Enhanced Viability of Endothelial Colony Forming Cells in Fibrin Microbeads for Sensor Vascularization
Abstract
:1. Introduction
2. Experimental Section
2.1. Cell Culture
2.2. Fibrin Bead Formation
2.3. Swelling Ratio and Imaging
2.4. Histology
2.5. Scanning Electron Microscopy
2.6. Live/Dead Assay
2.7. Viability Assay
2.8. Bioreactor Culture
2.9. Comet Assay to Assess DNA Damage
2.10. Statistics
3. Results
3.1. Formation and Control of Fibrin Beads

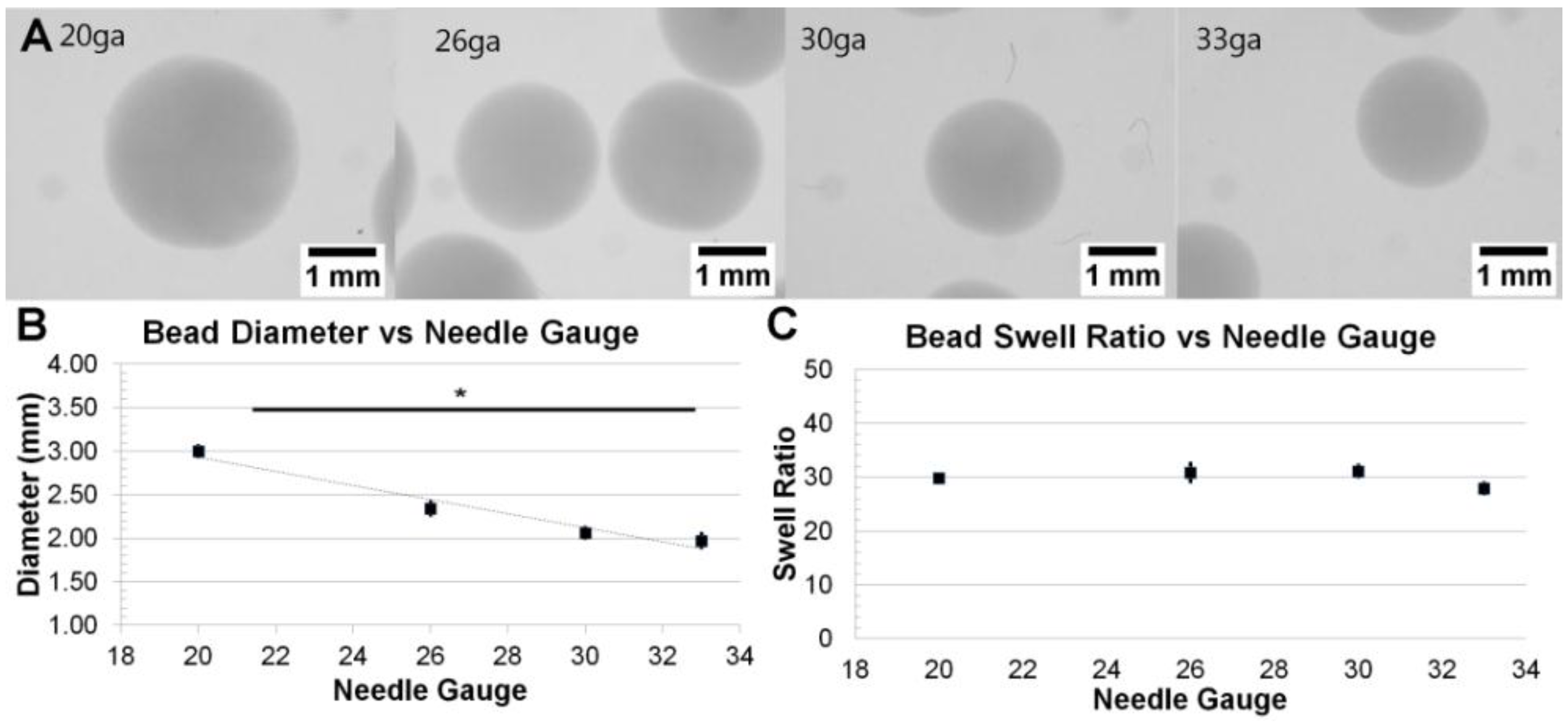
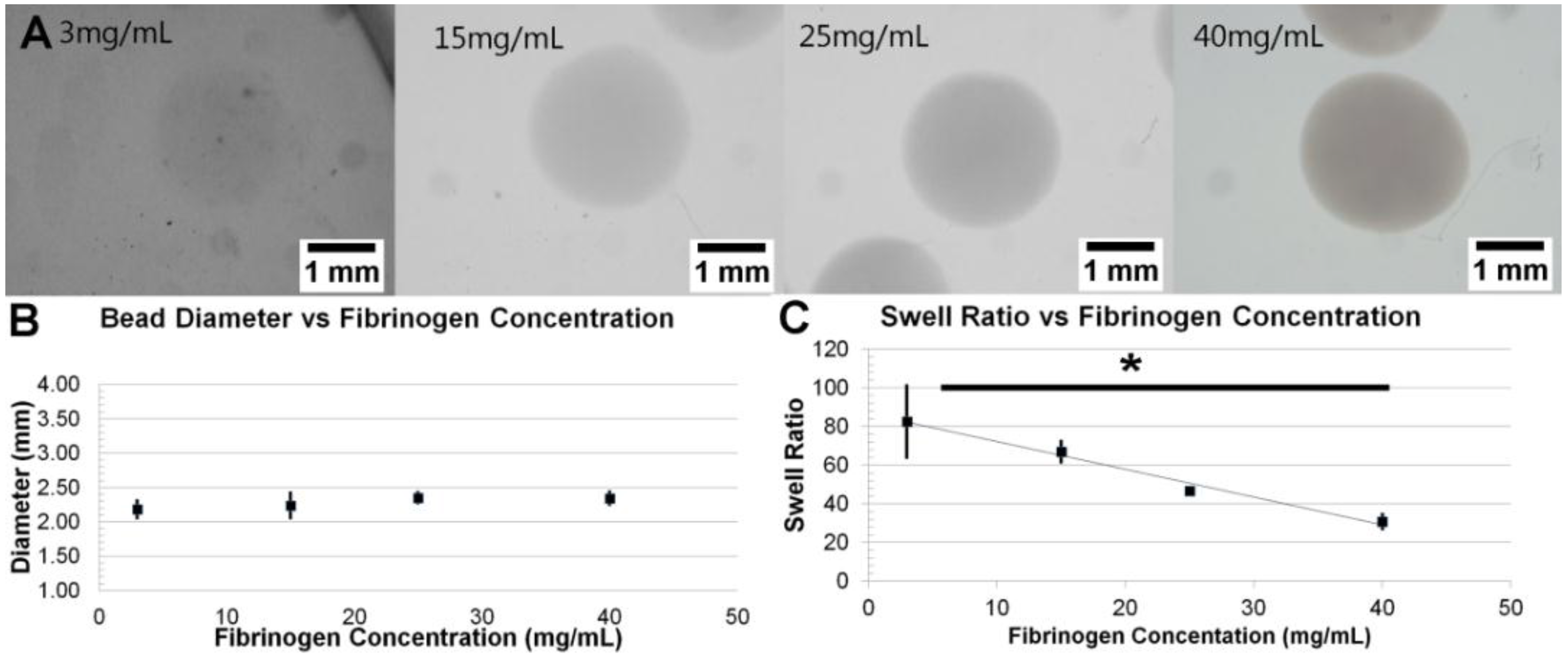
3.2. Fibril Formation Analysis of Fibrin Beads

3.3. Stability of Fibrin Beads in Culture

3.4. 24 h Cell Viability
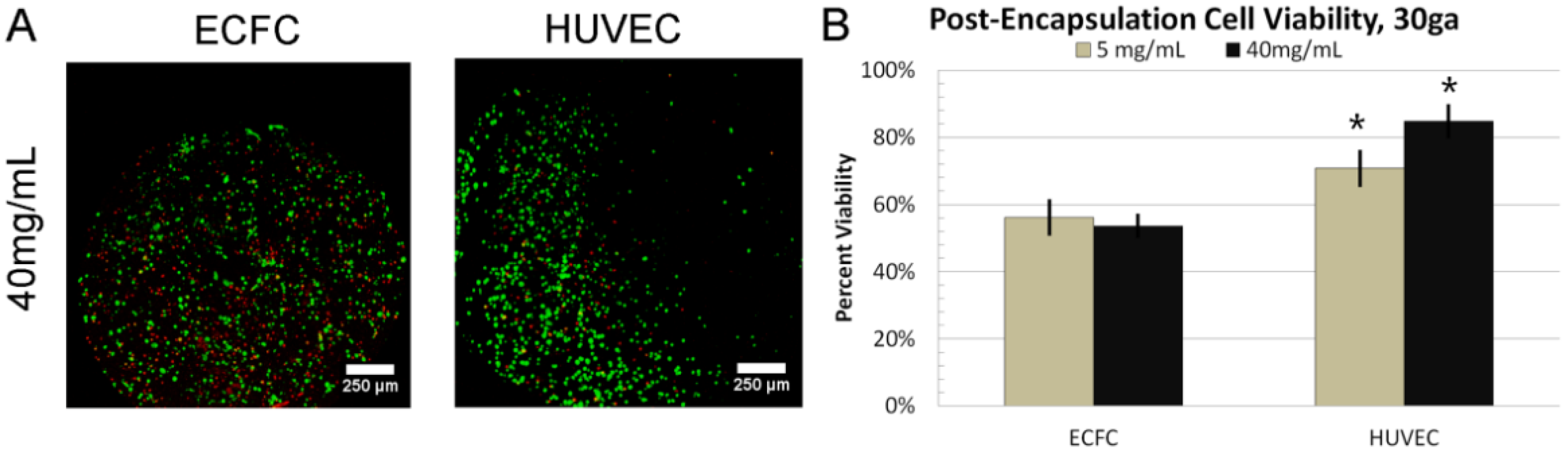
3.5. Dynamic Culture

3.6. DNA Damage

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Butt, O.I.; Carruth, R.; Kutala, V.K.; Kuppusamy, P.; Moldovan, N.I. Stimulation of peri-implant vascularization with bone marrow-derived progenitor cells: Monitoring by in vivo EPR oximetry. Tissue Eng. 2007, 13, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Klueh, U.; Dorsky, D.I.; Kreutzer, D.L. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials 2005, 26, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Smith, M.K.; Mooney, D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 2003, 24, 4023–4029. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Sainson, R.C.A.; Aoto, J.N.; Taylor, K.L.; Aitkenhead, M.; Pérez-del-Pulgar, S.; Carpenter, P.M.; Hughes, C.C.W. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: The role of fibroblasts and Angiopoietin-1. Microvasc. Res. 2003, 66, 102–112. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Davis, J.; Hughes, C.C.W. Optimized fibrin gel bead assay for the study of angiogenesis. J. Vis. Exp. 2007. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, M.C.; Magera, A.; Armani, C.; Chiellini, F.; Felice, F.; Dinucci, D.; Piras, A.M.; Minnocci, A.; Solaro, R.; Soldani, G.; et al. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell Prolif. 2011, 44, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Mead, L.E.; Prater, D.; Yoder, M.C.; Ingram, D.A. Isolation and characterization of endothelial progenitor cells from human blood. Curr. Protoc. Stem Cell Biol. 2008. [Google Scholar] [CrossRef]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Ingram, D.A.; Yoder, M.C. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Zigdon-Giladi, H.; Bick, T.; Lewinson, D.; Machtei, E.E. Co-Transplantation of Endothelial Progenitor Cells and Mesenchymal Stem Cells Promote Neovascularization and Bone Regeneration. Clin. Implant Dent. Relat. Res. 2013, 17, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.S.; Zhou, H.; Zeng, P.; Alge, D.; Li, W.; Finney, B.A.; Yoder, M.C.; Li, J. Blood vessel wall-derived endothelial colony-forming cells enhance fracture repair and bone regeneration. Calcif. Tissue Int. 2011, 89, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Bo, C.-J.; Jia, R.-P.; Liu, H.; Wu, R.; Wu, J.; Ge, Y.-Z.; Teng, G.-J. The renoprotective effect of bone marrow-derived endothelial progenitor cell transplantation on acute ischemia-reperfusion injury in rats. Transplant. Proc. 2013, 45, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.; Abou-Nassar, K.E.; Li, Y.; Filion, L.; Watpool, I.; McArdle, T.; McIntyre, L.; Ramsay, T.; Cheesman, J.; Touyz, R.; et al. Vascular progenitor recruitment in critically ill patients with acute kidney injury. Clin. Investig. Med. Médecine Clin. Exp. 2011, 34, E304–E313. [Google Scholar]
- Naruse, K.; Hamada, Y.; Nakashima, E.; Kato, K.; Mizubayashi, R.; Kamiya, H.; Yuzawa, Y.; Matsuo, S.; Murohara, T.; Matsubara, T.; et al. Therapeutic neovascularization using cord blood-derived endothelial progenitor cells for diabetic neuropathy. Diabetes 2005, 54, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Wang, S.; Han, Z.; Huang, X.; Li, S.; Chen, F.; Niu, R.; Dong, J.; Jiang, R.; et al. Transplantation of expanded endothelial colony-forming cells improved outcomes of traumatic brain injury in a mouse model. J. Surg. Res. 2013, 185, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Moubarik, C.; Guillet, B.; Youssef, B.; Codaccioni, J.-L.; Piercecchi, M.-D.; Sabatier, F.; Lionel, P.; Dou, L.; Foucault-Bertaud, A.; Velly, L.; et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011, 7, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Milbauer, L.C.; Enenstein, J.; Nguyen, J.; Pan, W.; Hebbel, R.P. Differential endothelial cell gene expression by African Americans versus Caucasian Americans: A possible contribution to health disparity in vascular disease and cancer. BMC Med. 2011, 9. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.; Kang, K.-T.; Bischoff, J. Rapid onset of perfused blood vessels after implantation of ECFCs and MPCs in collagen, PuraMatrix and fibrin provisional matrices. J. Tissue Eng. Regen. Med. 2015, 9, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Whittington, C.F.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol. Biosci. 2013, 13, 1135–1149. [Google Scholar] [CrossRef] [PubMed]
- Critser, P.J.; Voytik-Harbin, S.L.; Yoder, M.C. Isolating and defining cells to engineer human blood vessels. Cell Prolif. 2011, 44, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Critser, P.J.; Kreger, S.T.; Voytik-Harbin, S.L.; Yoder, M.C. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc. Res. 2010, 80, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-T.; Allen, P.; Bischoff, J. Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood 2011, 118, 6718–6721. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R. The use of fibrin based matrices and fibrin microbeads (FMB) for cell based tissue regeneration. Expert Opin. Biol. Ther. 2008, 8, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Perka, C.; Arnold, U.; Spitzer, R.S.; Lindenhayn, K. The use of fibrin beads for tissue engineering and subsequential transplantation. Tissue Eng. 2001, 7, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, G.; Lee, K.H.; Magalhães, R.; Wen, F.; Gouk, S.S.; Hutmacher, D.W.; Kuleshova, L.L. Cryopreservation of alginate-fibrin beads involving bone marrow derived mesenchymal stromal cells by vitrification. Biomaterials 2009, 30, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Fisher, J.P. Tubular perfusion system for the long-term dynamic culture of human mesenchymal stem cells. Tissue Eng. Part C Methods 2011, 17, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Both, S.K.; Yang, W.; Alghamdi, H.S.; Yang, F.; Fisher, J.P.; Jansen, J.A. In vivo bone regeneration using tubular perfusion system bioreactor cultured nanofibrous scaffolds. Tissue Eng. Part A 2014, 20, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Prasain, N.; Meador, J.L.; Yoder, M.C. Phenotypic and functional characterization of endothelial colony forming cells derived from human umbilical cord blood. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, P.; Yeatts, A.B.; Cardea, S.; Fisher, J.P.; Reverchon, E. Tubular perfusion system culture of human mesenchymal stem cells on poly-L-lactic acid scaffolds produced using a supercritical carbon dioxide-assisted process. J. Biomed. Mater. Res. A 2012, 100, 2563–2572. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Geibel, E.M.; Fears, F.F.; Fisher, J.P. Human Mesenchymal Stem Cell Position within Scaffolds Influences Cell Fate During Dynamic Culture. Biotechnol. Bioeng. 2012, 109, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Fisher, J.P. Bone tissue engineering bioreactors: Dynamic culture and the influence of shear stress. Bone 2011, 48, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Gordon, C.N.; Fisher, J.P. Formation of an aggregated alginate construct in a tubular perfusion system. Tissue Eng. Part C Methods 2011, 17, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Yeatts, A.B.; Choquette, D.T.; Fisher, J.P. Bioreactors to influence stem cell fate: Augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim. Biophys. Acta 2013, 1830, 2470–2480. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Anderson, D.; Yu, T.W.; Phillips, B.J.; Schmezer, P. The effect of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the COMET assay. Mutat. Res. 1994, 307, 261–271. [Google Scholar] [CrossRef]
- Zhao, H.; Ma, L.; Zhou, J.; Mao, Z.; Gao, C.; Shen, J. Fabrication and physical and biological properties of fibrin gel derived from human plasma. Biomed. Mater. Bristol Engl. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Sieminski, A.L.; Hebbel, R.P.; Gooch, K.J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp. Cell Res. 2004, 297, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Ronot, X.; Tracqui, P.; Usson, Y.; Tranqui, L. In vitro angiogenesis is modulated by the mechanical properties of fibrin gels and is related to alpha(v)beta3 integrin localization. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Nör, J.E.; Peters, M.C.; Christensen, J.B.; Sutorik, M.M.; Linn, S.; Khan, M.K.; Addison, C.L.; Mooney, D.J.; Polverini, P.J. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Investig. J. Tech. Methods Pathol. 2001, 81, 453–463. [Google Scholar] [CrossRef]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Corstorphine, L.; Sefton, M.V. Effectiveness factor and diffusion limitations in collagen gel modules containing HepG2 cells. J. Tissue Eng. Regen. Med. 2011, 5, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Chan, H.K.; Weaire, D.; Hutzler, S. Dense packings of spheres in cylinders: Simulations. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2012, 85. [Google Scholar] [CrossRef] [Green Version]
- Gautier, A.; Carpentier, B.; Dufresne, M.; Vu Dinh, Q.; Paullier, P.; Legallais, C. Impact of alginate type and bead diameter on mass transfers and the metabolic activities of encapsulated C3A cells in bioartificial liver applications. Eur. Cell. Mater. 2011, 21, 94–106. [Google Scholar] [PubMed]
- Kaunas, R.; Kang, H.; Bayless, K.J. Synergistic Regulation of Angiogenic Sprouting by Biochemical Factors and Wall Shear Stress. Cell. Mol. Bioeng. 2011, 4, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teoh, S.-H.; Chong, M.S.K.; Yeow, C.-H.; Kamm, R.D.; Choolani, M.; Chan, J.K.Y. Contrasting effects of vasculogenic induction upon biaxial bioreactor stimulation of mesenchymal stem cells and endothelial progenitor cells cocultures in three-dimensional scaffolds under in vitro and in vivo paradigms for vascularized bone tissue engineering. Tissue Eng. Part A 2013, 19, 893–904. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, J.K.; Zivkovic, L.; Fisher, J.P.; Yoder, M.C.; Brey, E.M. Enhanced Viability of Endothelial Colony Forming Cells in Fibrin Microbeads for Sensor Vascularization. Sensors 2015, 15, 23886-23902. https://doi.org/10.3390/s150923886
Gandhi JK, Zivkovic L, Fisher JP, Yoder MC, Brey EM. Enhanced Viability of Endothelial Colony Forming Cells in Fibrin Microbeads for Sensor Vascularization. Sensors. 2015; 15(9):23886-23902. https://doi.org/10.3390/s150923886
Chicago/Turabian StyleGandhi, Jarel K., Lada Zivkovic, John P. Fisher, Mervin C. Yoder, and Eric M. Brey. 2015. "Enhanced Viability of Endothelial Colony Forming Cells in Fibrin Microbeads for Sensor Vascularization" Sensors 15, no. 9: 23886-23902. https://doi.org/10.3390/s150923886
APA StyleGandhi, J. K., Zivkovic, L., Fisher, J. P., Yoder, M. C., & Brey, E. M. (2015). Enhanced Viability of Endothelial Colony Forming Cells in Fibrin Microbeads for Sensor Vascularization. Sensors, 15(9), 23886-23902. https://doi.org/10.3390/s150923886




