Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration
Abstract
:1. Introduction
2. Theory and Methods
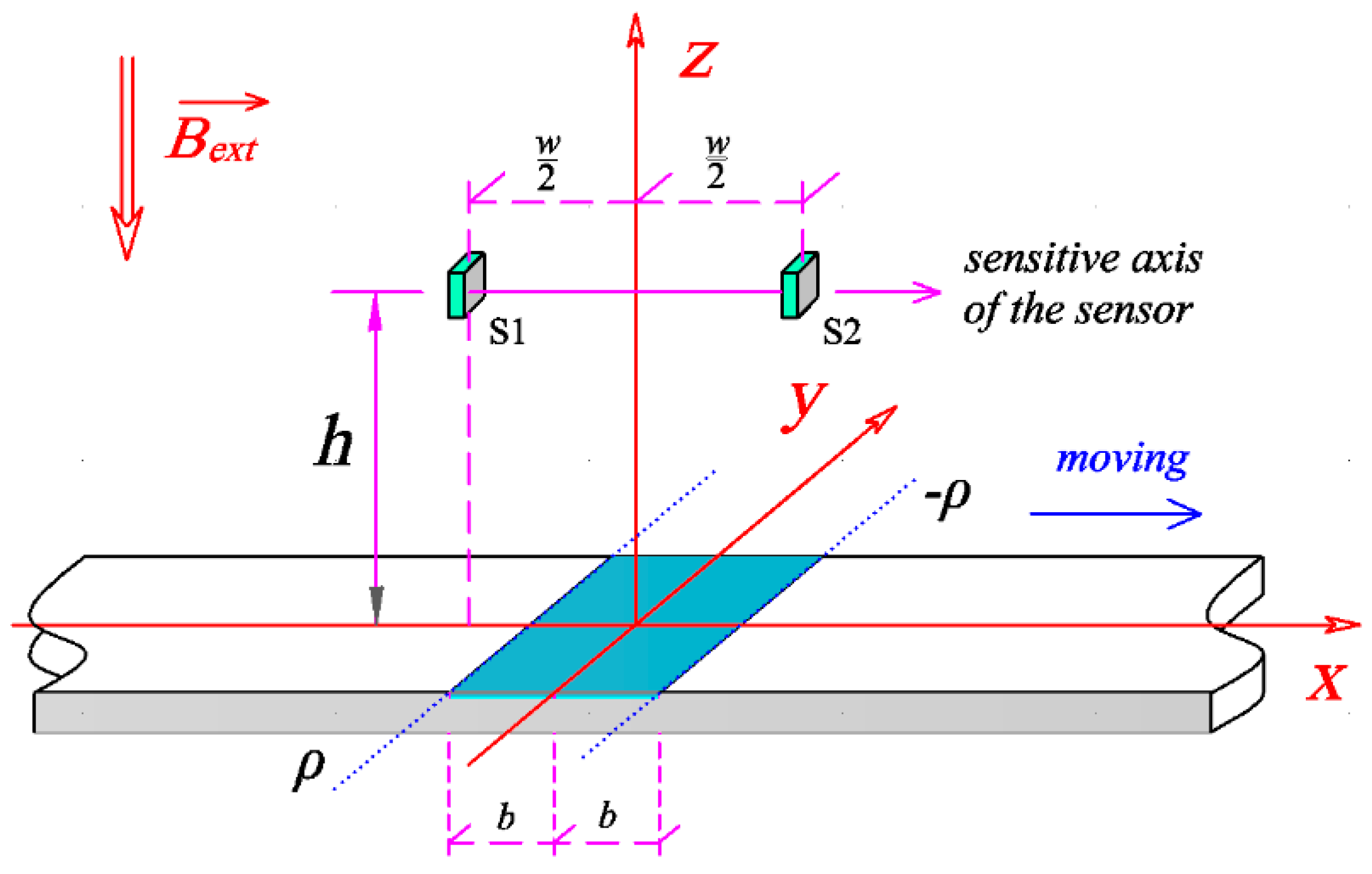
2.1. Analytical Signal Produced by Magnetic Strips
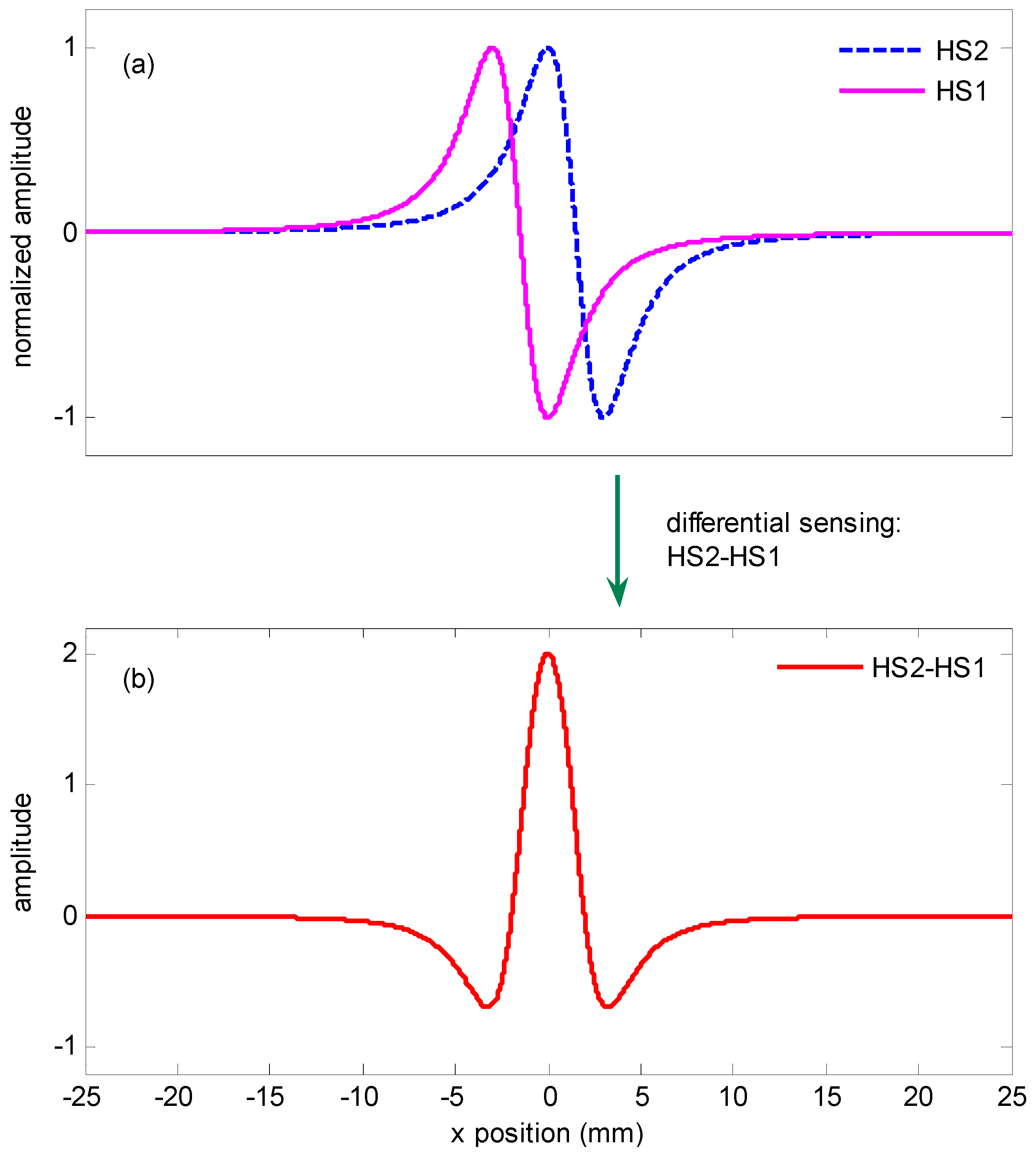
2.2. Differential Sensing Method
3. Experiments
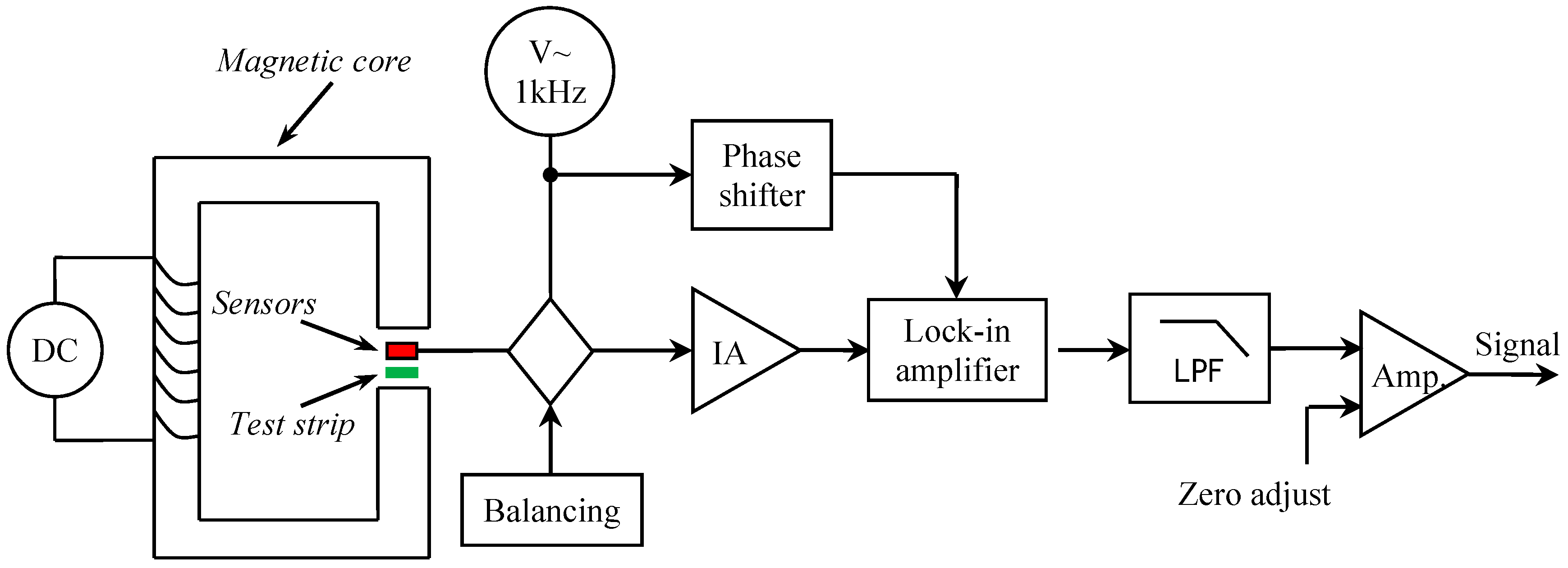
3.1. Experimental Prototype
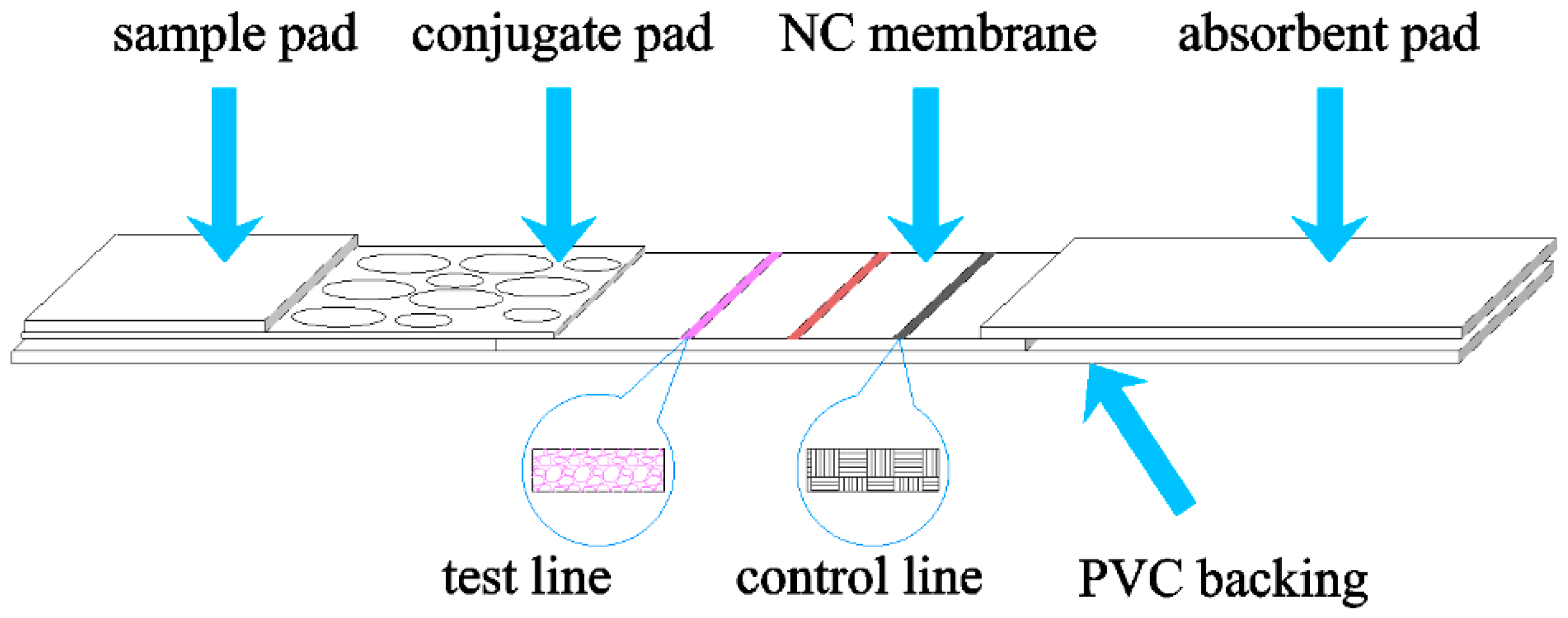
3.2. Sample Preparation
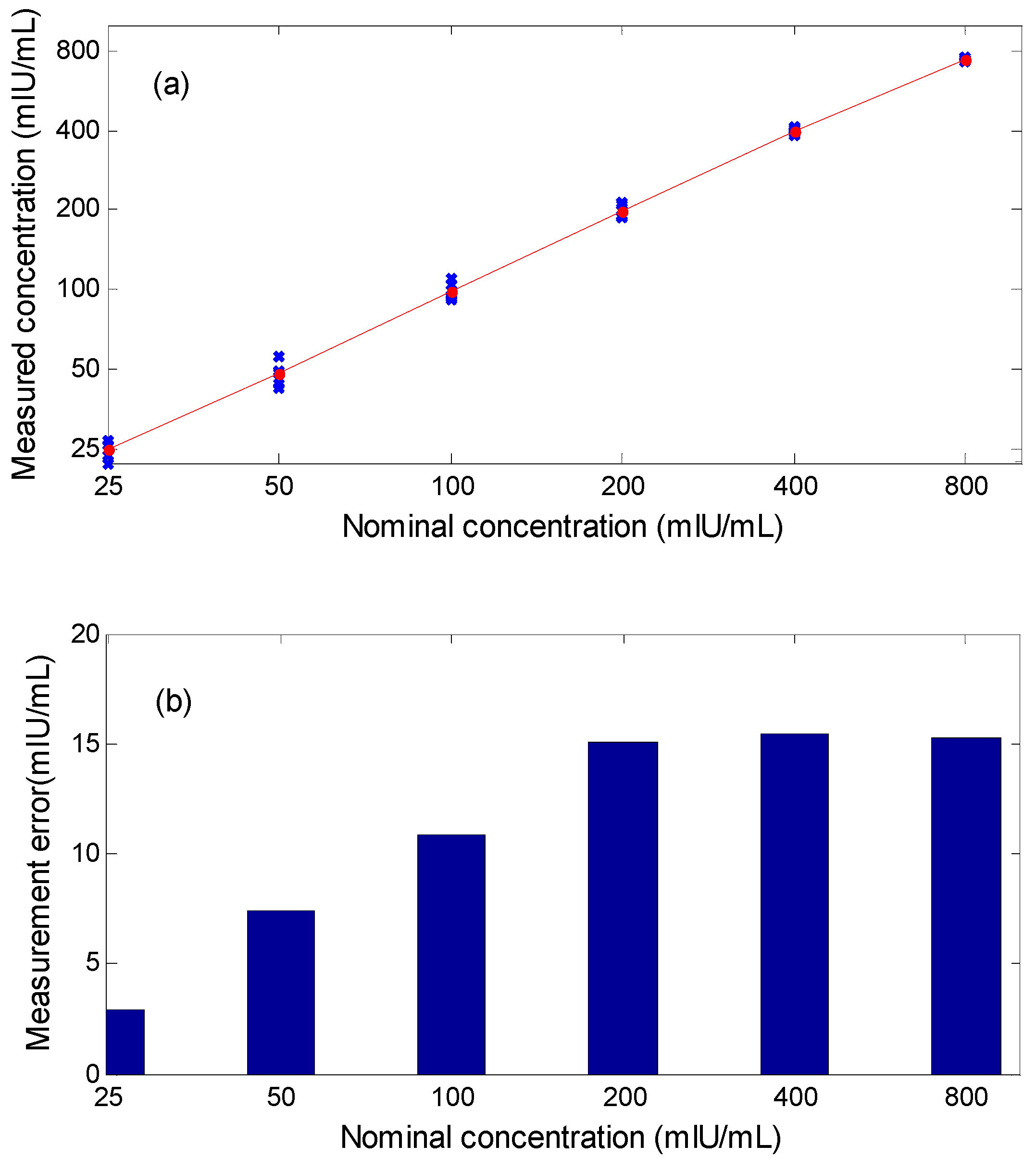
4. Results and Discussion
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yazdani, F.; Fattahi, B.; Azizi, N. Synthesis of functionalized magnetite nanoparticles to use as liver targeting MRI contrast agent. J. Magn. Magn. Mater. 2016, 406, 207–211. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Parit, S.B.; Chougale, A.D.; Sahoo, S.C.; Patil, P.S.; Patil, P.B. Immobilization of invertase on chitosan coated γ-Fe2O3 magnetic nanoparticles to facilitate magnetic separation. J. Colloid Interface Sci. 2016, 482, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, U.; Keskin, T.; TansIk, G.; Mutlu, P.; Yalcın, S.; Unsoy, G.; Yakar, A.; Khodadust, R.; Gunduz, G. Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer. Biomed. Pharmacother. 2014, 68, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Rezayan, A.H.; Mousavi, M.; Kheirjou, S.; Amoabediny, G.; Ardestani, M.S.; Mohammadnejad, J. Monodisperse magnetite(Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Magn. Mater. 2016, 420, 210–217. [Google Scholar] [CrossRef]
- Bahadir, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Yao, L.; Teng, J.; Zhu, M.; Zheng, L.; Zhong, Y.; Liu, G.; Xue, F.; Chen, W. MWCNTs based high sensitive lateral flow strip biosensor for rapid determination of aqueous mercury ions. Biosens. Bioelectron. 2016, 85, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Marquina, C.; de Teresa, J.M.; Serrate, D.; Marzo, J.; Cardoso, F.A.; Saurel, D.; Cardoso, S.; Freitas, P.P.; Ibarra, M.R. GMR sensors and magnetic nanoparticles for immuno-chromatographic assays. J. Magn. Magn. Mater. 2012, 324, 3495–3498. [Google Scholar] [CrossRef]
- Krause, H.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.F.; Hartmann, M.; Keusgen, M. Magnetic particle detection by frequency mixing for immunoassay applications. J. Magn. Magn. Mater. 2007, 311, 436–444. [Google Scholar] [CrossRef]
- Rivas, M.; Lago-Cachón, D.; Martínez-García, J.C.; García, J.A.; Calleja, A.J. Eddy-current sensing of superparamagnetic nanoparticles with spiral-like copper circuits. Sens. Actuators A. 2014, 216, 123–127. [Google Scholar] [CrossRef]
- Visscher, M.; Waanders, S.; Krooshoop, H.J.G.; ten Haken, B. Selective detection of magnetic nanoparticles in biomedical applications using differential magnetometry. J. Magn. Magn. Mater. 2014, 365, 31–39. [Google Scholar] [CrossRef]
- Wu, K.; Wang, Y.; Feng, Y.; Yu, L.; Wang, J. Colorize magnetic nanoparticles using a search coil based testing method. J. Magn. Magn. Mater. 2015, 380, 251–254. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Manteca, A.; Mujika, M.; Arana, S. GMR sensors: Magnetoresistive behaviour optimization for biological detection by means of superparamagnetic nanoparticles. Biosens. Bioelectron. 2011, 26, 3705–3709. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Xu, L.; Wilson, R.J.; Wang, S.X. A novel zero-drift detection method for highly sensitive GMR biochips. IEEE Trans. Magn. 2006, 42, 3560–3562. [Google Scholar] [CrossRef]
- Lee, C.; Lai, M.; Huang, H.; Lin, C.; Wei, Z. Wheatstone bridge giant-magnetoresistance based cell counter. Biosens. Bioelectron. 2014, 57, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Q.; Gao, X.Y.; Leng, F.F.; Lü, M.; Guo, P.Z.; Zhao, G.X.; Li, S.D. Detection of the concentration of MnFe2O4 magnetic microparticles using giant magnetoresistance sensors. IEEE Trans. Magn. 2016, 52. [Google Scholar] [CrossRef]
- Park, J. Superparamagnetic nanoparticle quantification using a giant magnetoresistive sensor and permanent magnets. J. Magn. Magn. Mater. 2015, 389, 56–60. [Google Scholar] [CrossRef]
- Quynh, L.K.; Tu, B.D.; Dang, D.X.; Viet, D.Q.; Hien, L.T.; Giang, D.T.H.; Duc, N.H. Detection of magnetic nanoparticles using simple AMR sensors in Wheatstone bridge. J. Sci. Adv. Mater. Devices. 2016, 1, 98–102. [Google Scholar] [CrossRef]
- Choi, J.; Gani, A.; Bechstein, D.J.B.; Lee, J.; Utz, P.J.; Wang, S.X. Portable, one-step, and rapid GMR biosensor platform with smartphone interface. Biosens. Bioelectron. 2016, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Albisetti, E.; Petti, D.; Cantoni, M.; Damin, F.; Torti, A.; Chiari, M.; Bertacco, R. Conditions for efficient on-chip magnetic bead detection via magnetoresistive sensors. Biosens. Bioelectron. 2013, 47, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, M.P.; Vetoshko, P.M.; Brusentsov, N.A.; Nikitin, P.I. Highly sensitive room-temperature method of non-invasive in vivo detection of magnetic nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1658–1661. [Google Scholar] [CrossRef]
- Useinov, A.N.; Useinov, N.K.; Ye, L.X.; Wu, T.; Lai, C.H. Tunnel magnetoresistance in magnetic tunnel junctions with embedded nanoparticles. IEEE Trans. Magn. 2015, 51. [Google Scholar] [CrossRef]
- Albisetti, E.; Pett, D.; Damin, F.; Cretich, M.; Torti, A.; Chiari, M.; Bertacco, R. Photolithographic bio-patterning of magnetic sensors for biomolecular recognition. Sens. Actuators B 2014, 200, 39–46. [Google Scholar] [CrossRef]
- Zatsepin, N.N.; Shcherbinin, V.E. On calculation of magnetostatic field generated by surface flaws. I. Topography of fields in flaws models. Defektoskopiya 1966, 5, 50–59. [Google Scholar]
- Lei, H.; Tian, G. Broken wire detection in coated steel belts using magnetic flux leakage method. Insight 2013, 55, 126–131. [Google Scholar] [CrossRef]
- Li, G.; Sun, S.; Wang, S.X. Spin valve biosensors: Signal dependence on nanoparticle position. J. Appl. Phys. 2006, 99. [Google Scholar] [CrossRef]
- Fodil, K.; Denoual, M.; Dolabdjian, C. Experimental and analytical investigation of a 2-D magnetic imaging method using magnetic nanoparticles. IEEE Trans. Magn. 2016, 52. [Google Scholar] [CrossRef]
- Xiao, L.; Li, J.; Brougham, D.F.; Fox, E.K.; Feliu, N.; Bushmelev, A. Water-soluble superparamagnetic magnetite nanoparticles with biocompatible coating for enhanced magnetic resonance imaging. ACS Nano 2011, 5, 6315–6324. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, K.; Huang, P.; Zhang, Y.; Zhi, X.; Bao, C.; Cui, D. Superparamagnetic Fe3O4-Ag hybrid nanocrystals as a potential contrast agent for CT imaging. CrystEngComm 2012, 14, 7556–7559. [Google Scholar] [CrossRef]
- Workman, S.; Wells, S.K.; Pau, C.; Owen, S.M.; Dong, X.F.; LaBorde, R.; Granade, T.C. Rapid detection of HIV-1 p24 antigen using magnetic immuno-chromatography (MICT). J. Virol. Methods 2009, 160, 14–21. [Google Scholar] [CrossRef] [PubMed]
- MagnaBioScience. Available online: http://www.magnabiosciences.com/ (accessed on 5 October 2016).




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Wang, K.; Ji, X.; Cui, D. Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration. Sensors 2016, 16, 2130. https://doi.org/10.3390/s16122130
Lei H, Wang K, Ji X, Cui D. Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration. Sensors. 2016; 16(12):2130. https://doi.org/10.3390/s16122130
Chicago/Turabian StyleLei, Huaming, Kan Wang, Xiaojun Ji, and Daxiang Cui. 2016. "Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration" Sensors 16, no. 12: 2130. https://doi.org/10.3390/s16122130





