of hydrazine by electrocatalytic oxidation at cobalt tetraphenylporphyrin modified electrode with heat treatment. Talanta 1992, 39, 45–50. [Google Scholar]
- Malaki, E.A.; Koupparis, M.K. Kinetic study of the determination of hydrazines, isoniazid and sodium azide by monitoring their reactions with 1-fluoro-2,4-dinitrobenzene, by means of a fluoride-selective electrode. Talanta 1989, 36, 431–436. [Google Scholar]
- Dias, F.; Olojola, A.S.; Joselskis, B. Spectrophotometric determination of micro amounts of hydrazine and hydroxylamine alone and in the presence of each other. Talanta 1979, 26, 47–49. [Google Scholar]
- Pournaghi-Azar, M.H.; Sabzi, R. Electrochemical characteristics of a cobalt pentacyanonitrosylferrate film on a modified glassy carbon electrode and its catalytic effect on the electrooxidation of hydrazine. J. Electroanal. Chem. 2003, 543, 115–125. [Google Scholar]
- Ozoemena, K.I.; Nyokong, T. Electrocatalytic oxidation and detection of hydrazine at gold electrode modified with iron phthalocyanine complex linked to mercaptopyridine self-assembled monolayer. Talanta 2005, 67, 162–168. [Google Scholar]
- Zhang, J.; Tse, Y-H.; Pietro, W.J.; Lever, A.B.P. Electrocatalytic activity of NNNN-tetramethyltetra-3,4-pyridoporphyrazinocobalt(II) adsorbed on a graphite electrode towards the oxidation of hydrazine and hydroxylamine. J. Electroanal. Chem. 1996, 406, 203–211. [Google Scholar]
- Isaacs, M.; Aguirre, M.J.; Toro-Labbe, A.; Costamagna, J.; Paez, M.; Zagal, J.H. Comparative study of the electrocatalytic activity of cobalt phthalocyanine and cobalt naphthalocyanine for the reduction of oxygen and the oxidation of hydrazine. Electrochim. Acta 1998, 43, 1821–1827. [Google Scholar]
- Li, X.; Zhang, S.; Sun, C. Fabrication of covalently attached multiplayer film electrode containing cobalt phthalocyanine and its electrocatalytic oxidation of hydrazine. J. Electroanal. Chem. 2003, 553, 139–145. [Google Scholar]
- Hou, W.; Ji, H.; Wang, E. Amperometric flow-injection analysis of hydrazine by electrocatalytic oxidation at cobalt tetraphenylporphyrin-modified electrode with heat treatment. Talanta 1992, 39, 45–50. [Google Scholar]
- Batchelor-McAuley, C.; Banks, C.E.; Simm, A.O.; Jones, T.G.J.; Compton, R.G. A comparison of the use of palladium nanoparticles supported on a boron-doped diamond and palladium plated BDD microdisc array. Analyst 2006, 131, 106–110. [Google Scholar]
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Volume 1–20, Academic Press: Boston, 1999–2003.
- Vasuvedan, P.; Poughat, N.; Shuklat, A.K. Metal phthalocyanines as electrocatalysyts for redox reactions. Appl. Organomet. Chem. 1996, 10, 591–604. [Google Scholar]
- Ozoemena, K.I.; Nyokong, T. Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: California, 2006; Volume 3, Chapter E; pp. 157–200. [Google Scholar]
- Bedioui, F.; Devynck, J.; Bied-Charreton, C. Immobilization of metalloporphyrins in electropolymerized films: design and applications. Acc. chem. Res. 1995, 28, 30–36. [Google Scholar]
- Zagal, J.H.; Gulppi, M.A.; Caro, C.A.; Cárdenas-Jirón, G.I. Paradoxical effect of the redox potential of adsorbed metallophthalocyanines on their activity for the oxidation of 2-mercaptoethanol. Inner versus outer sphere electrocatalysis. Electrochem. Commun. 1999, 1, 389–393. [Google Scholar]
- Ozoemena, K.I.; Nyokong, T.; Westbroek, P. Self-assembled monolayers of cobalt and iron phthalocyanine complexes on gold electrodes: Comparative surface electrochemistry and electrocatalytic interaction with thiols and thiocyanate. Electroanalysis 2003, 15, 1762–1770. [Google Scholar]
- Griveau, S.; Gulppi, M.; Bedioui, F.; Zagal, J.H. Electropolymerized cobalt macrocomplex-based films for thiols electro-oxidation: effect of the film formation conditions and the nature of the macrocyclic ligand. Solid State Ionics 2004, 169, 59–63. [Google Scholar]
- Maree, S.; Nyokong, T. Electrocatalytic behavior of substituted cobalt phthalocyanines towards the oxidation of cysteine. J. Electroanal. Chem. 2000, 492, 120–127. [Google Scholar]
- Nyokong, T.; Vilakazi, S. Phthalocyanines and related complexes as electrocatalysts for the detection of nitric oxide. Talanta 2003, 61, 27–35. [Google Scholar]
- Oni, J.; Diab, N.; Radtke, I.; Schuhmann, W. Detection of NO release from endothelial cells using Pt micro electrodes modified with a pyrrole-functionalised Mn(II) porphyrin. Electrochim. Acta 2003, 48, 3349–3354. [Google Scholar]
- Pereira-Rodrigues, N.; Albin, V.; Koudelka-Hep, M.; Auger, V.; Pailleret, A.; Bedioui, F. Nickel tetrasulfonated phthalocyanine based platinum microelectrode array for nitric oxide oxidation. Electrochem. Commun. 2002, 4, 922–927. [Google Scholar]
- Cosnier, S.; Gondran, C.; Wessel, R.; Montforts, F-P.; Wedel, M. A poly(pyrrole-cobalt(II)deuteroporphyrin) electrode for the potentiometric determination of nitrite. Sensors 2003, 3, 213–222. [Google Scholar]
- D'Souza, F.; Hsieh, Y.Y.; Wickman, H.; Kutner, W. New sensor for dissolved dioxygen: a gold electrode modified with a condensation polymer film of β-cyclodextrin hosting cobalt tetraphenylporphyrin. Chem. Commun. 1997, 1191–1192. [Google Scholar]
- Janda, P.; Lam, H.; Pietro, W.J.; Lever, A.B.P. Monomeric and polymeric tetraaminophthalocyaninatocobalt(II) modified electrodes: electrocatalytic reduction of oxygen. J. Porphyrins phthalocyanines 1997, 1, 3–16. [Google Scholar]
- Zhao, Z.X.; Ozoemena, K.I.; Maree, D.M.; Nyokong, T. Synthesis and electrochemical studies of a covalently linked cobalt(II) phthalocyanine-cobalt(II) porphyrin conjugate. Dalton Trans. 2005, 1241–1248. [Google Scholar]
- Ozoemena, K.I.; Zhao, Z.X.; Nyokong, T. Immobilized cobalt (II) phthalocyanine–cobalt (II) porphyrin pentamer at a glassy carbon electrode: Applications to efficient amperometric sensing of hydrogen peroxide in neutral and basic media. Electrochem. Commun. 2005, 7, 679–684. [Google Scholar]
- Ozoemena, K.I.; Nyokong, T. Novel amperometric glucose biosensor based on an ether-linked cobalt(II) phthalocyanine–cobalt(II) tetraphenylporphyrin pentamer as a redox mediator. Electrochim. Acta 2006, 51, 5131–5136. [Google Scholar]
- Mantai, K.E.; Hind, G. Mechanism and stoichiometry of the oxidation of hydrazine by illuminated chloroplasts. Plant Physiol. 1971, 48, 5–8. [Google Scholar]
- Nevin, W.A.; Hempetead, M.R.; Liu, W.; Leznoff, C.C.; Lever, A.B.P. Electrochemistry and spectroelectrochemistry of mononuclear and binuclear cobalt phthalocyanines. Inorg. Chem. 1987, 26, 570–578. [Google Scholar]
- Katz, E.; ; Wilner, I. Ultrathin Electrochemical Chemo- and Biosensors. Technology and Performance; Mirsky, V.M., Ed.; Springer-Verlag: New York, 2004; Chapter 4; pp. 68–116. [Google Scholar]
- Bisquert, J.; Garcia-Belmonte, G.; Bueno, P.; Longo, E.; Bulhoes, L.O.S. Impedance of constant phase element (CPE)-blocked diffusion in film electrodes. J. Electroanal. Chem. 1998, 452, 229–234. [Google Scholar]
- Sabatini, E.; Rubinstein, I. Organized self-assembled monolayers on electrodes. 2. Monolayer-based ultramicroelectrodes for the study of very rapid electrode kinetics. J. Phys. Chem. 1987, 91, 6663–6669. [Google Scholar]
- Adams, J.Q.; Thomas, J.R. Electron Paramagnetic Resonance of a Hydrazine Radical Ion. J. Chem. Phys. 1963, 39, 1904–1906. [Google Scholar]
- Zhao, Y.-D.; Zhang, W.-D.; Chen, H.; Luo, Q.-M. Anodic oxidation of hydrazine at carbon nanotube powder microelectrode and its detection. Talanta 2002, 58, 529–534. [Google Scholar]
- Golabi, S.M.; Zare, H.R.; Hamzehloo, M. Electrocatalytic oxidation of hydrazine at a pyrocatechol violet (PCV) chemically modified electrode. Microchem. J. 2001, 69, 13–23. [Google Scholar]
- Zen, J.-M.; Kumar, A. S.; Chang, M.-R. Electrocatalytic oxidation and trace detection of amitrole using a Nafion/lead–ruthenium oxide pyrochlore chemically modified electrode. Electrochim. Acta 2000, 45, 1691–1700. [Google Scholar]
- Wermeckers, B.; Beck, F. ; Anodic oxidation of β-cyanoethyl ethers. Electrochim. Acta 1985, 30, 1491–1500. [Google Scholar]
- Siswana, M.; Ozoemena, K.I.; Nyokong, T. Electrocatalytic behaviour of carbon paste electrode modified with iron (II) phthalocyanine (FePc) nanoparticles towards the detection of amitrole. Talanta 2006, 69, 1136–1142. [Google Scholar]

©2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Ozoemena, K.I. Anodic Oxidation and Amperometric Sensing of Hydrazine at a Glassy Carbon Electrode Modified with Cobalt (II) Phthalocyanine–cobalt (II) Tetraphenylporphyrin (CoPc- (CoTPP)4) Supramolecular Complex. Sensors 2006, 6, 874-891. https://doi.org/10.3390/s6080874
Ozoemena KI. Anodic Oxidation and Amperometric Sensing of Hydrazine at a Glassy Carbon Electrode Modified with Cobalt (II) Phthalocyanine–cobalt (II) Tetraphenylporphyrin (CoPc- (CoTPP)4) Supramolecular Complex. Sensors. 2006; 6(8):874-891. https://doi.org/10.3390/s6080874
Chicago/Turabian StyleOzoemena, Kenneth I. 2006. "Anodic Oxidation and Amperometric Sensing of Hydrazine at a Glassy Carbon Electrode Modified with Cobalt (II) Phthalocyanine–cobalt (II) Tetraphenylporphyrin (CoPc- (CoTPP)4) Supramolecular Complex" Sensors 6, no. 8: 874-891. https://doi.org/10.3390/s6080874



