Volatile Organic Compound Optical Fiber Sensors: A Review
Abstract
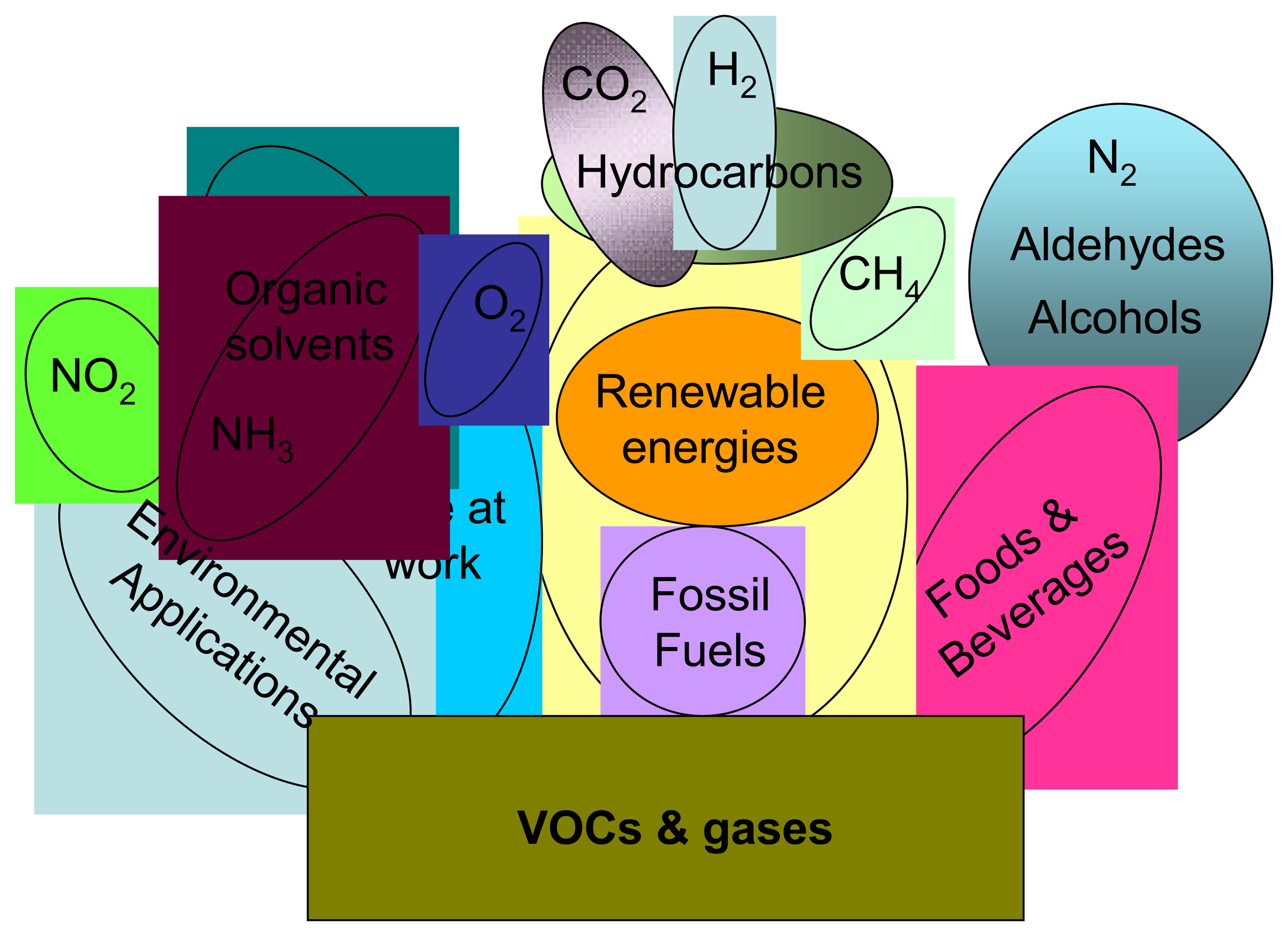
:1. Introduction
2. Sensing Architectures
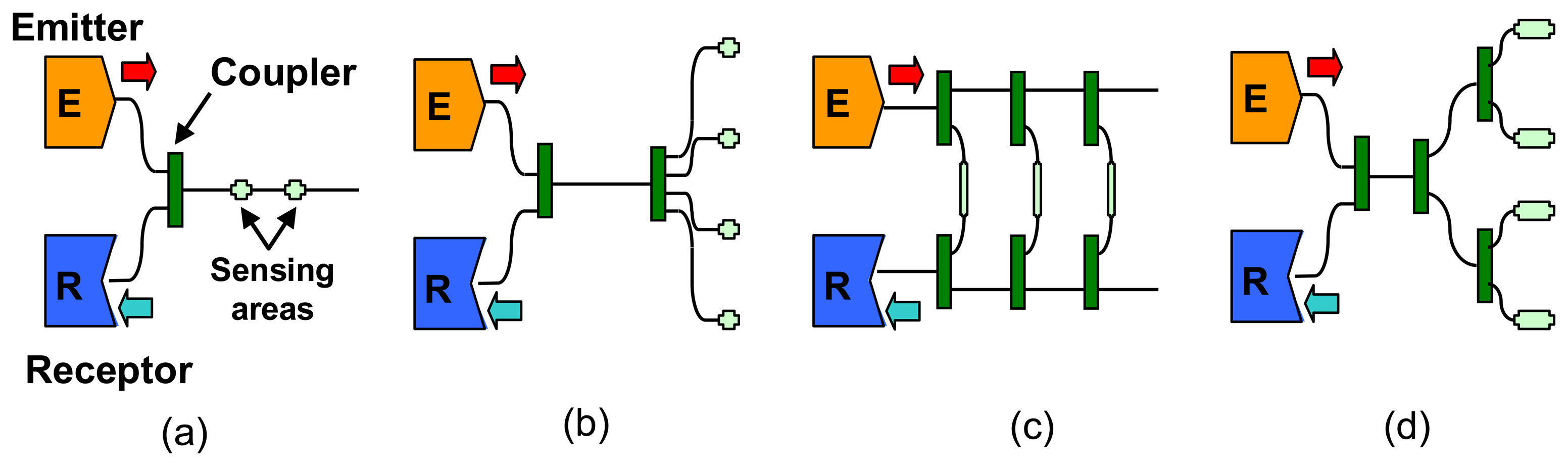
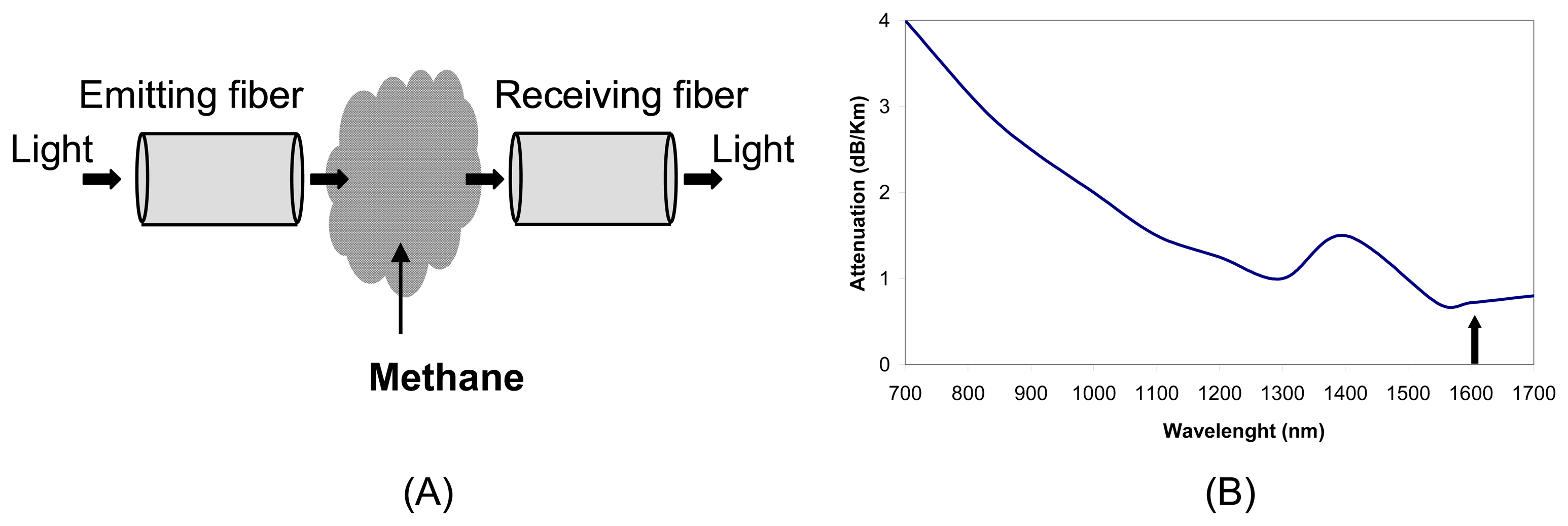
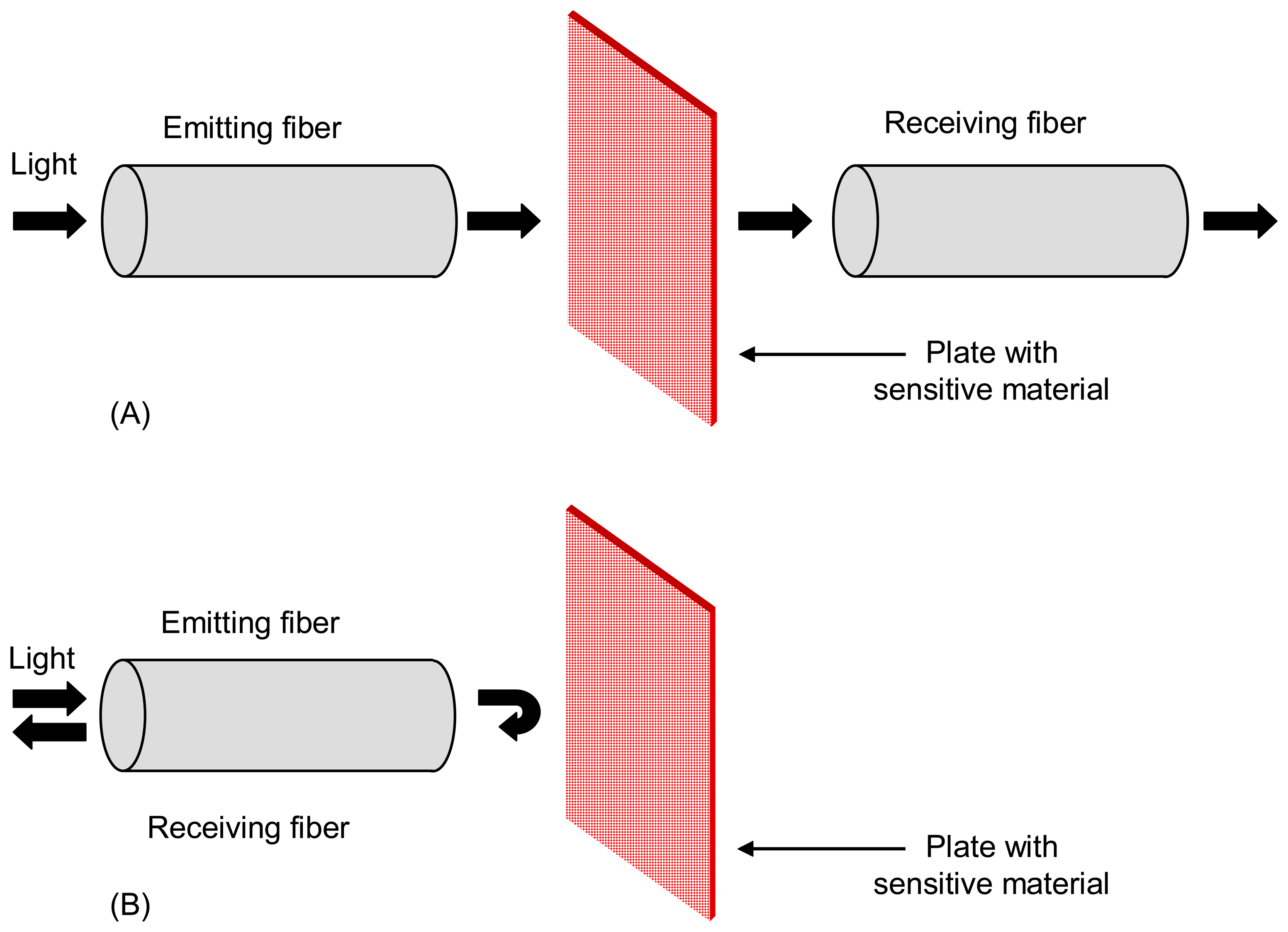
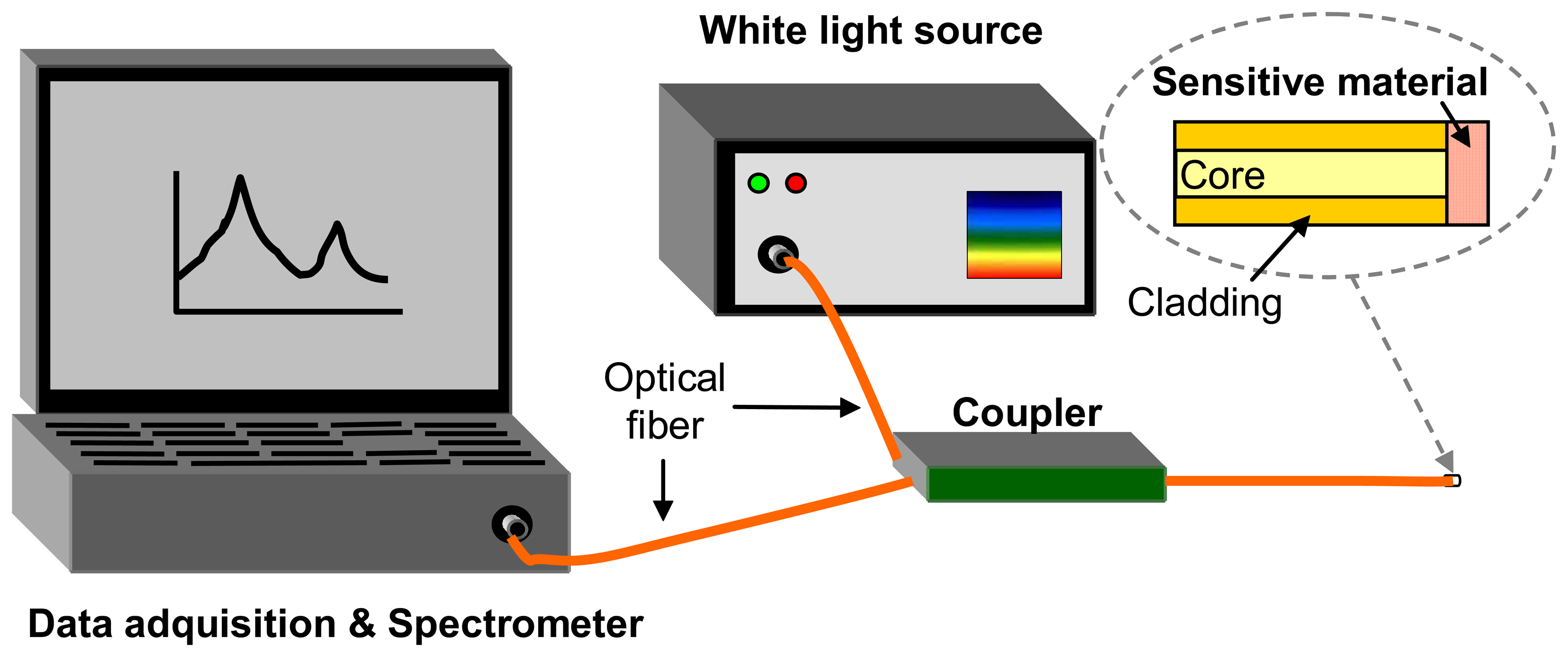
2.1 Extrinsic sensors
2.2 Intrinsic sensors
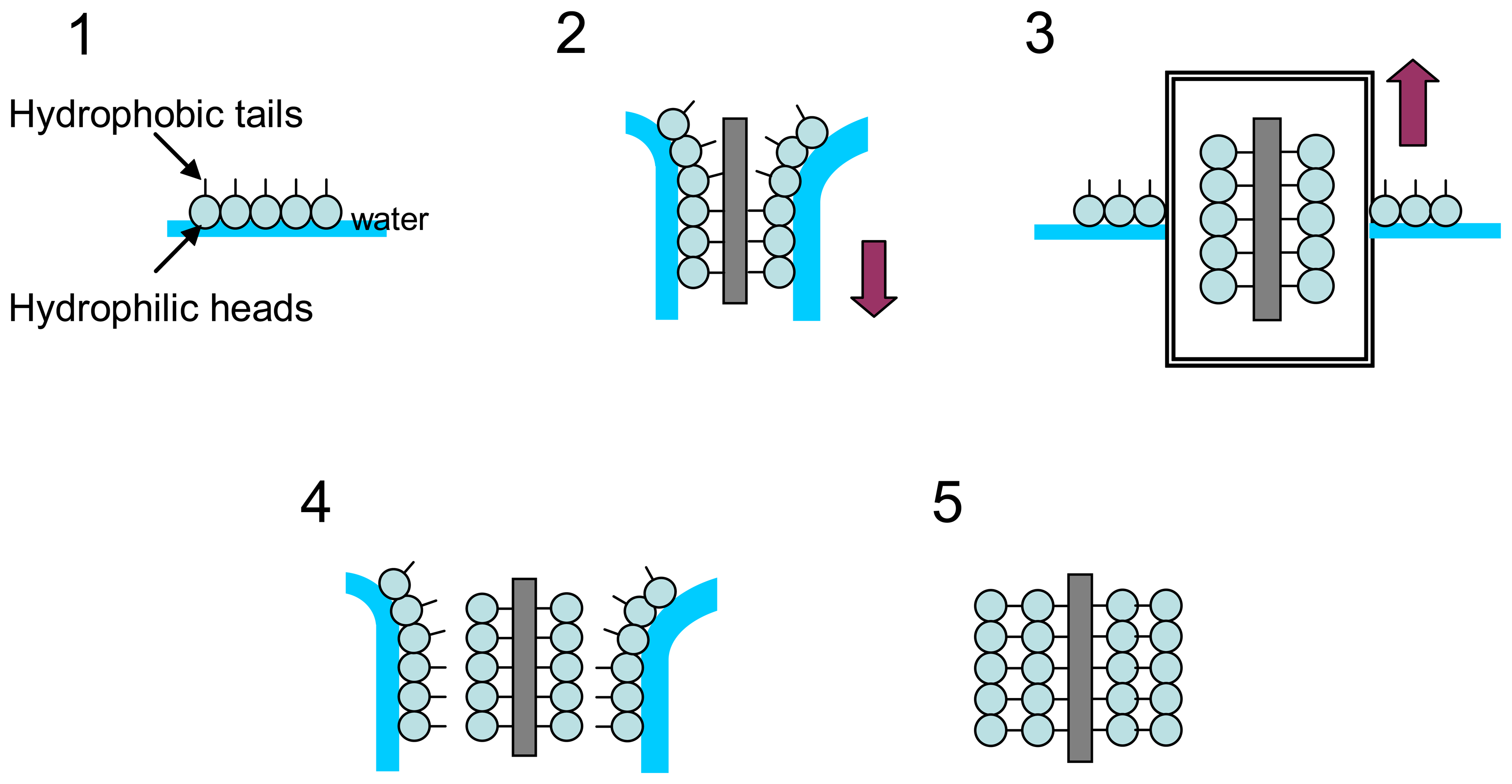
2.2.1 Evanescent wave intrinsic sensors
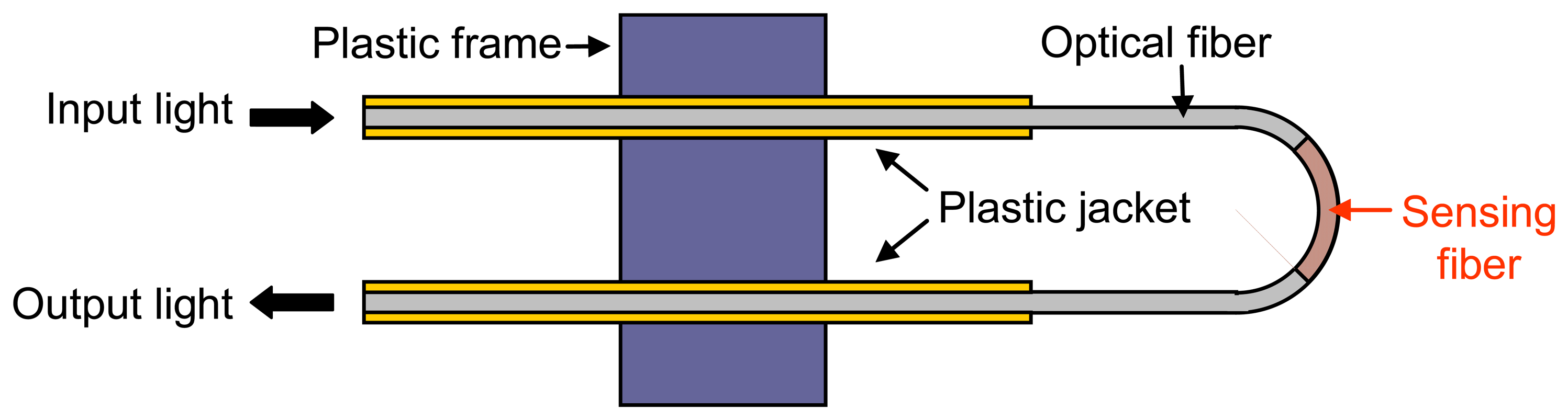
2.2.1.1 Bended evanescent wave sensors
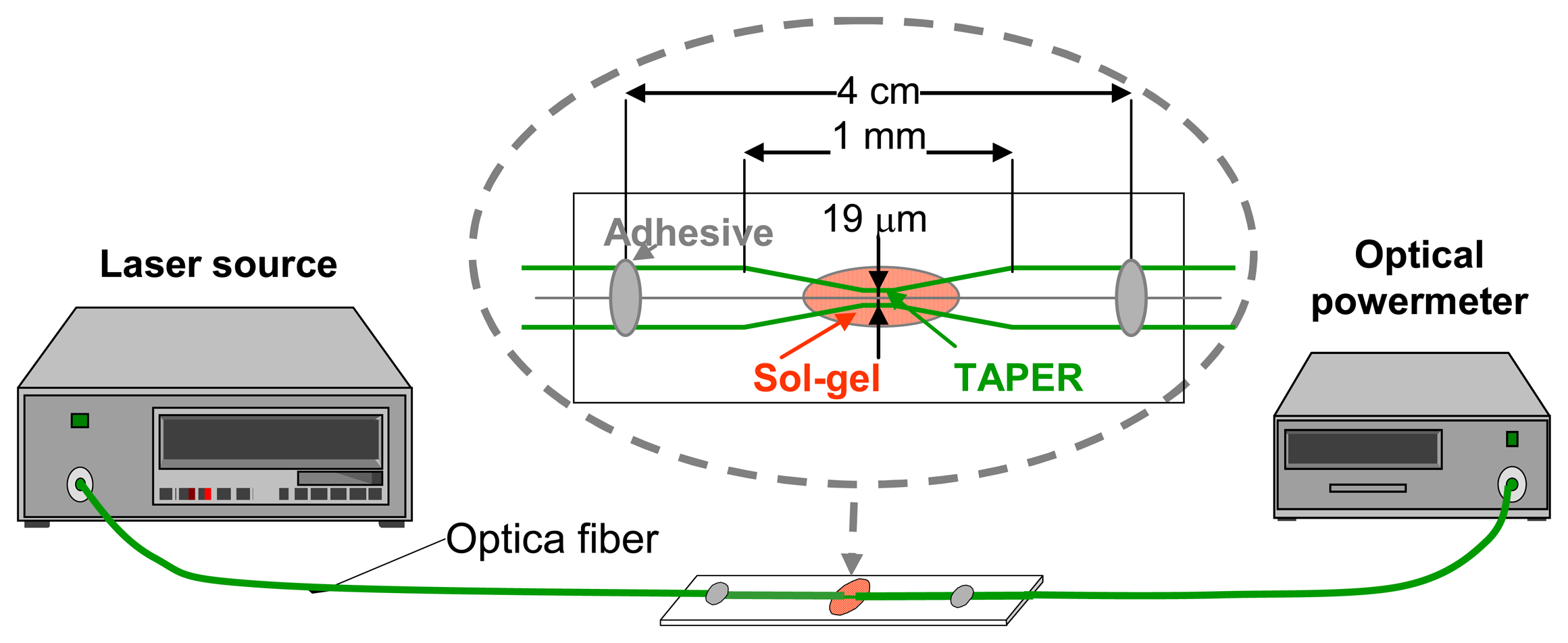
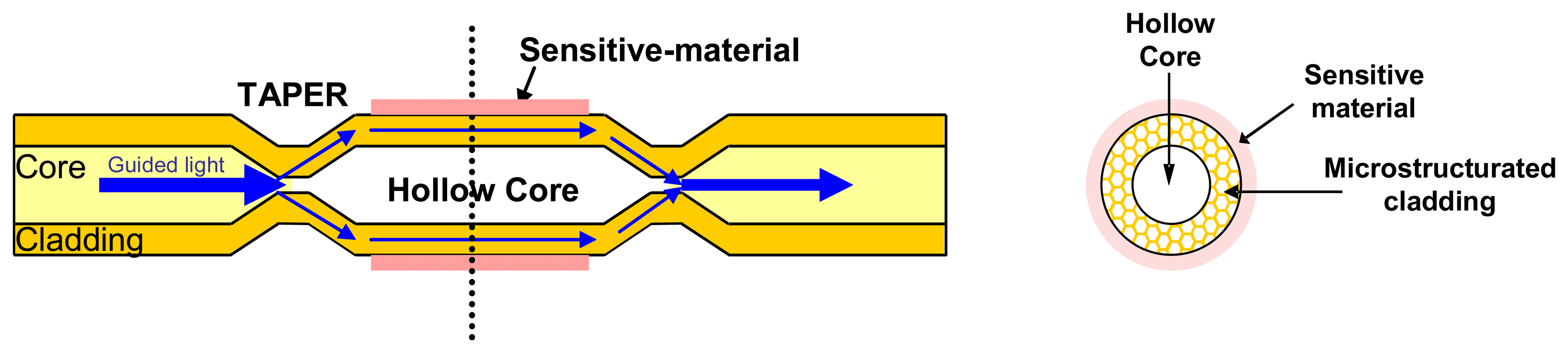
2.2.1.2 Evanescent wave sensors based on special fibers
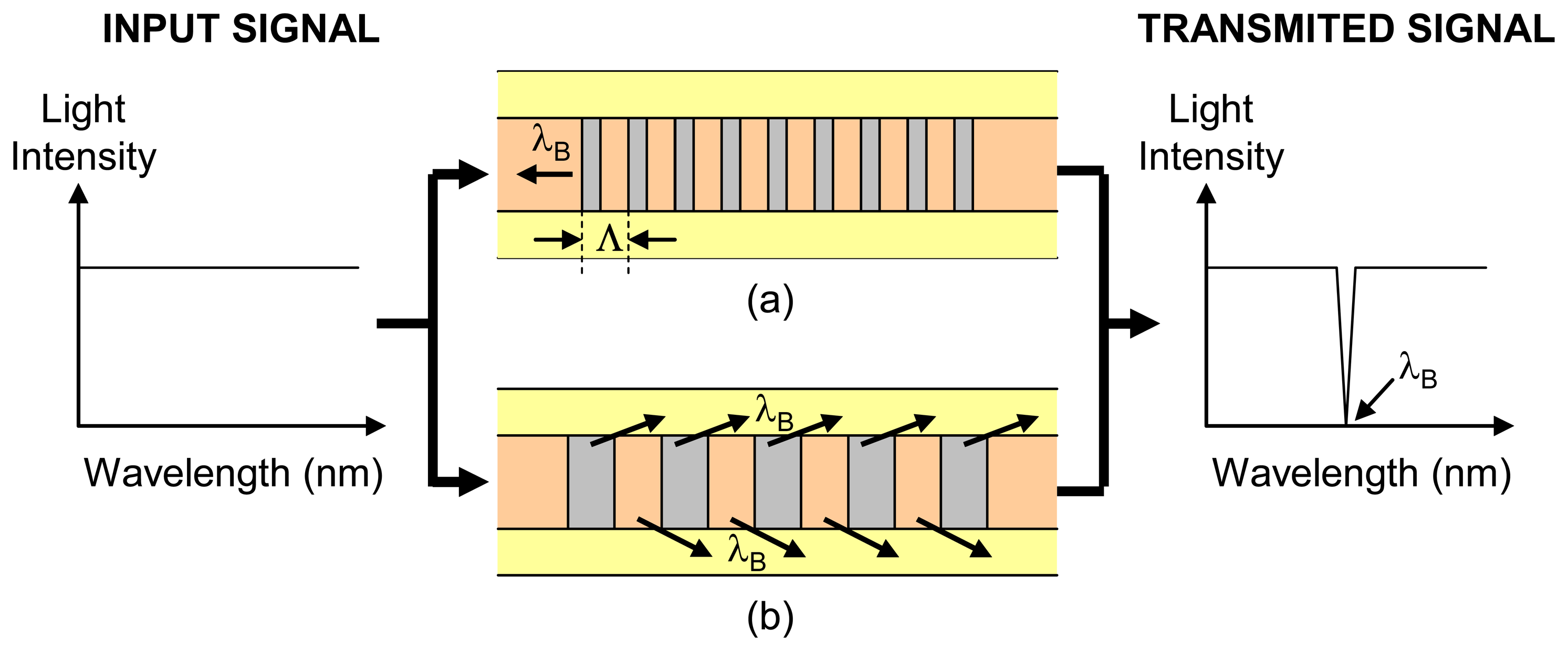
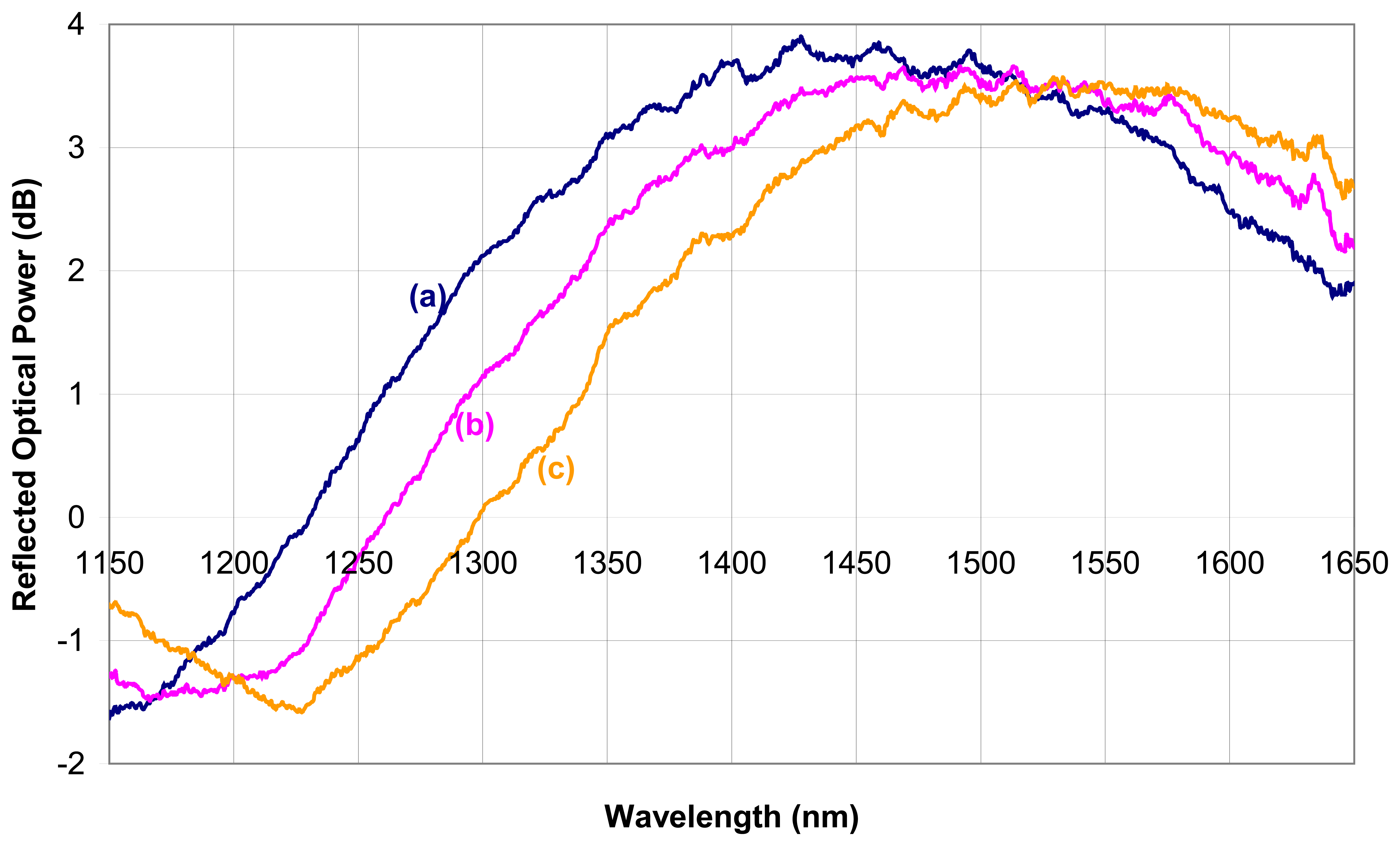
2.2.1.3 Bragg grating intrinsic sensors
2.2.2 Active fiber core intrinsic sensors
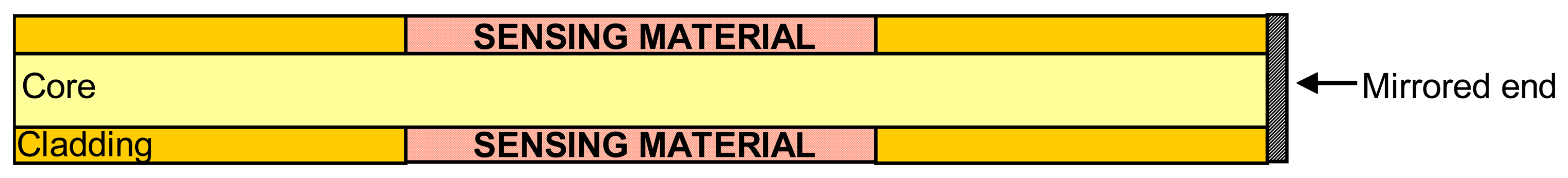
2.2.3 Reflection intrinsic sensors
2.2.4 Hybrid intrinsic sensors
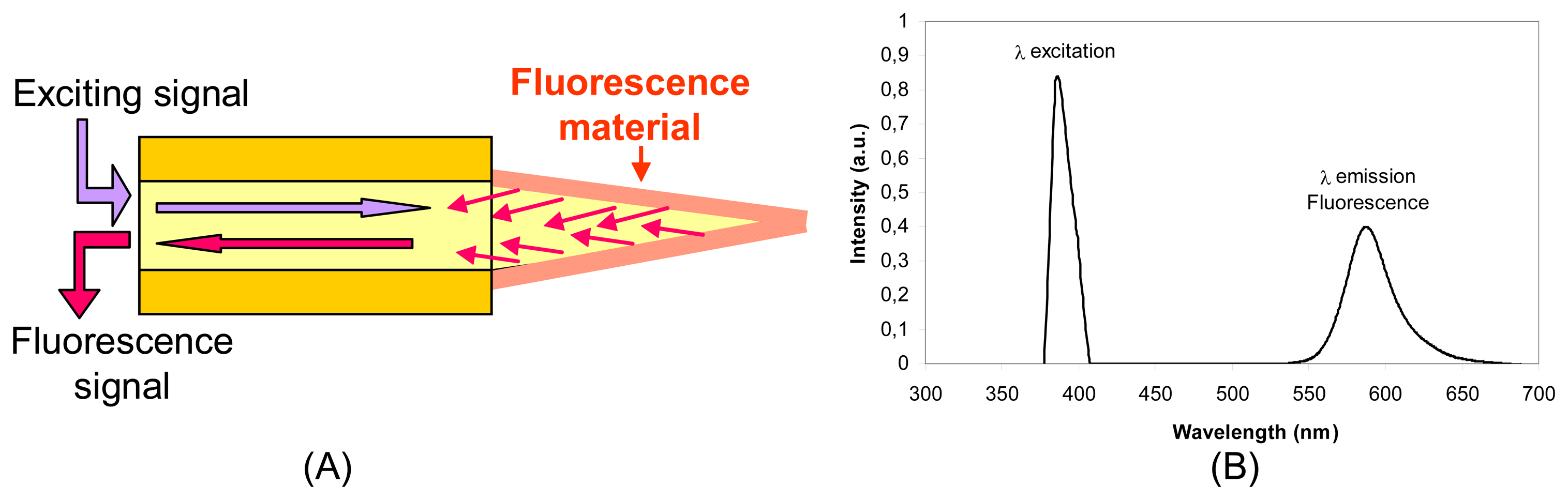
2.2.5 Fluorescence sensors
3. More relevant applications and future challenges
4. Conclusions
Acknowledgments
References
- Lee, B. Review of the present status of optical fiber sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar]
- Kovacs, G.T. Micromachined Transducers Sourcebook; McGraw-Hill: New York, 1998. [Google Scholar]
- Culshaw, B. Research to reality: bringing fibre optic sensors into applications. P. SPIE 2005, 5952, 595201. [Google Scholar]
- Culshaw, B. Optical Fiber Sensor Technologies: Opportunities and-Perhaps-Pitfalls. IEEE J. Lightwav. Technol. 2004, 22, 39–50. [Google Scholar]
- Spadavecchia, J.; Ciccarella, G.; Rella, R.; Capone, S.; Siciliano, P. Metallophthalocyanines thin films in array configuration for electronic optical nose applications. Sensor. Actuat. B-Chem. 2003, 96, 489–497. [Google Scholar]
- Semmelroch, P.; Grosh, W. Studies on Character Impact Odorants of Coffee Brews. J. Agr. Food Chem. 1996, 44, 537–543. [Google Scholar]
- Bohnert, K.; Gabus, P.; Kostovic, J.; Brämdle, H. Optical fiber sensors for the electric power industry. Opt. Laser. Eng. 2005, 43, 511–526. [Google Scholar]
- Hotate, K. Fiber Sensor Technology Today. Jpn. J. Appl. Phys. 2006, 45, 6616–6625. [Google Scholar]
- Sumdia, S.; Okazaki, S.; Asakura, S.; Nakagawa, H.; Murayama, H.; Hasegawa, T. Distributed hydrogen determination with fiber-optic sensor. Sensor. Actuat. B-Chem 2005, 108, 508–514. [Google Scholar]
- McKinley, B.A.; Parmley, C.L. Clinical Trial an Ex vivo Arterial Blood Gas Monitor. J. Crit. Care 1998, 13, 190–197. [Google Scholar]
- Giuliani, J.F.; Wohltjen, H.; Jarvis, N.L. Reversible optical waveguide sensor for ammonia vapors. Opt. Lett. 1983, 8, 54–56. [Google Scholar]
- Matias, I.R.; Arregui, F.J.; Claus, R.O. Optical Fiber Sensors. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2006; Vol. 9, pp. 1–19. [Google Scholar]
- Culshaw, B. Fiber optics in sensing and measurement. IEEE J. Sel. Top. Quant. 2000, 6, 1014–1021. [Google Scholar]
- Udd, E. Fiber Optic Sensors; Wiley: USA, 1992. [Google Scholar]
- Spooncer, R.C. Handbook of Measurement Science; Wiley: Chichester, UK, 1992. [Google Scholar]
- Culshaw, B.; Stewart, G.; Dong, F.; Tandy, C.; Moodie, D. Fibre optic techniques for remote spectroscopic methane detection-from concept to system realisation. Sensor. Actuat. B-Chem. 1998, 51, 25–37. [Google Scholar]
- Chambers, P.; Austin, E. A. D.; Dakin, J.P. Theoretical analysis of methane gas detection system, using the complementary source modulation method of correlation spectroscopy. Meas. Sci. Tech. 2004, 15, 1629–1636. [Google Scholar]
- Stewart, G.; Tandy, C.; Moodie, D.; Morante, M.A.; Dong, F. Design of a fibre optic multi-point sensor for gas detection. Sensor. Actuat. B-Chem 1998, 51, 227–232. [Google Scholar]
- Ho, H.L.; Jin, W.; Yu, H.B.; Chan, K.C.; Chan, C.C.; Demokan, M.S. Experimental demonstration of a Fiber-Optic Gas Sensor Network Addressed by FMCW. IEEE Photon. Tech. Lett. 2000, 12, 1546–1548. [Google Scholar]
- Whitenett, G.; Stewart, G.; Atherton, K.; Culshaw, B.; Johnstone, W. Optical fibre instrumentation for environmental monitoring applications. J. Opt. A-Pure Appl. Opt. 2003, 5, S140–S145. [Google Scholar]
- Cheung, A.; Johnstone, W.; Moodie, D. Gas detection based on optical correlation spectroscopy using a single light source. Meas. Sci. Tech. 2006, 17, 1107–1112. [Google Scholar]
- Xiao, G.Z.; Adnet, A.; Zhang, Z.; Sun, F.G.; Grover, C.P. Monitoring changes in the refractive index of gases by means of a fiber optic Fabry-Perot interferometer sensor. Sensor. Actuat. A-Phys. 2005, 118, 177–182. [Google Scholar]
- Christie, S.; Scorsone, E.; Persaud, K.; Kvasnik, F. Remote detection of gaseous ammonia using near infrared transmission properties of polyaniline. Sensor. Actuat. B-Chem. 2003, 90, 163–169. [Google Scholar]
- Kondratowicz, B.; Narayanaswamy, R.; Persaud, K.C. An investigation into the use of electrochromic polymers in optical fibre gas sensors. Sensor. Actuat. B-Chem. 2001, 74, 138–144. [Google Scholar]
- Akarajas, A.U.; Mat, Salleh M.; Yahaya, M. Enriching the selectivity of metalloporphyrins chemical sensors by means of optical technique. Sensor. Actuat. B-Chem. 2002, 85, 191–196. [Google Scholar]
- Khijwania, S.K.; Gupta, B.D. Fiber optic evanescent field absorption sensor with high sensitivity and linear dynamic range. Opt. Commun. 1998, 152, 259–262. [Google Scholar]
- Khalil, S.; Bansal, L.; El-Sherif, M. Intrinsic fiber optic chemical sensor for the detection of dimethyl methylphosphonate. Opt. Eng. 2004, 43, 2683–2688. [Google Scholar]
- Dickinson, T.A.; Michael, K.L.; Kauer, J.S.; Walt, D.R. Convergent, Self-Encoded Bead Sensor Arrays in the design of an Artificial Nose. Anal. Chem. 1999, 71, 2192–2198. [Google Scholar]
- Yuan, J.; El-Sherif, A. Fiber-Optic Chemical Sensor Using Polyaniline as Modified Cladding Material. IEEE Sensor. J. 2003, 3, 5–12. [Google Scholar]
- Messica, A.; Greenstein, A.; Katzir, A. Theory of fiber-optic, evanescent-wave spectroscopy and sensors. Appl. Opt. 1996, 35, 2274–2284. [Google Scholar]
- Schwotzer, G.; Latka, I.; Lehmann, H.; Willsch, R. Optical sensing of hydrocarbons in air or in water using UV absorption in the evanescent field of fibers. Sensor. Actuat. B-Chem. 1997, 38-39, 150–153. [Google Scholar]
- Shadaram, M.; Espada, L.; Garcoa, F. Modeling and performance evaluation of ferrocene-based polymer clad tapered optical fiber gas sensors. Opt. Eng. 1998, 37, 1124–1129. [Google Scholar]
- Barmenkov, Y.O. Time-domain fiber laser hydrogen sensor. Opt. Lett. 2004, 29, 2461–2463. [Google Scholar]
- Lacroix, S.; Bourbonnais, R.; Gonthier, F.; Bures, J. Tapered monomode optical fibers: understanding large power transfer. Appl. Opt. 1986, 25, 4421–4425. [Google Scholar]
- Willer, U.; Scheel, D.; Kostjucenko, I.; Bohling, C.; Schade, W.; Faber, E. Fiber-optic evanescent-field laser sensor for in-situ gas diagnostics. Spectrochim. Acta A 2002, 58, 2427–2432. [Google Scholar]
- Senosiain, J.; Díaz, I.; Gastón, A.; Sevilla, J. High Sensitivity Temperature Sensor Based on Side-Polished Optical Fiber. IEEE Trans. Instrum. Meas. 2001, 50, 1656–1660. [Google Scholar]
- Gastón, A.; Pérez, F.; Sevilla, J. Optical fiber relative-humidity sensor with polyvinyl alcohol film. Appl. Opt. 2004, 43, 4127–4132. [Google Scholar]
- Gastón, A.; Lozano, I.; Pérez, F.; Auza, F.; Sevilla, J. Evanescent Wave Optical-Fiber Sensing (Temperature, Relative Humidity and pH Sensors). IEEE Sensor. J. 2003, 3, 806–811. [Google Scholar]
- Segawa, H.; Ohnishi, E.; Arai, Y.; Yoshida, K. Sensitivity of fiber-optic carbon dioxide sensors utilizing indicator dye. Sensor. Actuat. B-Chem. 2003, 94, 276–281. [Google Scholar]
- Cherif, K.; Mrazek, J.; Hleli, S.; Matejec, V.; Abdelghani, A.; Chomat, M.; Jaffrezic-Renault, N.; Kasik, I. Detection of aromatic hydrocarbons in air and water by using xerogel layers coated on PCS fibers excited by an inclined collimated beam. Sensor. Actuat. B-Chem. 2003, 95, 97–106. [Google Scholar]
- Malis, C.; Landl, M.; Simon, P.; MacCraith, B.D. Fiber optic ammonia sensing employing novel near infrared dyes. Sensor. Actuat. B-Chem. 1998, 51, 359–367. [Google Scholar]
- Potyrailo, R.A.; Hieftje, G.M. Oxygen detection by fluorescence quenching of tetraphenylporphyrin immobilized in the original cladding of an optical fiber. Anal. Chim. Acta 1998, 370, 1–8. [Google Scholar]
- Okazaki, S.; Nakagawa, H.; Asakura, S.; Tomiuchi, Y.; Tsuji, N.; Murayama, H.; Washiya, M. Sensing Characteristics of an optical fiber sensor for hydrogen leak. Sensor. Actuat. B-Chem. 2003, 93, 142–147. [Google Scholar]
- Scorsone, E.; Christie, S.; Persaud, K.C.; Simon, P.; Kvasnik, F. Fibre-optic evanescent sensing of gaseous ammonia with two forms of a new near-infrared dye in comparison to phenol red. Sensor. Actuat. B-Chem. 2003, 90, 37–45. [Google Scholar]
- Sekimoto, S.; Nakagawa, H.; Okazaki, S.; Fukuda, K.; Asakura, S.; Shigemori, T.; Takahashi, S. A Fiber-optic evanescent-wave hydrogen gas sensor using palladium-supported tungsten oxide. Sensor. Actuat. B-Chem. 2000, 66, 142–145. [Google Scholar]
- Jorge, P.A.S.; Caldas, P.; Rosa, C.C.; Oliva, A.G.; Santos, J.L. Optical fiber probes for fluorescente based oxygen sensing. Sensor. Actuat. B-Chem. 2004, 130, 290–299. [Google Scholar]
- Grant, S.A.; Satcher, J.H., Jr.; Bettencourt, K. Development of sol-gel-based fiber nitrogen dioxide gas sensors. Sensor. Actuat. B-Chem. 2000, 69, 132–137. [Google Scholar]
- Abdelghani, A.; Chovelon, J.M.; Jaffrezic-Renault, N.; Lacroix, M.; Gagnaire, H.; Veillas, C.; Berkova, B.; Chomat, M.; Matejec, V. Optical fibre sensor coated with porous silica layers for gas and chemical vapour detection. Sensor. Actuat. B-Chem. 1997, 44, 495–498. [Google Scholar]
- Abdelmalek, F.; Chovelon, J.M.; Lacroix, M.; Jaffrezic-Renault, N.; Matajec, V. Optical fibre sensors sensitized by phenyl-modified porous silica prepared by sol-gel. Sensor. Actuat. B-Chem. 1999, 56, 234–242. [Google Scholar]
- Bariain, C.; Matias, I.R.; Romeo, I.; Garrido, J.; Laguna, M. Detection of volatile organic compund vapors by using a vapochromic material on a tapered optical fiber. Appl. Phys. Lett. 2000, 77, 2274–2276. [Google Scholar]
- Bariain, C.; Matias, I.R.; Romeo, I.; Garrido, J.; Laguna, M. Behavioral experimental studies of a novel vapochromic material towards development of optical fiber organic compounds sensor. Sensor. Actuat. B-Chem. 2001, 76, 25–31. [Google Scholar]
- Hu, W.; Liu, Y.; Xu, Y.; Liu, S.; Zhou, S.; Zeng, P.; Zhu, D.B. The gas sensitivity of Langmuir-Blodgett films of a new asymmetrically substituted phthalocyanine. Sensor. Actuat. B-Chem. 1999, 56, 228–233. [Google Scholar]
- Bariain, C.; Matias, I.R.; Fernandez-Valdivielso, C.; Arregui, F.J.; Rodríguez-Méndez, M.L.; De Saja, J.A. Optical fiber sensor based on lutetium bisphthalocyanine for the detection of gases using standard telecommunication wavelengths. Sensor. Actuat. B-Chem. 2003, 93, 153–158. [Google Scholar]
- Gutierrez, N.; Rodríguez-Méndez, M.L.; De Saja, J.A. Array of sensors based on lanthanide bisphthalocyanine Langmuir-Blodgett films for the detection of olive oil aroma. Sensor. Actuat. B-Chem. 2001, 77, 437–442. [Google Scholar]
- Khijwania, S.K.; Gupta, B.D. Maximum achievable sensitivity of the fiber optic evanescent field absorption sensor based on the U-shaped probe. Opt. Comm. 2000, 175, 135–137. [Google Scholar]
- Khijwania, S.K.; Gupta, B.D. Fiber optic evanescent field absorption sensor: Effect of fiber parameters and geometry of the probe. Opt. Quant. Electron. 1999, 31, 625–636. [Google Scholar]
- Otsuki, S.; Adachi, K.; Taguchi, T. A novel fiber-optic gas-sensing configuration using extremely curved optical fibers and an attempt for optical humidity detection. Sensor. Actuat. B-Chem. 1998, 53, 91–96. [Google Scholar]
- Tao, S.; Xu, L.; Fanguy, J.C. Optical fiber ammonia sensing probes using reagent immobilized porous silica coating as transducers. Sensor. Actuat. B-Chem. 2006, 115, 158–163. [Google Scholar]
- Scorsone, E.; Christie, S.; Persaud, K.C.; Kvasnik, F. Evanescent sensing of alkaline and acidic vapours using a plastic clad silica fibre doped with poly(o-methoxyaniline). Sensor. Actuat. B-Chem. 2004, 97, 174–181. [Google Scholar]
- Peng, W.; Pickrell, G.R.; Shen, F.; Wang, A. Experimental Investigation of Optical Waveguide-Based Multigas Sensing. IEEE Photon. Tech. Lett. 2004, 16, 2317–2319. [Google Scholar]
- Matias, I.R.; Bravo, J.; Arregui, F.J.; Corres, J.M. Nanofilms onto a hollow core fiber. Opt. Eng. Lett. 2006, 45, 050503-1–3. [Google Scholar]
- Hoo, Y.L.; Jin, W.; Wang, D.N. Evanescent-wave gas sensing microstructure fiber. Opt. Eng. 2002, 41, 8–9. [Google Scholar]
- Hoo, Y.L.; Jin, W.; Ho, H.L.; Wang, D.N. Measurement of Gas Diffusion Coefficient Using Photonic Crystal Fiber. IEEE Photon. Tech. Lett. 2003, 15, 1434–1436. [Google Scholar]
- Charlton, C.; Temelkuran, B.; Dellemann, G. Midinfrared sensors meet nanotechnology: Trace gas sensing with quantum cascade laser inside photonic band-gap hollow waveguides. Appl. Phys. Lett. 2005, 86, 194102. [Google Scholar]
- Konorov, S.O.; Fedetov, A.B.; Zheltikov, A.M. Phase. Phase-Matched four-wave mixing and sensing of water molecules by coherent anti-stokes Raman scattering in large-core-area hollow photonic-crystal fibers. Opt. Soc. Am. 2005, 22, 2049–2053. [Google Scholar]
- Hoo, Y.L.; Jin, W.; Ho, H.L.; Ju, J.; Wang, D.N. Gas diffusion measurement using hollow-core photonic bandgap fiber. Sensor. Actuat. B-Chem. 2005, 105, 183–186. [Google Scholar]
- Arregui, F.J.; Claus, R.O.; Cooper, K.L.; Fernandez-Valdivielso, C.; Matias, I.R. Optical Fiber Gas Sensor Based on Self-Assembled Gratings. J. Lightwav. Technol. 2001, 19, 1932–1937. [Google Scholar]
- Shinquan, T.; Winstead, C.B.; Jindal, R.; Singh, J.P. Optical-Fiber Sensor Using Tailored Porous Sol-Gel Fiber Core. IEEE Sensor. J. 2004, 4, 322–328. [Google Scholar]
- Othenos, A.; Kalli, K. Fiber Bragg Gratings; Artech House: Norwood and London, 1999. [Google Scholar]
- Maier, R.R.J.; Barton, J.S.; Jones, J.D.C.; McCulloch, S.; Jones, B.J.S.; Burnell, G. Palladium-based hydrogen sensing for monitoring of ageing materials. Meas. Sci. Technol. 2006, 17, 1118–1123. [Google Scholar]
- Wu, X.; Li, S.; Zhang, G.; Zhang, Z. Optical fiber sensor monitoring fault gas based on modulated fiber grating model filter. Proc. SPIE 1998, 3538, 147–154. [Google Scholar]
- Luo, S.; Liu, Y.; Sucheta, A.; Evans, M.; Tassell, R. Applications to LPG fiber optical sensors for relative humidity and chemical warfare agents monitoring. Proc. SPIE 2002, 4920, 193–204. [Google Scholar]
- Falate, R.; Kamikawachi, R.C.; Müller, M.; Kalinowski, H.J.; Fabris, J.L. Fiber optic sensors for hydrocarbon detection. Sensor. Actuat. B-Chem. 2005, 105, 430–436. [Google Scholar]
- Neurauter, G.; Klimant, I.; Wolfbeis, O.S. Fiber-optic microsensor for high resolution pCO2 sensing in marine environment. Fresen. J. Anal. Chem. 2000, 366, 481–487. [Google Scholar]
- Wolfbeis, O.S.; Kovacs, B.; Goswami, K.; Klainer, S.M. Fiber-Optic Fluorescence Carbon Dioxide Sensor for Environmental Monitoring. Mikrochim. Acta 1998, 129, 181–188. [Google Scholar]
- Giordano, M.; Russo, M.; Cusano, A.; Mensitieri, G. An high sensivity optical sensor for chloroform vapours detection base don nanometric film of δ-form syndiotactic polystyrene. Sensor. Actuat. B-Chem. 2005, 107, 140–147. [Google Scholar]
- Mechery, S.J.; Singh, J.P. Fiber optic based gas sensor with nanoporous structure for the selective detection of NO2 in air samples. Anal. Chim. Acta 2006, 557, 123–129. [Google Scholar]
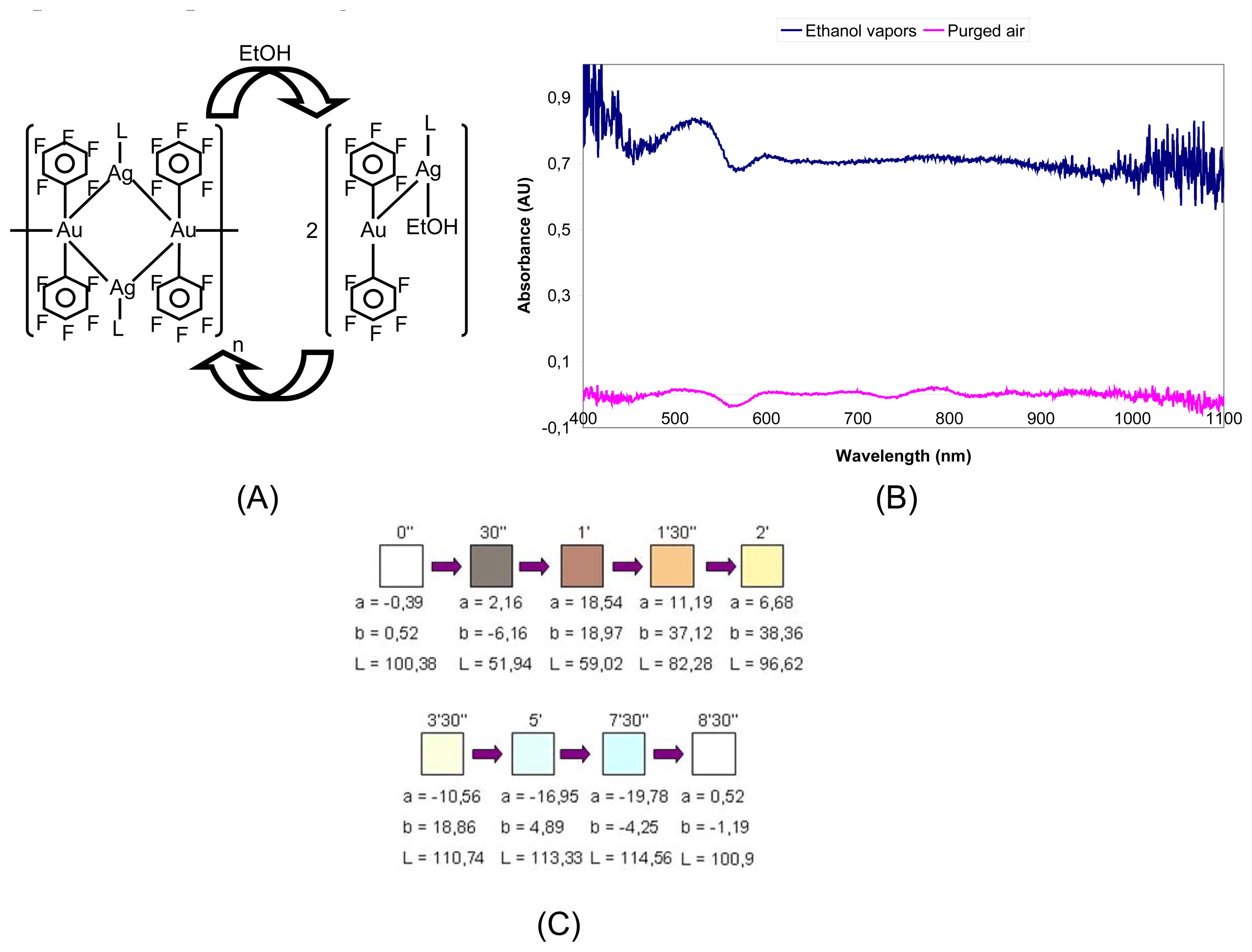
- Luquin, A.; Bariain, C.; Vergara, E.; Cerrada, E.; Garrido, J.; Matias, I.R.; Laguna, M. New preparation of gold-silver complexes and optical fibre environmental sensors based on vapochromic [Au2Ag2(C6F5)4(phen)2]n. Appl. Organomet. Chem. 2005, 19, 1232–1238. [Google Scholar]
- Bariain, C.; Matias, I.R.; Fernandez-Valdivielso, C.; Elosua, C.; Luquin, A.; Garrido, J.; Laguna, M. Optical fibre sensor base don vapochromic gold complexes for environmental applications. Sensor. Actuat. B-Chem. 2005, 108, 535–541. [Google Scholar]
- Casado, S.; Elosua, C.; Bariain, C.; Segura, A.; Matias, I.R.; Fernandez, A.; Luquin, A.; Garrido, J.; Laguna, M. Volatile-organic-compound optic fiber sensor using a gold-silver vapochromic complex. Opt. Eng. 2006, 45, 044401. [Google Scholar]
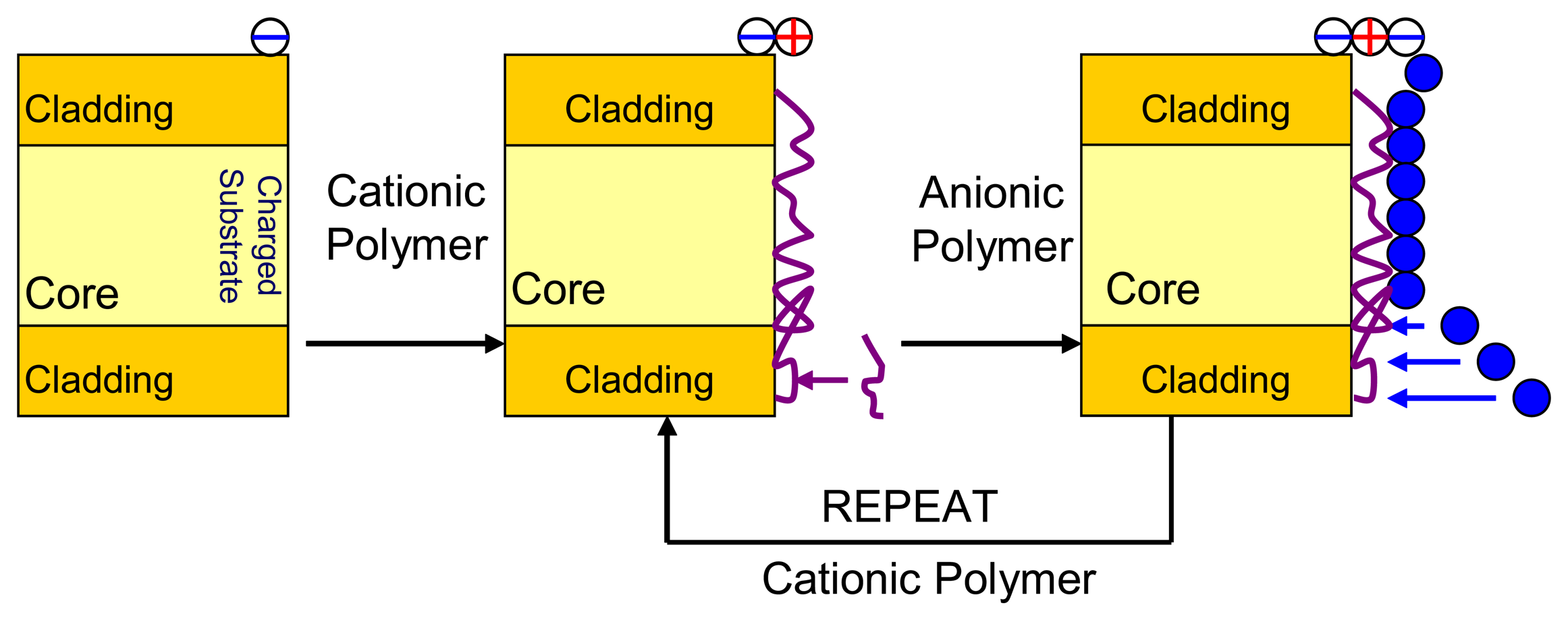
- Arregui, F.J.; Matias, I.R.; Liu, Y.; Lenahan, M.; Claus, R.O. Optical fiber nanometer-scale Fabry-Perot interferometer formed by the ionic self-assembly monolayer process. Opt. Lett. 1999, 24, 596–598. [Google Scholar]
- Choi, J.; Rubner, M.F. Influence of the degree of ionization on weak polyelectrolyte multilayer assembly. Macromolecules 2005, 38, 124–166. [Google Scholar]
- Elosua, C.; Bariain, C.; Matias, I.R.; Arregui, F.J.; Luquin, A.; Laguna, M. Volatile alcoholic compunds fibre optic nanosensor. Sensor. Actuat. B-Chem. 2006, 115, 444–449. [Google Scholar]
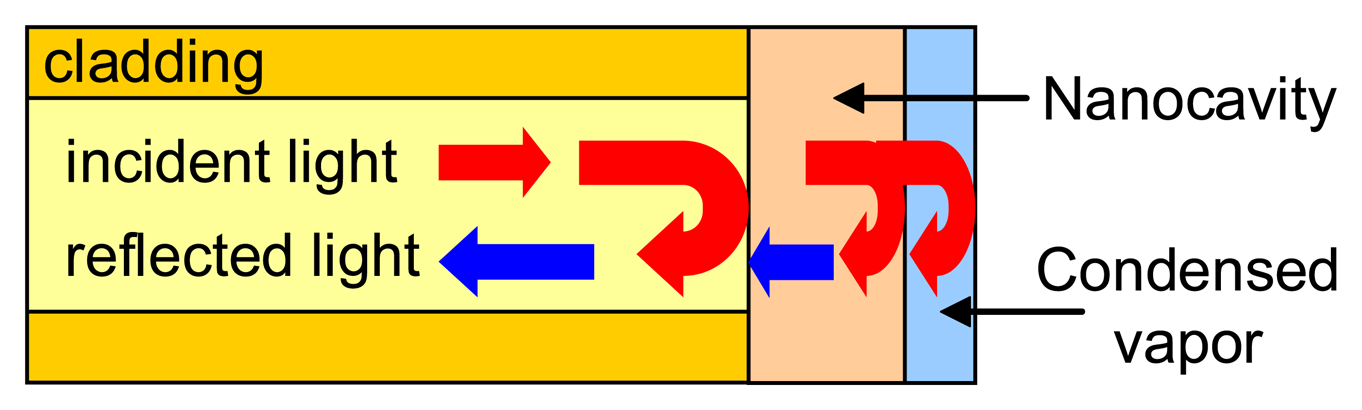
- Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Development of an In-Fiber Nanocavity Towards Detection of Volatile Organic Gases. Sensors 2006, 6, 578–592. [Google Scholar]
- Galbarra, D.; Arregui, F.J.; Matias, I.R.; Claus, R.O. Ammonia optical fiber sensor based on self-assembled zirconia thin films. Smart Mater. Struct. 2005, 14, 739–744. [Google Scholar]
- Arregui, F.J.; Matias, I.R.; Claus, R.O. Optical Fiber Gas Sensor Based on Hydrophobic Alumina Thin Films Formed by the Electrostatic Self-Assembly Monolayer Process. IEEE Sensor. J. 2003, 3, 56–61. [Google Scholar]
- Mitsubayashi, K.; Kon, T.; Hashimoto, Y. Optical bio-sniffer for ethanol using an oxygen-sensitive optical fiber. Biosens. Bioelectron. 2003, 19, 193–198. [Google Scholar]
- Mitsubayashi, K.; Minamide, T.; Otsuka, K.; Kudo, H.; Saito, H. Optical bio-sniffer for methyl mercaptan in halitosis. Anal. Chim. Acta 2006, 573-573, 75–80. [Google Scholar]
- Kim, Y.C.; Peng, W.; Banerji, S.; Booksh, K.S. Tapered fiber optic surface plasmon resonance sensor for analyses of vapor and liquid phases. Opt. Lett. 2005, 30, 2218–2220. [Google Scholar]
- O'Neal, P.D.; Meledeo, A.; Davis, J.R.; Ibey, B.L.; Gant, V.A.; Pishko, M.V.; Cote, G.L. Oxygen Sensor Based on the Fluorescence Quenching of a Ruthenium Complex Immobilized in a Biocompatible Poly(Ethylene Glycol) Hydrogel. IEEE Sensor. J. 2004, 4, 728–734. [Google Scholar]
- Moreno, J.; Arregui, F.J.; Matias, I.R. Fiber optic ammonia sensing employing novel thermoplastic polyurethane membranes. Sensor. Actuat. B-Chem. 2005, 105, 419–424. [Google Scholar]
- Grattan, K.T.V.; Zhang, Z.Y. Fiber Optic Fluorescence Thermometry; Chapman & Hall: London, 1995. [Google Scholar]
- Dybko, A. Errors in Chemical Sensor Measurements. Sensors 2001, 1, 29–37. [Google Scholar]
- Boilot, P.; Hines, E.L.; Gongora, M.A.; Folland, R.S. Electronic noses inter-comparison, data fusion and sensor selection in discrimination of standard fruit solutions. Sensor. Actuat. B-Chem. 2003, 88, 80–88. [Google Scholar]
- Taurino, A.M.; Distante, C.; Siciliano, P.; Vasanelli, L. Quantitative and qualitative analysis of VOCs mixtures by means of a microsensors array and different evaluation methods. Sensor. Actuat. B-Chem. 2003, 93, 117–125. [Google Scholar]
- Pardo, M.; Sberveglieri, G.; Gardini, S.; Dalcanale, E. A hierarchical classification scheme for an Electronic Nose. Sensor. Actuat. B-Chem. 2000, 69, 359–365. [Google Scholar]
- Pardo, M.; Niederjaufner, G.; Benussi, G.; Comini, E.; Faglia, G.; Sberveglieri, G.; Holmberg, M.; Lundstrom, I. Data preprocessing enhances the classification of different brands of Espresso coffee with an electronic nose. Sensor. Actuat. B-Chem. 2000, 69, 397–403. [Google Scholar]
- Luo, D.; Hosseini, H.G.; Stewart, J.R. Application of an ANN with extracted parameters from an electronic nose in cigarette brand identification. Sensor. Actuat. B-Chem. 2004, 99, 253–257. [Google Scholar]
- Zhang, H.; Blaban, M.O.; Principe, J.C. Improving pattern recognition of electronic nose data with time-delay neural networks. Sensor. Actuat. B-Chem. 2003, 96, 385–389. [Google Scholar]
- Carmel, L.; Seve, N.; Lancet, D.; Harel, D. An eNose algorithm for identifying chemicals and determinig their concentration. Sensor. Actuat. B-Chem. 2003, 93, 77–83. [Google Scholar]
- Brudzewski, K.; Osowski, S.; Markiewicz, T. Classification of milk by means of an electronic nose and SVM neural network. Sensor. Actuat. B-Chem. 2004, 98, 291–298. [Google Scholar]
- Walt, D.R.; Dickinson, T.; White, J.; Kauer, J.; Stephen, J.; Engelhardt, H.; Sutter, J.; Jurs, P. Optical sensor arrays for odor recognition. Biosens. Bioelectron. 1999, 13, 697–699. [Google Scholar]
- Bakken, G.A.; Kauffman, G.W.; Jurs, P.C.; Albert, K.J.; Stitzel, S.S. Pattern recognition analysis of optical sensor array data to detect nitroaromatic compounds vapors. Sensor. Actuat. B-Chem. 2001, 79, 1–10. [Google Scholar]



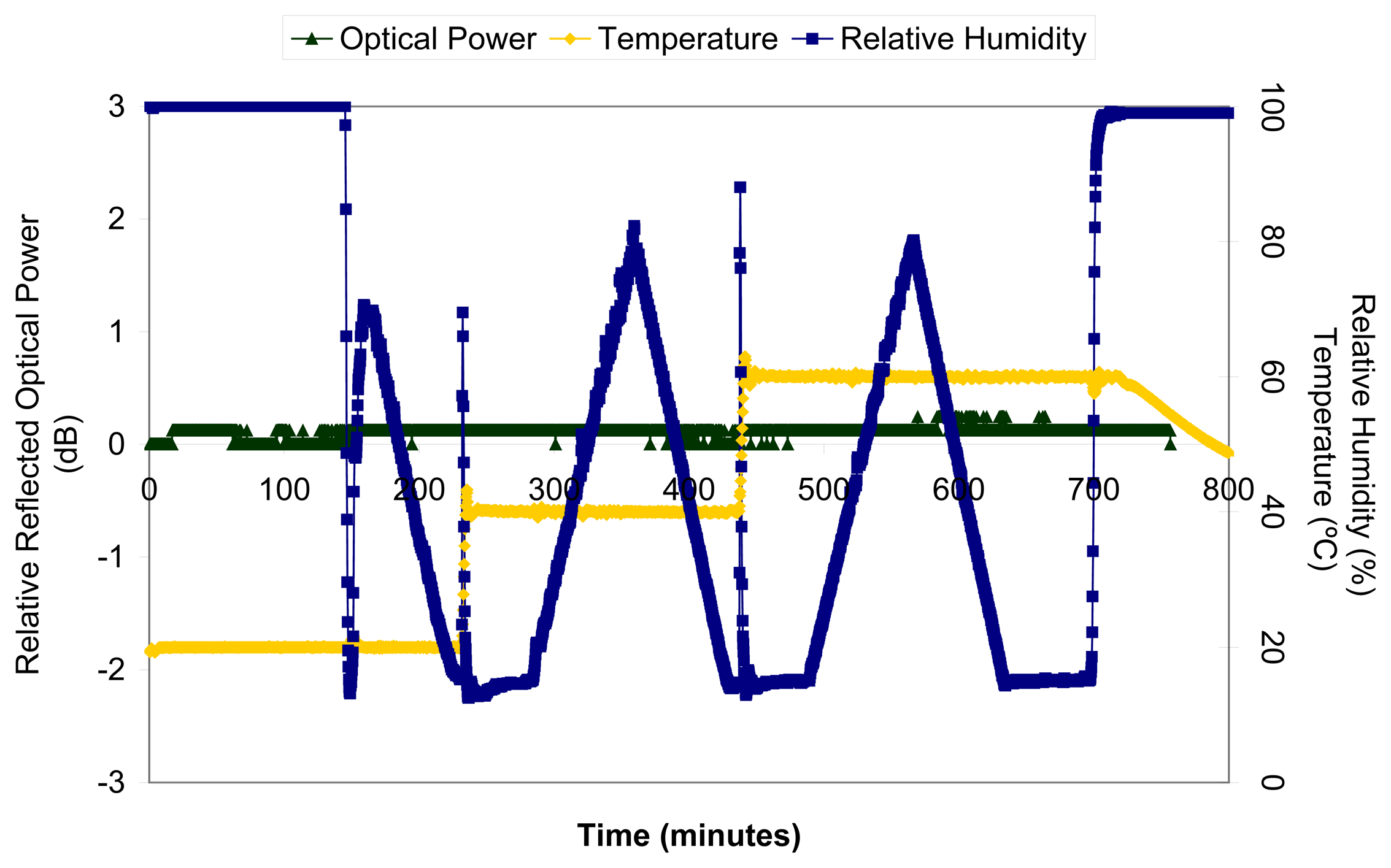
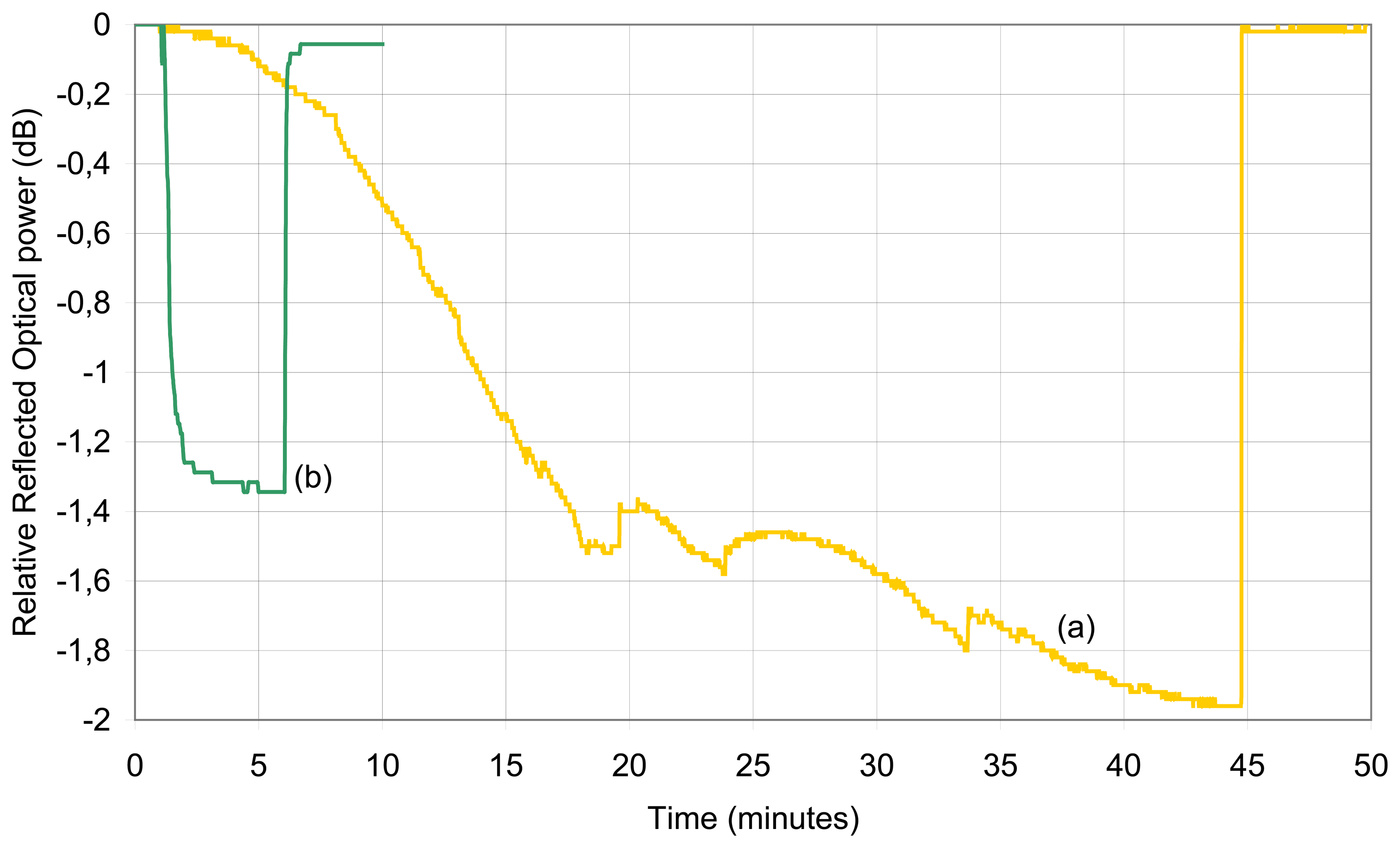

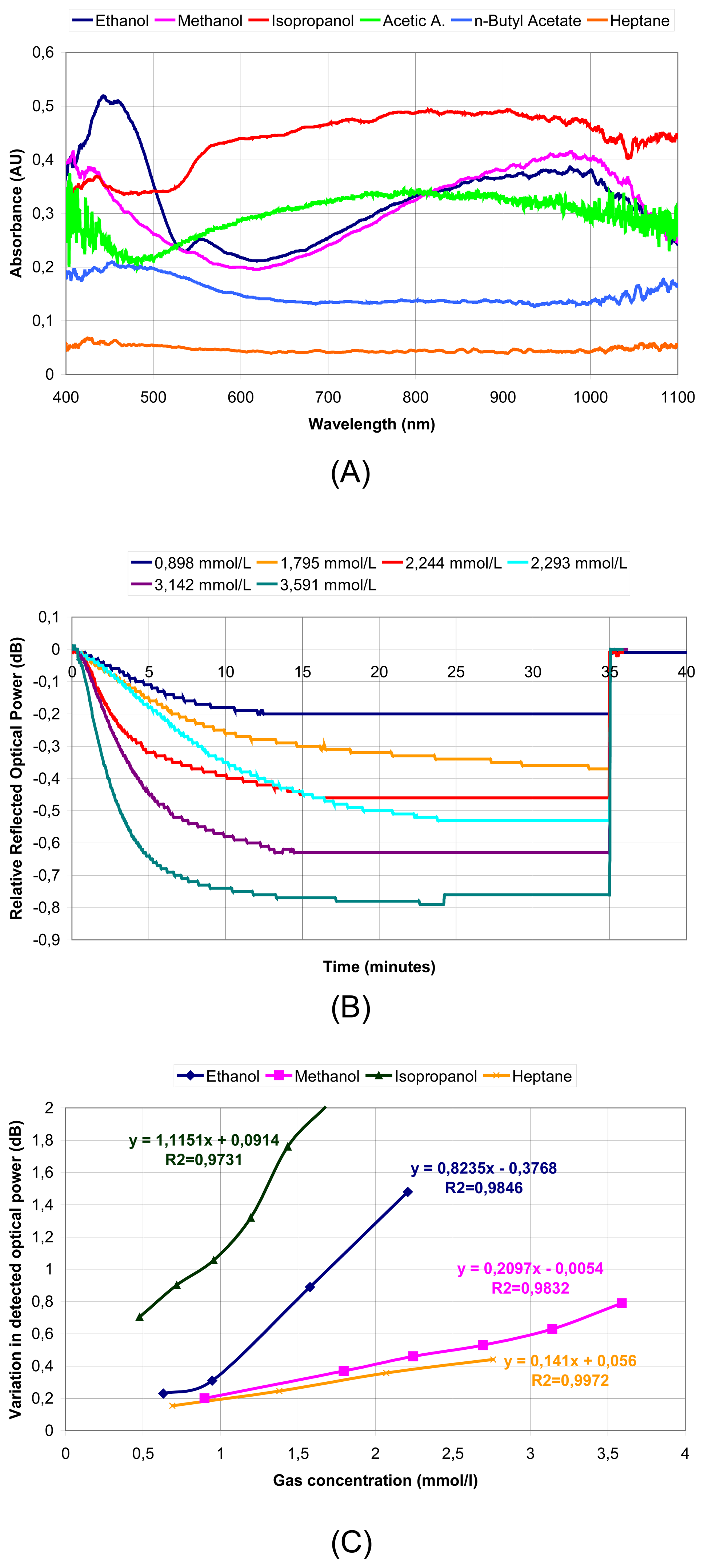
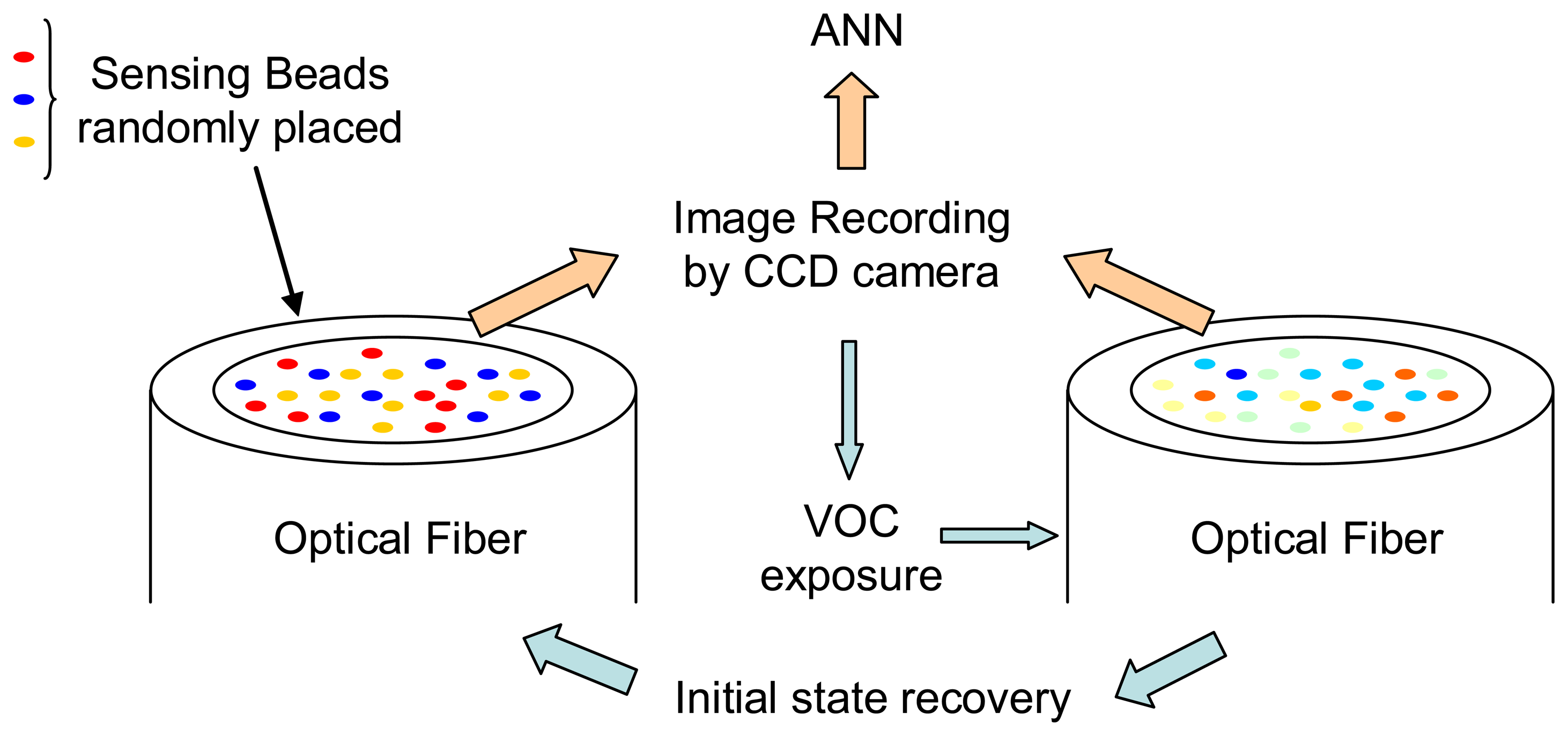
© 2006 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Elosua, C.; Matias, I.R.; Bariain, C.; Arregui, F.J. Volatile Organic Compound Optical Fiber Sensors: A Review. Sensors 2006, 6, 1440-1465. https://doi.org/10.3390/s6111440
Elosua C, Matias IR, Bariain C, Arregui FJ. Volatile Organic Compound Optical Fiber Sensors: A Review. Sensors. 2006; 6(11):1440-1465. https://doi.org/10.3390/s6111440
Chicago/Turabian StyleElosua, Cesar, Ignacio R. Matias, Candido Bariain, and Francisco J. Arregui. 2006. "Volatile Organic Compound Optical Fiber Sensors: A Review" Sensors 6, no. 11: 1440-1465. https://doi.org/10.3390/s6111440
APA StyleElosua, C., Matias, I. R., Bariain, C., & Arregui, F. J. (2006). Volatile Organic Compound Optical Fiber Sensors: A Review. Sensors, 6(11), 1440-1465. https://doi.org/10.3390/s6111440





