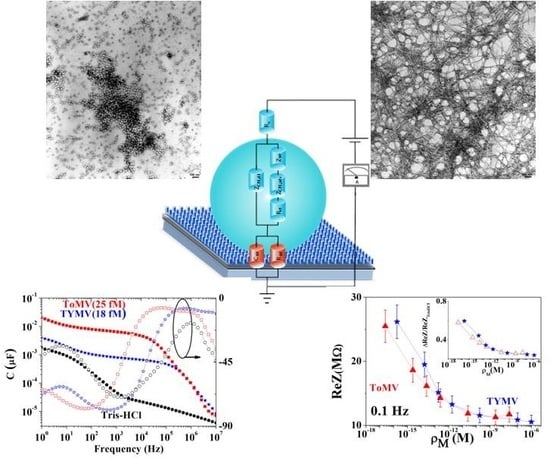
Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices
Abstract
:1. Introduction
2. Materials and Methods
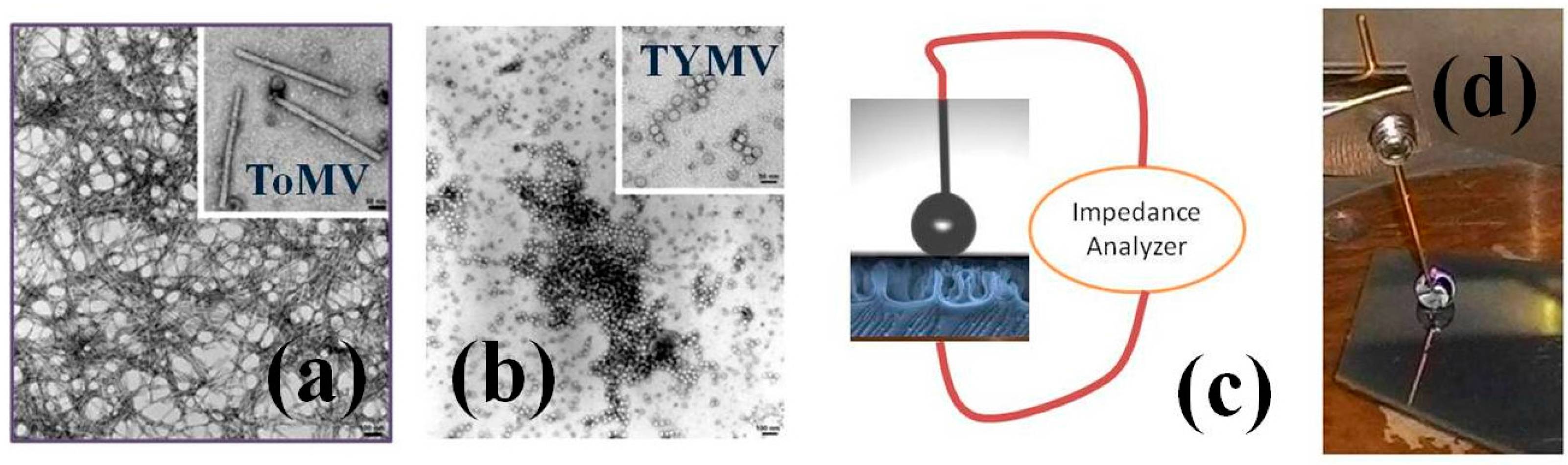
2.1. Virus Description and Morphological Characterization
2.2. Virus Extraction, Characterization, and Suspension Preparation
2.3. Surface Texturing
2.4. Device Configuration and Measurement Procedure
2.5. Impedance Measurements
3. Results
3.1. NPs Inspection
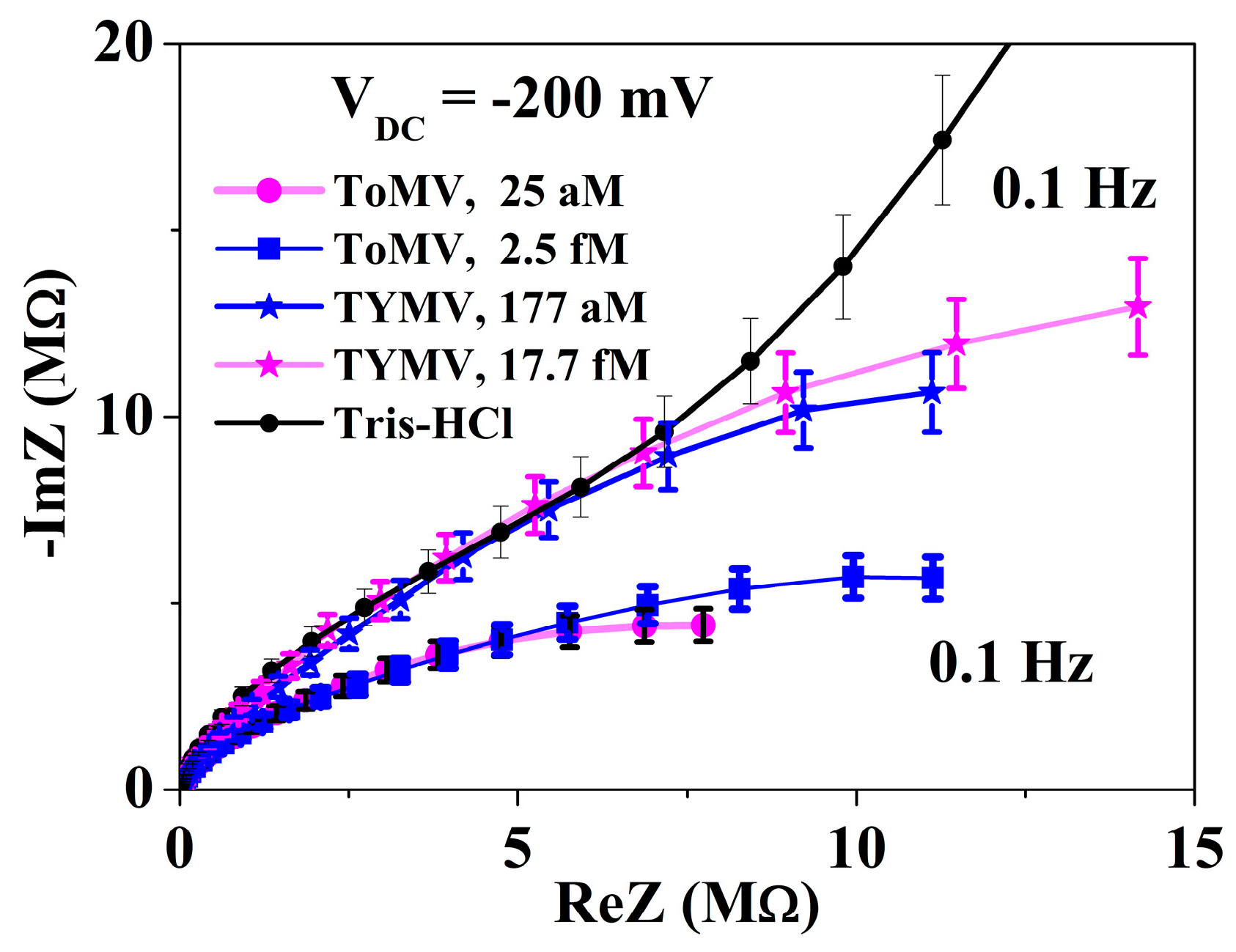
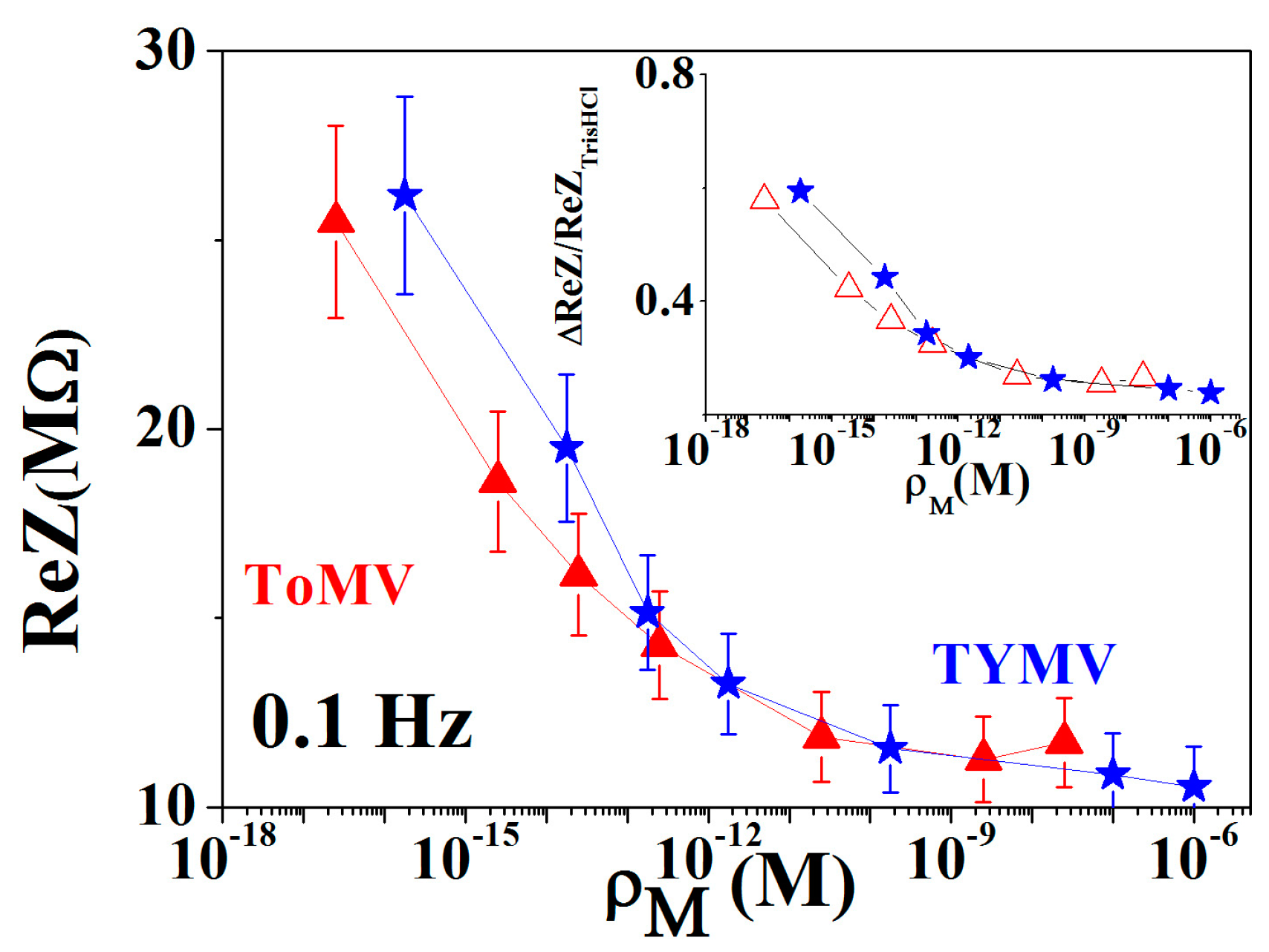
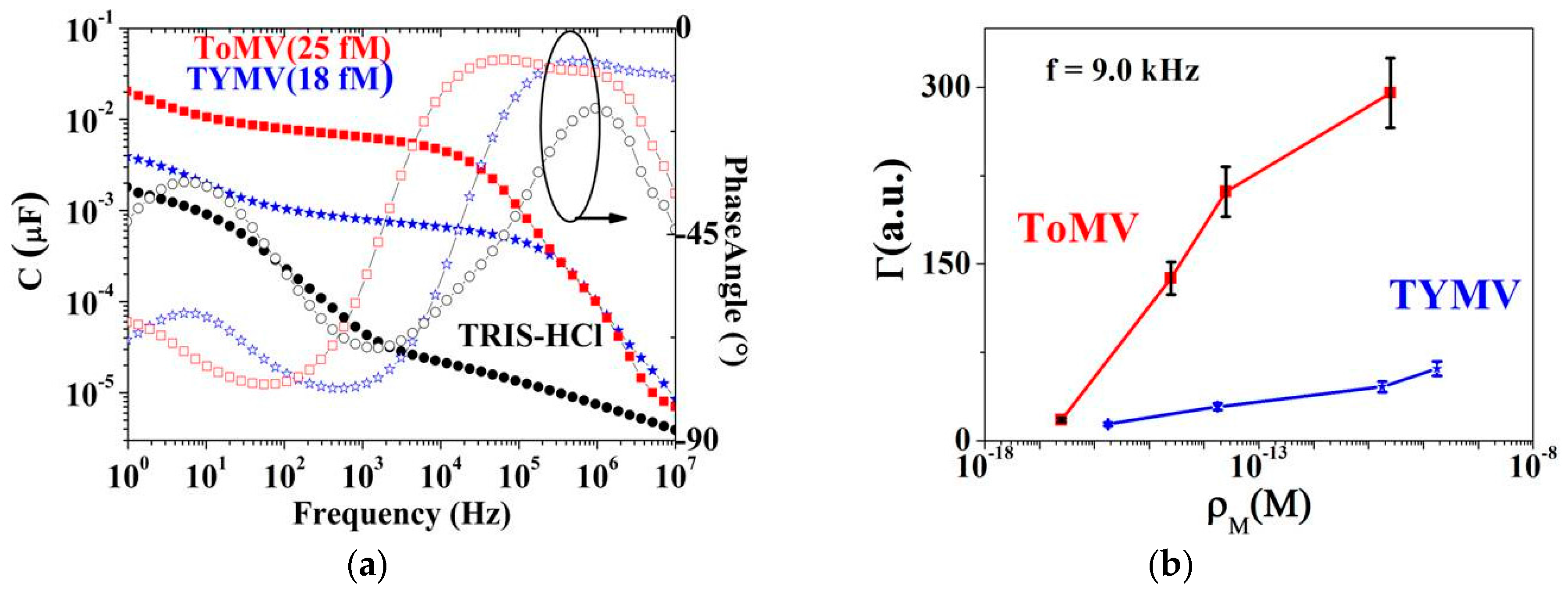
3.2. Calibration Curve Extraction and Virus Recognition via Real-Time Measurements
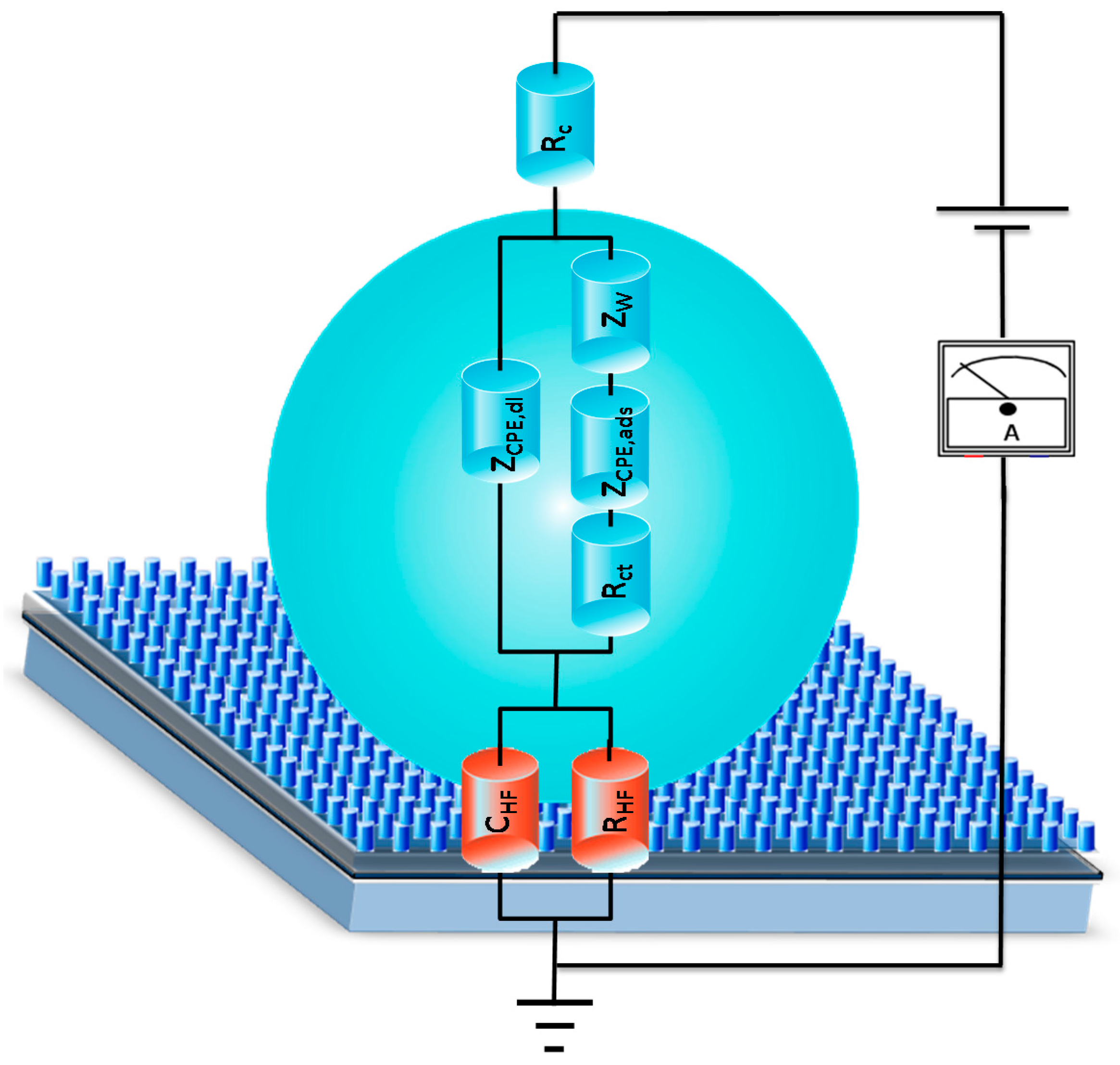
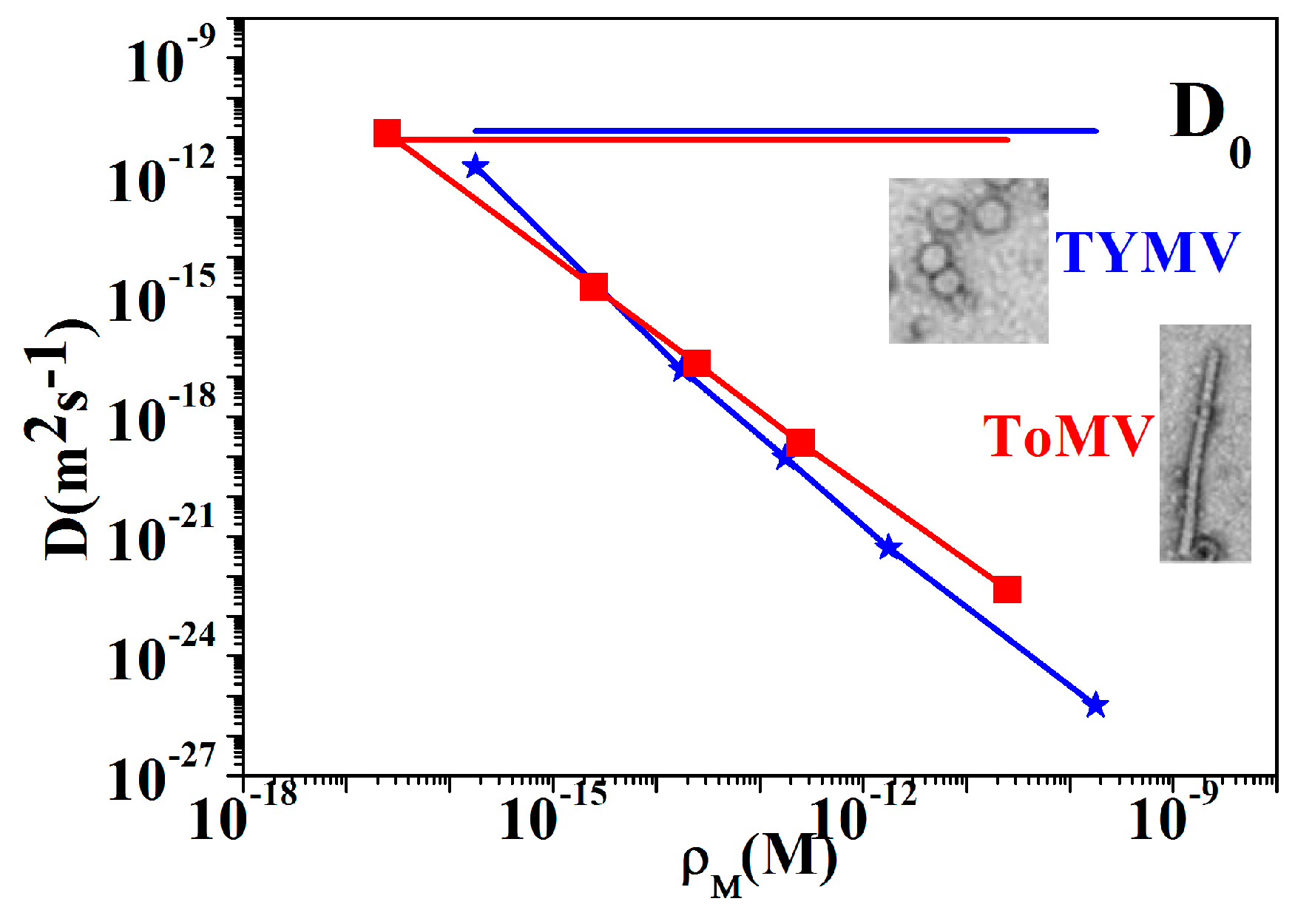
3.3. NPs Analysis: Compact Model and Virus Dynamics in the Droplets
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Makkouk, K.M.; Hsu, H.T.; Kumari, S.G. Detection of three plant viruses by dot-blot and tissue-blot immunoassays using chemiluminescent and chromogenic substrates. J. Phytopathol. 1993, 139, 97–102. [Google Scholar] [CrossRef]
- Fenby, N.S.; Scott, N.W.; Slater, A.; Elliott, M.C. PCR and non-isotopic labeling techniques for plant virus detection. Cell. Mol. Biol. 1995, 41, 639–652. [Google Scholar] [PubMed]
- McLaughlin, M.R.; Barnett, O.W.; Burrows, P.M.; Baum, R.H. Improved ELISA conditions for detection of plant viruses. J. Virol. Methods 1981, 3, 13–25. [Google Scholar] [CrossRef]
- Mullis, K.F.; Faloona, F.; Scharf, S.; Saiki, R.; Horn, G.; Erlich, H. Specific enzymatic amplification of DNA in vitro: The polymerase chain reaction. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.M.; French, R. The polymerase chain reaction and plant disease diagnosis. Annu. Rev. Phytopathol. 1993, 31, 81–109. [Google Scholar] [CrossRef] [PubMed]
- Eun, A.J.-C.; Huang, L.; Chew, F.T.; Li, S.F.-Y.; Wong, S.M. Detection of two orchid viruses using quartz crystal microbalance (QCM) immunosensors. J. Virol. Methods 2002, 99, 71–79. [Google Scholar] [CrossRef]
- Boltovets, P.M.; Snopok, B.A.; Boyko, V.R.; Shevchenko, T.P.; Dyachenko, N.S.; Shirshov, Y.M. Detection of plant viruses using a surface plasmon resonance via complexing with specific antibodies. J. Virol. Methods 2004, 121, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.K.; Carvalhal, R.F.; Stach-Machado, D.R.; Kubota, L.T. Surface plasmon resonance immunosensor for early diagnosis of Asian rust on soybean leaves. Biosens. Bioelectron. 2009, 24, 2483–2487. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Esteban-Ferrer, D.; Cuervo, A.; Carrascosa, J.L.; Gomila, G. Label-free identification of single dielectric nanoparticles and viruses with ultraweak polarization forces. Nat. Mater. 2012, 11, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Mustafa, F.; Ali, L.M.; Rizvi, T.A. Virus detection and quantification using electrical parameters. Sci. Rep. 2014, 4, 6831. [Google Scholar] [CrossRef] [PubMed]
- Al Ahmad, M.; Mustafa, F.; Ali, L.M.; Karakkat, J.V.; Rizvi, T.A. Label-free capacitance-based identification of viruses. Sci. Rep. 2015, 5, 9809. [Google Scholar] [CrossRef] [PubMed]
- Birnbaumer, G.M.; Lieberzeit, P.A.; Richter, L.; Schirhagl, R.; Milnera, M.; Dickert, F.L.; Bailey, A.; Ertl, P. Detection of viruses with molecularly imprinted polymers integrated on a microfluidic biochip using contact-less dielectric microsensors. Lab Chip 2009, 9, 3549–3556. [Google Scholar] [CrossRef] [PubMed]
- Jarocka, U.; Radecka, H.; Malinowski, T.; Michalczuk, L.; Radecki, J. Detection of Prunus Necrotic Ringspot Virus in Plant Extracts with Impedimetric Immunosensor Based on Glassy Carbon Electrode. Electroanalysis 2013, 25, 433–438. [Google Scholar] [CrossRef]
- Cahill, B.P.; Wiedemeier, S.; Gastrock, G. Measurement of droplet impedance in segmented flow. In Tagungsband, Proceedings of Dresdner Sensor-Symposium 2013, Dresden, Germany, 9–11 December 2013; AMA Service GmbH: Dresden, Germany, 2013; pp. 276–278. [Google Scholar]
- Simon, M.G.; Lin, R.; Lopez-Prieto, J.; Lee, A.P. Label-free detection of dna amplification in droplets using electrical impedance. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences 2011 (MicroTAS 2011), Seattle, WA, USA, 2–6 October 2011; Landers, J.P., Bienvenue, J., Herr, A., Eds.; Curran Associates: Red Hook, NY, USA, 2011; Volume 1, p. 1683. [Google Scholar]
- Wang, X.; Ma, S.; Su, Y.; Zhang, Y.; Bi, H.; Zhang, L.; Han, X. High Impedance Droplet–Solid Interface Lipid Bilayer Membranes. Anal. Chem. 2015, 87, 2094–2099. [Google Scholar] [CrossRef] [PubMed]
- Dak, P.; Ebrahimi, A.; Alam, M.A. Non-faradaic impedance characterization of an evaporating droplet for microfluidic and biosensing applications. Lab Chip 2014, 14, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Dak, P.; Salm, E.; Dash, S.; Garimella, S.V.; Bashir, R.; Alam, M.A. Nanotextured superhydrophobic electrodes enable detection of attomolar-scale DNA concentration within a droplet by non-faradaic impedance spectroscopy. Lab Chip 2013, 13, 4248–4256. [Google Scholar] [CrossRef] [PubMed]
- Turnip Yellow Mosaic Virus. Available online: http://www.dpvweb.net/dpv/showdpv.php?dpvno=230 (accessed on 17 November 2016).
- Tomato Mosaic Virus. Available online: http://www.dpvweb.net/dpv/showdpv.php?dpvno=156 (accessed on 17 November 2016).
- Foster, G.D.; Taylor, S.C. Plant Virology Protocols: From Virus Isolation to Transgenic Resistance; Humana Press: Totowa, NJ, USA, 1998. [Google Scholar]
- Brakke, M.K. Density-Gradient Centrifugation. In Methods in Virology 2; Maramorosch, K., Koprowski, H., Eds.; Academic Press: New York, NY, USA, 1967; pp. 93–118. [Google Scholar]
- Di Mundo, R.; Ambrico, M.; Ambrico, P.F.; D’Agostino, R.; Italiano, F.; Palumbo, F. Single-Step Plasma Process Producing Anti-Reflective and Photovoltaic Behavior on Crystalline Silicon. Plasma Process. Polym. 2011, 8, 239–245. [Google Scholar] [CrossRef]
- Ambrico, M.; Ambrico, P.F.; Cardone, A.; Cicco, S.R.; Palumbo, F.; Ligonzo, T.; Di Mundo, R.; Petta, V.; Augelli, V.; Favia, P.; et al. Melanin-like polymer layered on a nanotextured silicon surface for a hybrid biomimetic interface. J. Mater. Chem. C 2014, 2, 573–582. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A.; Osipovich, N.P.; Streltsov, E.A. Potentiodynamic electrochemical impedance spectroscopy of lead upd on polycrystalline gold and on selenium atomic underlayer. Electrochem. Commun. 2005, 7, 631–636. [Google Scholar] [CrossRef]
- Bondarenko, A.S.; Ragoisha, G.A. EIS Spectrum Analyser. Available online: http://www.abc.chemistry.b-su.by/vi/analyser/ (accessed on 17 November 2016).
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Song, J.; Bazant, M.Z. Effects of Nanoparticle Geometry and Size Distribution on Diffusion Impedance of Battery Electrodes. J. Electrochem. Soc. 2012, 160, A15–A24. [Google Scholar] [CrossRef]
- Maxakato, N.W.; Ozoemena, K.I.; Arendse, C.J. Dynamics of Electrocatalytic Oxidation of Ethylene Glycol, Methanol and Formic Acid at MWCNT Platform Electrochemically Modified with Pt/Ru Nanoparticles. Electroanalysis 2010, 22, 519–529. [Google Scholar] [CrossRef]
- Yun, H.J.; Lee, H.; Joo, J.B.; Kim, W.; Yi, J. Influence of Aspect Ratio of TiO2 Nanorods on the Photocatalytic Decomposition of Formic Acid. J. Phys. Chem. C 2009, 113, 3050–3055. [Google Scholar] [CrossRef]
- Bonanni, A.; Pumera, M.; Miyahara, Y. Influence of gold nanoparticle size (2–50 nm) upon its electrochemical behavior: An electrochemical impedance spectroscopic and voltammetric study. Phys. Chem. Chem. Phys. 2011, 13, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Woehl, T.J.; Prozorov, T. The Mechanisms for nanoparticle surface diffusion and chain self-assembly determined from real-time nanoscale kinetics in liquid. J. Phys. Chem. C 2015, 119, 21261–21269. [Google Scholar] [CrossRef]
- Moore, W.J. Physical Chemistry; Longman: London, UK, 1978. [Google Scholar]
- Cassagnau, P. Linear viscoelasticity and dynamics of suspensions and molten polymers filled with nanoparticles of different aspect ratios. Polymer 2013, 54, 4762–4775. [Google Scholar] [CrossRef]
- Sergio, R.A. Forward Depolarized Light Scattering from Wormlike Chains. Macromolecules 1987, 20, 370–380. [Google Scholar]
- Senyuk, B.; Glugla, D.; Smalyukh, I.I. Rotational and translational diffusion of anisotropic gold nanoparticles in liquid crystals controlled by varying surface anchoring. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. Plant Viruses. In Methods of Enzymatic Analysis, Volume XI. Antigens and Antibodies 2; Wiley-VCH: Weinheim, Germany, 1986; pp. 474–481. [Google Scholar]
- Candresse, T.; Hammond, R.W.; Hadidi, A. Detection and identi-fication of plant viruses and viroids using polymerase chain reaction (PCR). In Plant Virus Disease Control; Hadidi, A., Khetarpal, R.K., Koganezawa, H., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1998; pp. 409–410. [Google Scholar]

| ToMV | |||||
| ρM (M) | CHF (nF) | RHF (MΩ) | Rads (kΩ) | ZCPE,ads * | ZW § |
| 25 aM | 33 | 0.7 | 10 | 2.5 (0.99) | 104 |
| 250 aM | 36 | 1.1 | 6.8 | 8.0 (0.94) | 88 |
| 2.5 fM | 48 | 1.0 | 6.4 | 12 (0.94) | 81 |
| 25 fM | 74 | 0.9 | 5.8 | 17 (0.93) | 79 |
| 25 pM | 80 | 1.5 | 13 | 32 (0.90) | 55 |
| TRIS-HCl | 0.86 (0.82) | 4.1 | - | 1.9 × 10−3 (1) | |
| TYMV | |||||
| ρM (M) | CHF (nF) | RHF (MΩ) | Rads (kΩ) | ZCPE,ads * | ZW § |
| 177 aM | 12 | 2.9 | 14 | 4.9(0.94) | 38 |
| 17.7 fM | 33 | 1.6 | 6.8 | 9.1 (0.95) | 135 |
| 177 fM | 80 | 1.0 | 5.4 | 21 (0.91) | 170 |
| 1.77 pM | 97 | 1.1 | 6.5 | 25 (0.90) | 228 |
| 177 pM | 99 | 1.2 | 7.5 | 29 (0.90) | 218 |
| TRIS-HCl | 0.86 (0.82) | 4.1 | - | 1.9 × 10−3 (1) | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrico, M.; Ambrico, P.F.; Minafra, A.; De Stradis, A.; Vona, D.; Cicco, S.R.; Palumbo, F.; Favia, P.; Ligonzo, T. Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices. Sensors 2016, 16, 1946. https://doi.org/10.3390/s16111946
Ambrico M, Ambrico PF, Minafra A, De Stradis A, Vona D, Cicco SR, Palumbo F, Favia P, Ligonzo T. Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices. Sensors. 2016; 16(11):1946. https://doi.org/10.3390/s16111946
Chicago/Turabian StyleAmbrico, Marianna, Paolo Francesco Ambrico, Angelantonio Minafra, Angelo De Stradis, Danilo Vona, Stefania R. Cicco, Fabio Palumbo, Pietro Favia, and Teresa Ligonzo. 2016. "Highly Sensitive and Practical Detection of Plant Viruses via Electrical Impedance of Droplets on Textured Silicon-Based Devices" Sensors 16, no. 11: 1946. https://doi.org/10.3390/s16111946