Diamond Nanowires: A Novel Platform for Electrochemistry and Matrix-Free Mass Spectrometry
Abstract
:1. Introduction
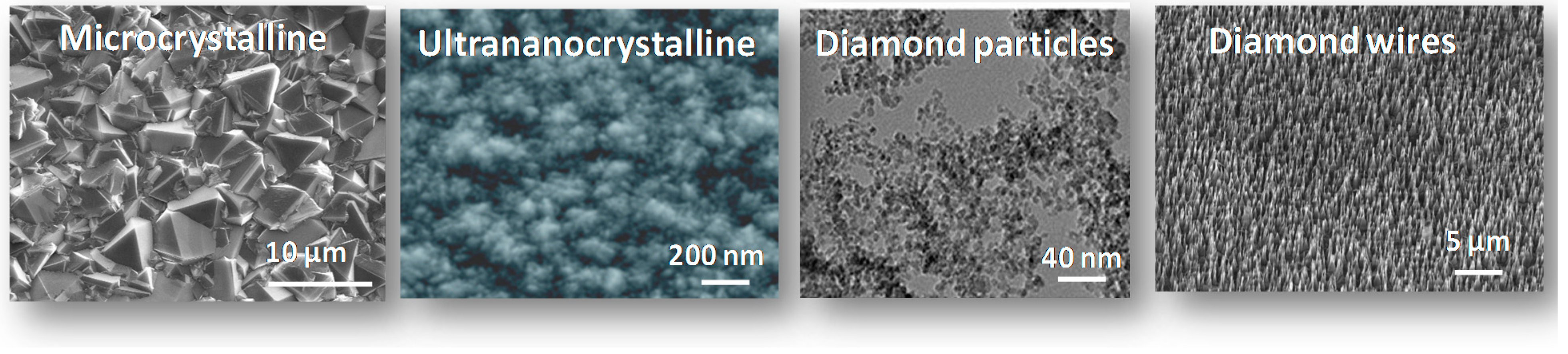
2. Synthetic Routes of Diamond Nanowires
2.1. Top-Down Approach
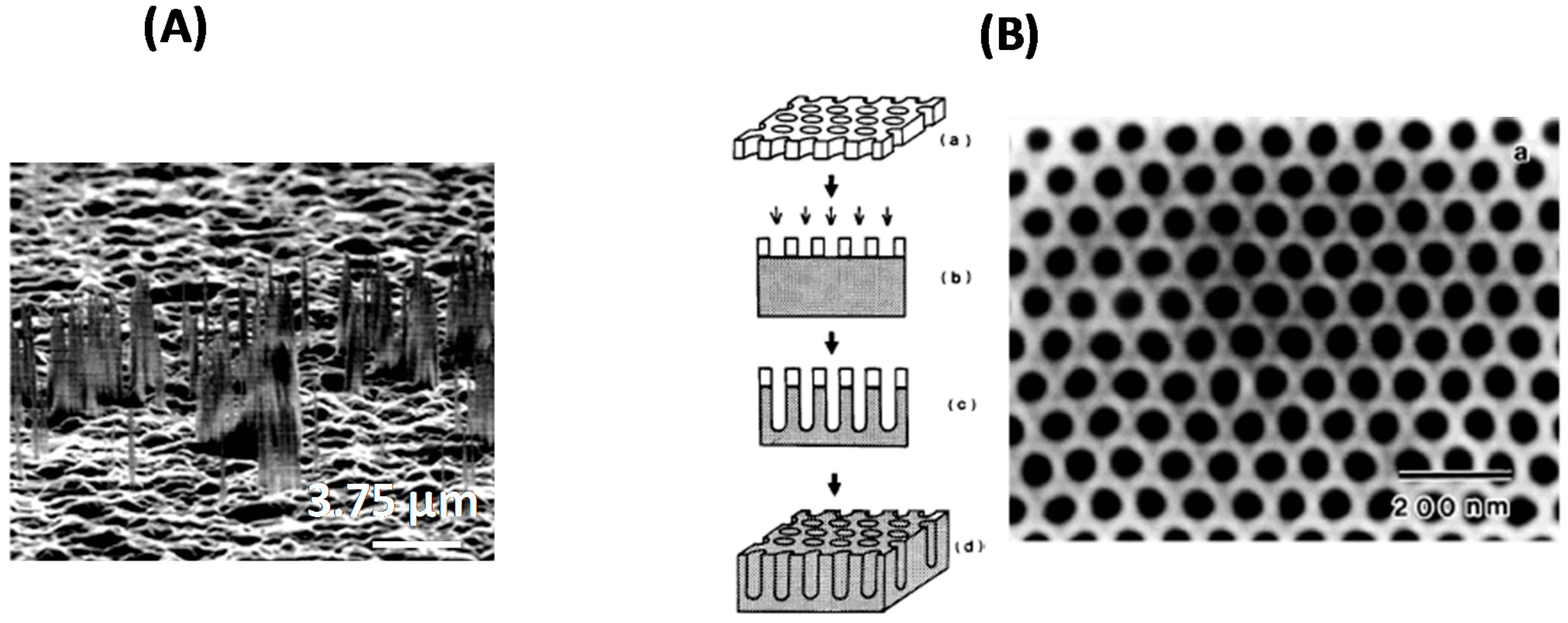
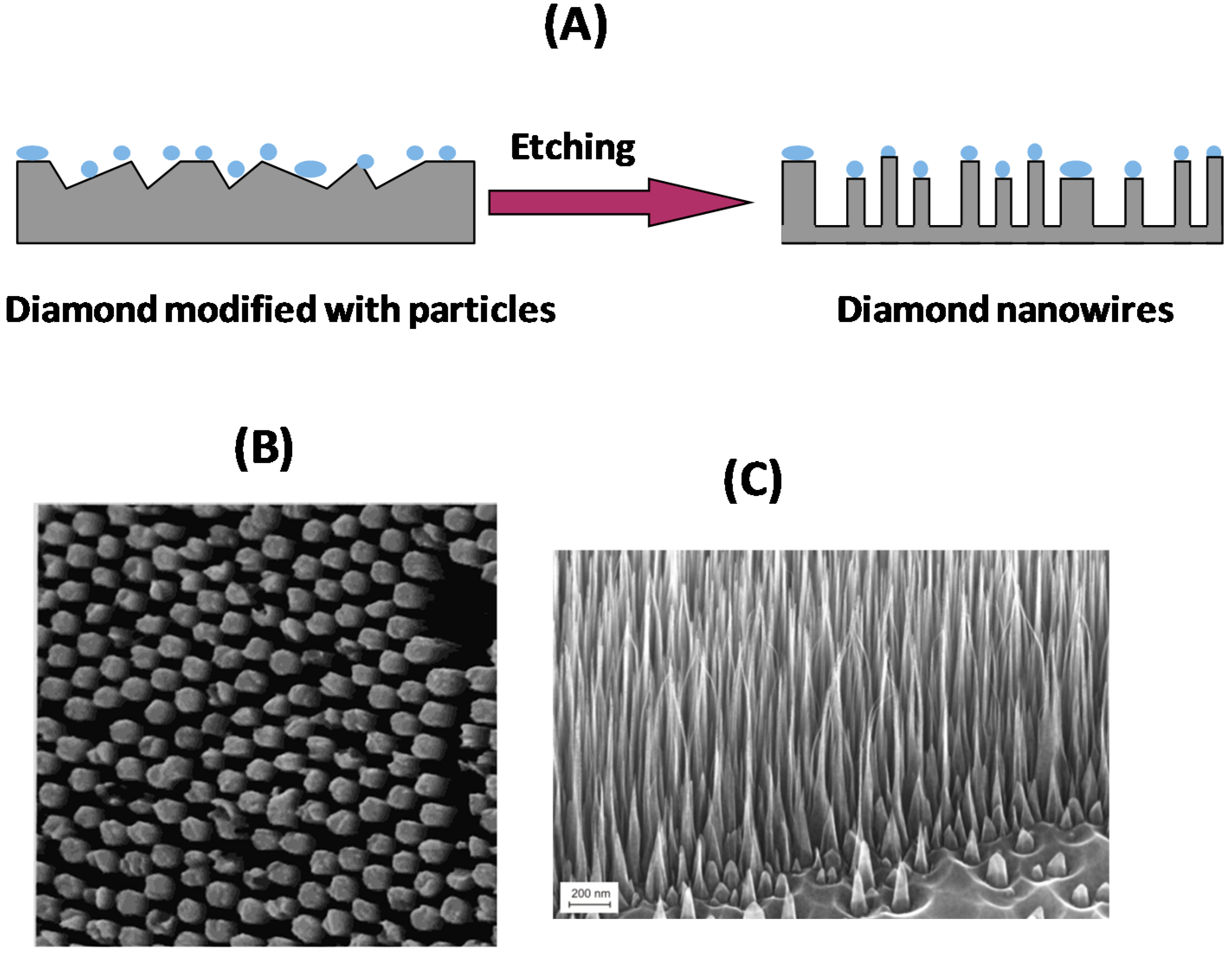
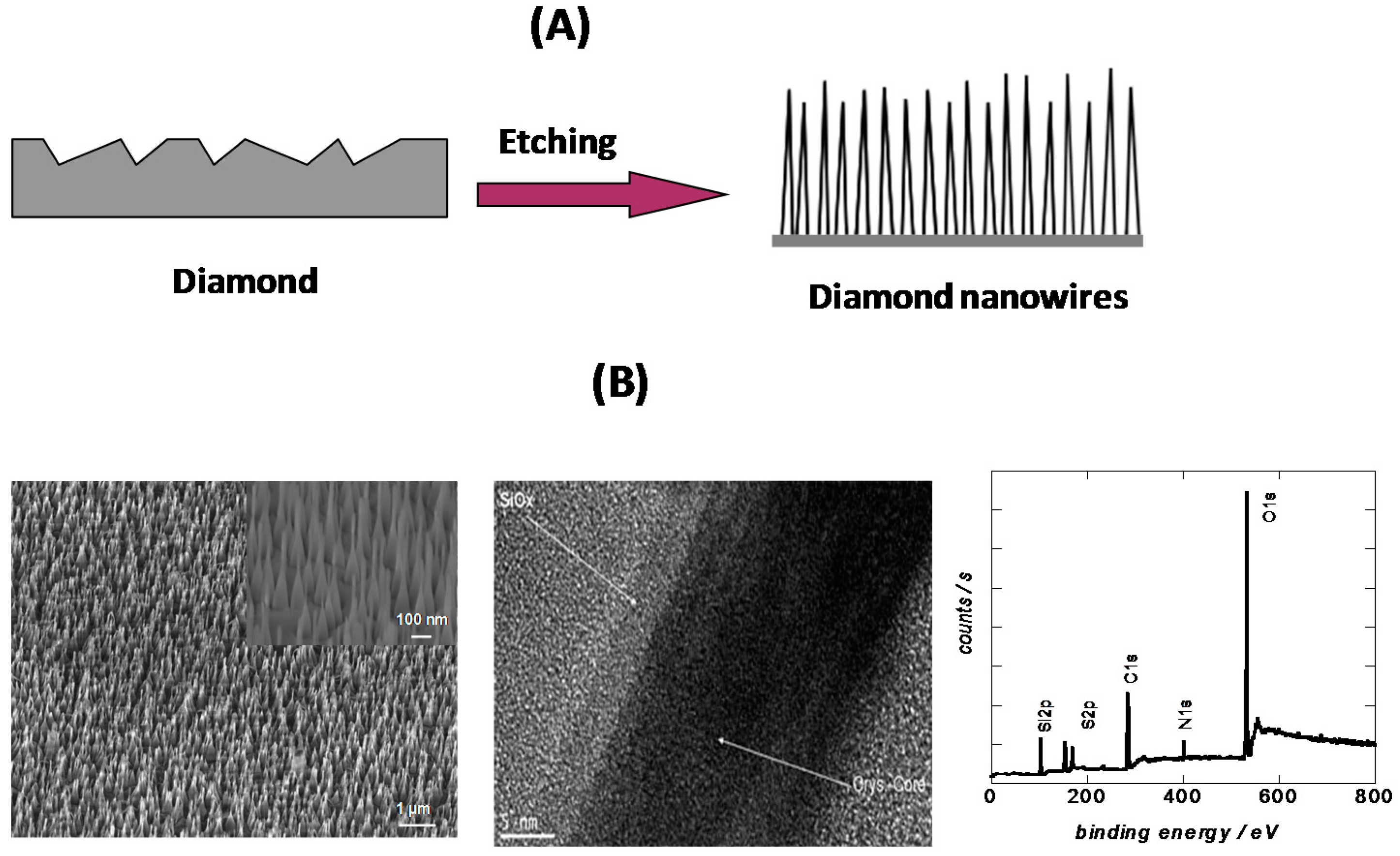
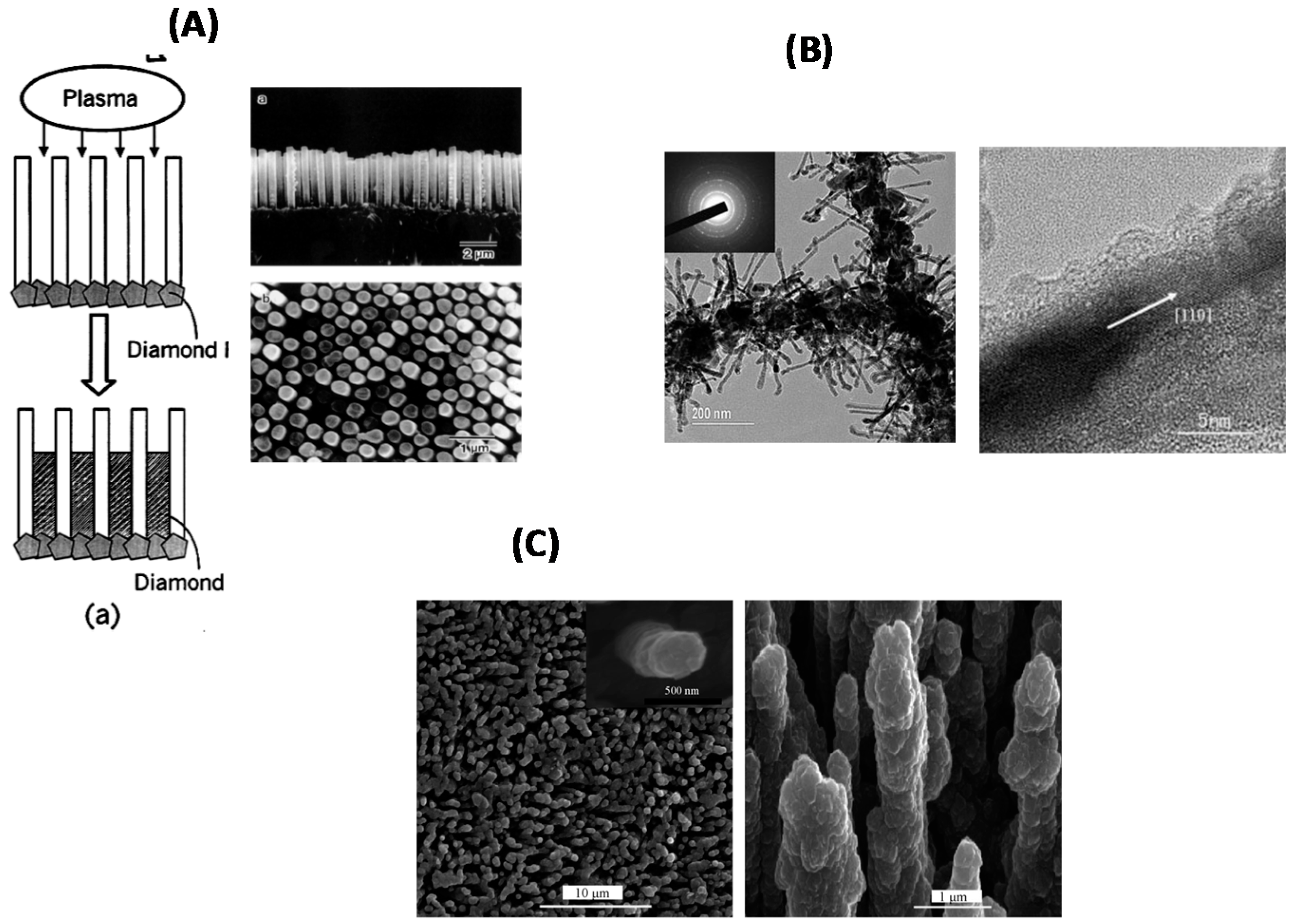
2.2. Bottom-Up Approach
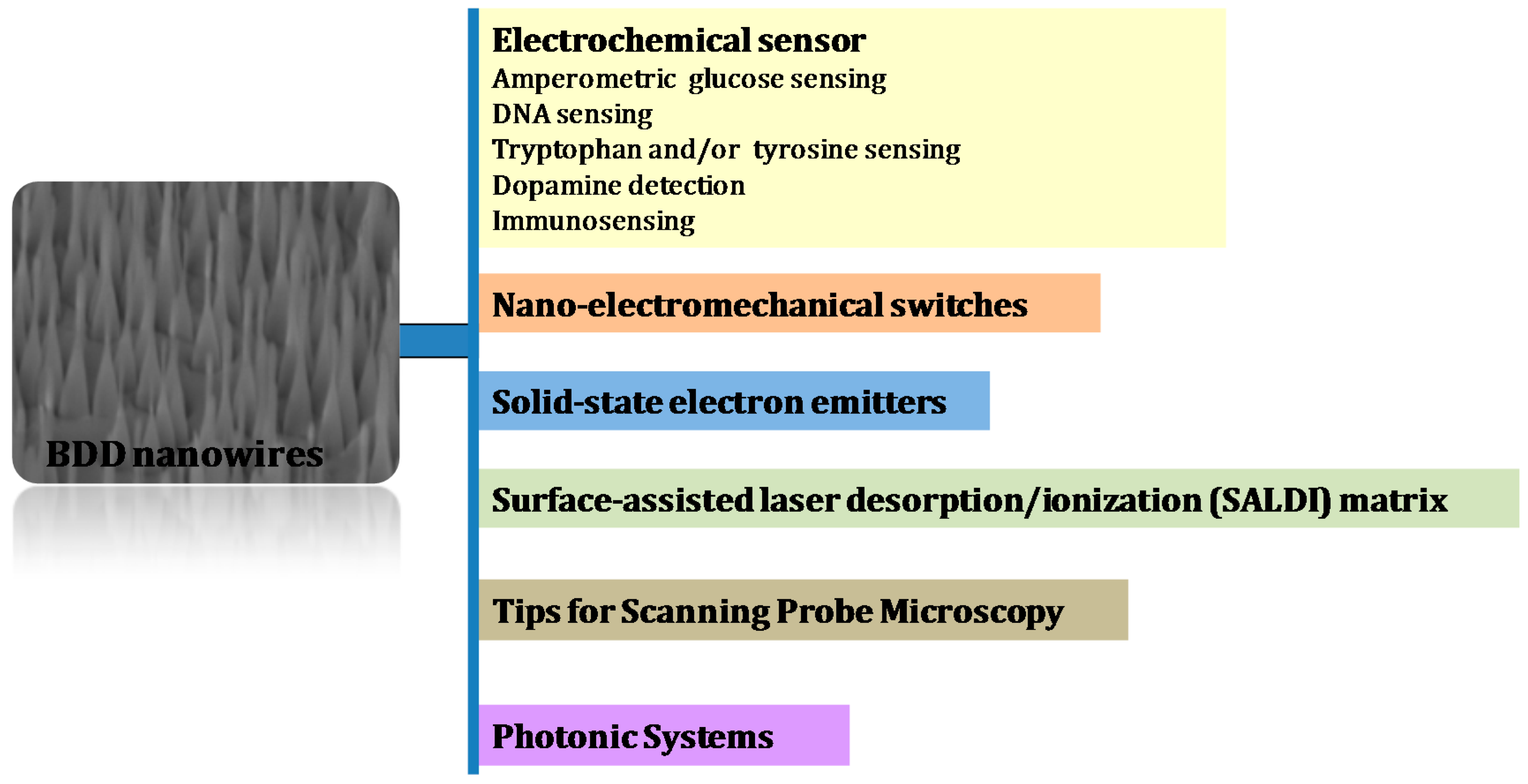
3. Applications of Diamond Nanowires
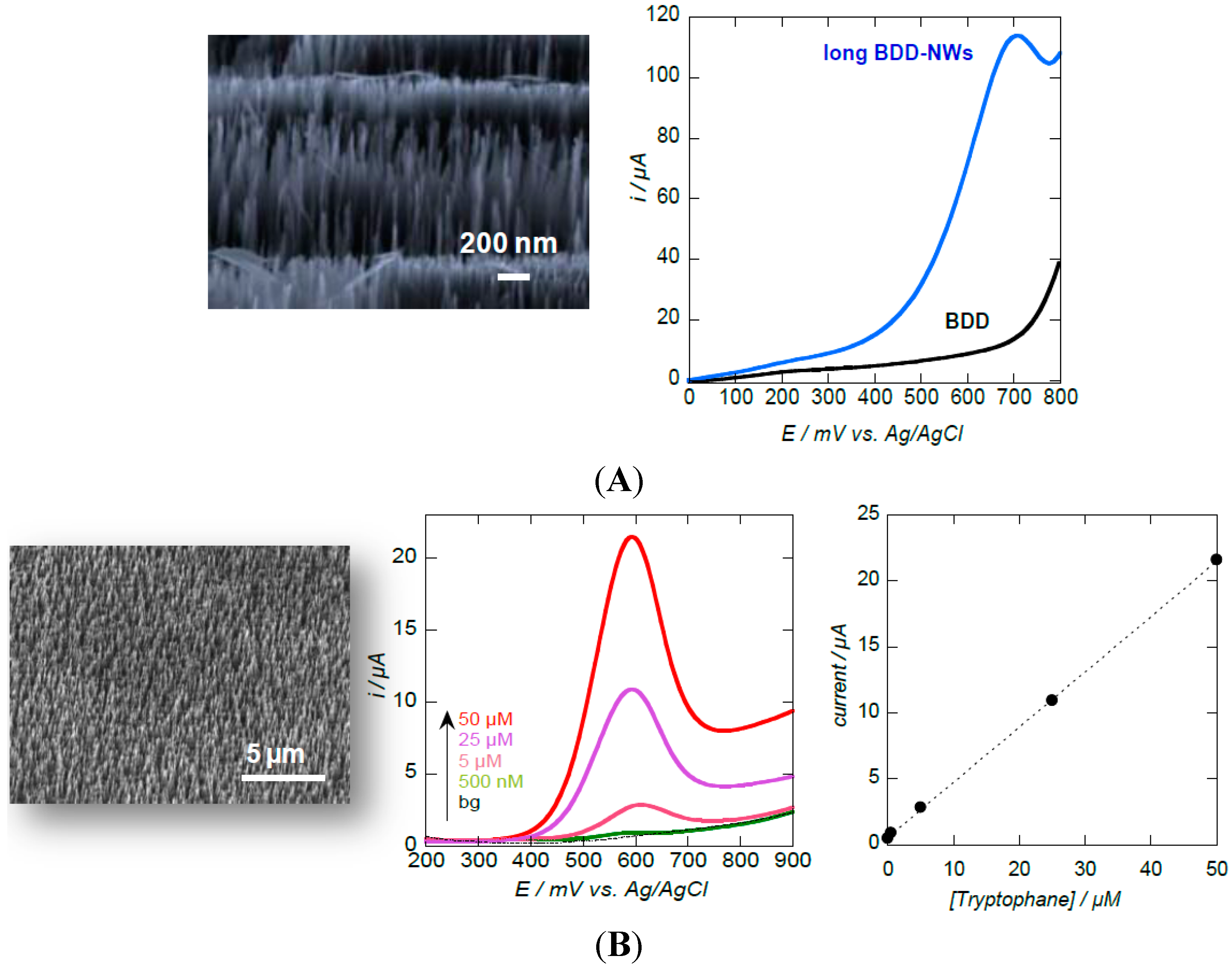
3.1. Diamond Nanowires for Electrochemical Sensing
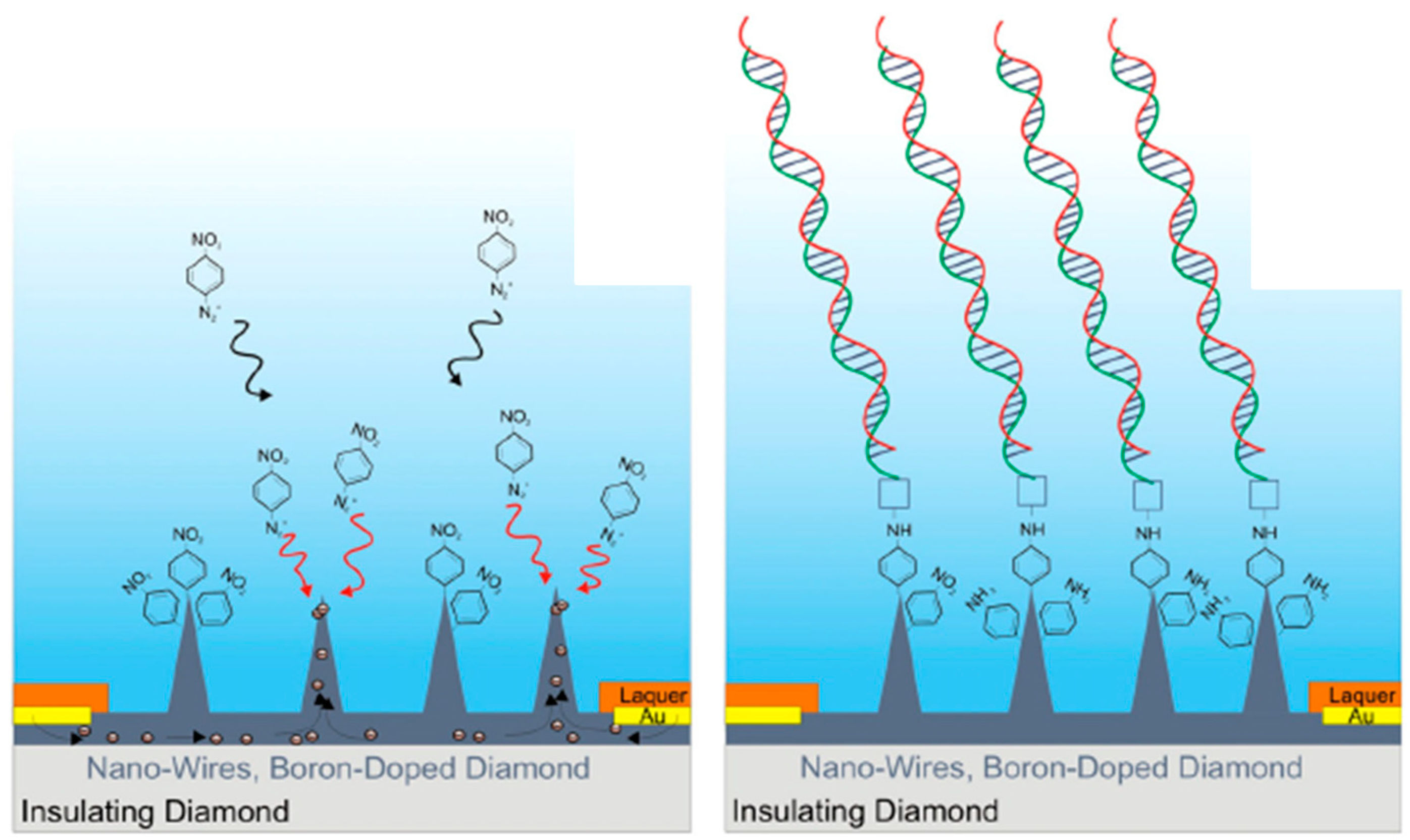

3.2. Diamond Nanowires for Matrix-Free Mass Spectrometry
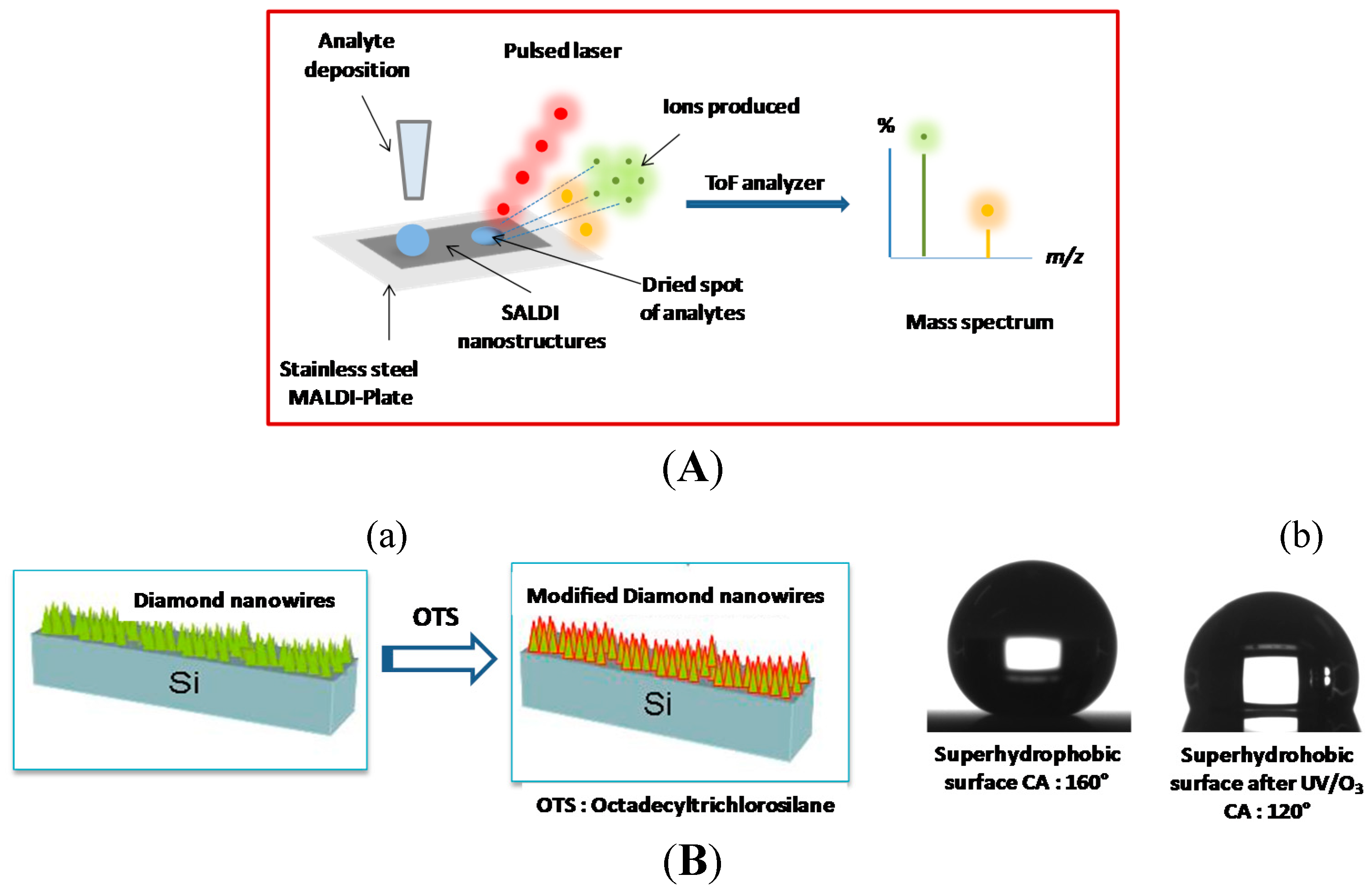
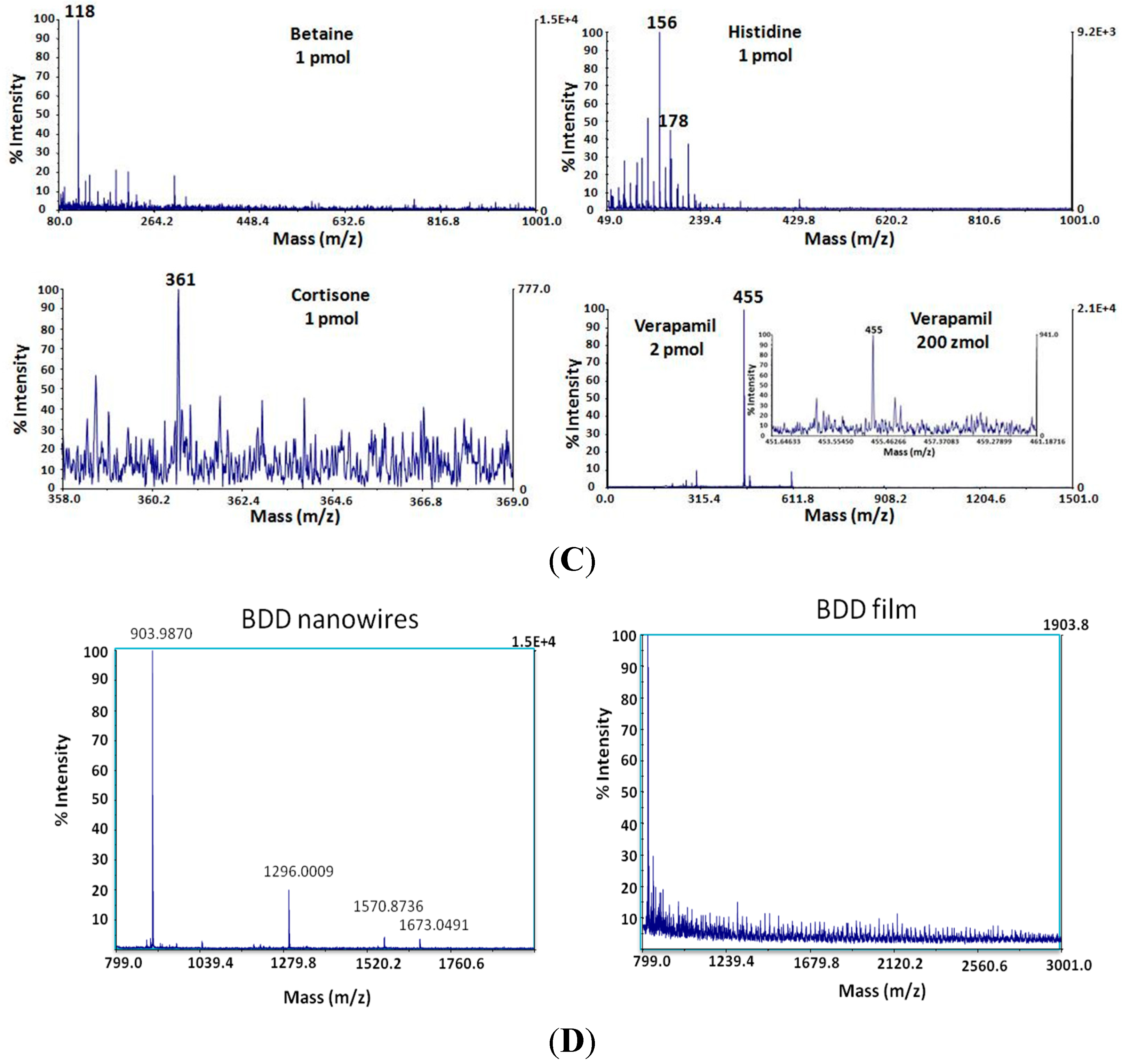
3.3. Future Trend: Coupled Electrochemistry-Mass Spectrometry Analysis on Diamond Nanowires
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Nemanich, R.J.; Carlisle, J.A.; Hirata, A.; Haenen, K. CVD diamond—Research, applications, and challenges. MRS Bull. 2014, 39, 490–548. [Google Scholar] [CrossRef]
- Fortin, E.; Chane-Tune, J.; Mailley, P.; Szunerits, S.; Marcus, B.; Petit, J.P.; Mermoux, M.; Vieil, E. Nucleosides and ODN electrochemical detection onto boron-doped diamond electrodes. Bioelectrochemistry 2004, 63, 303–306. [Google Scholar] [CrossRef] [PubMed]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
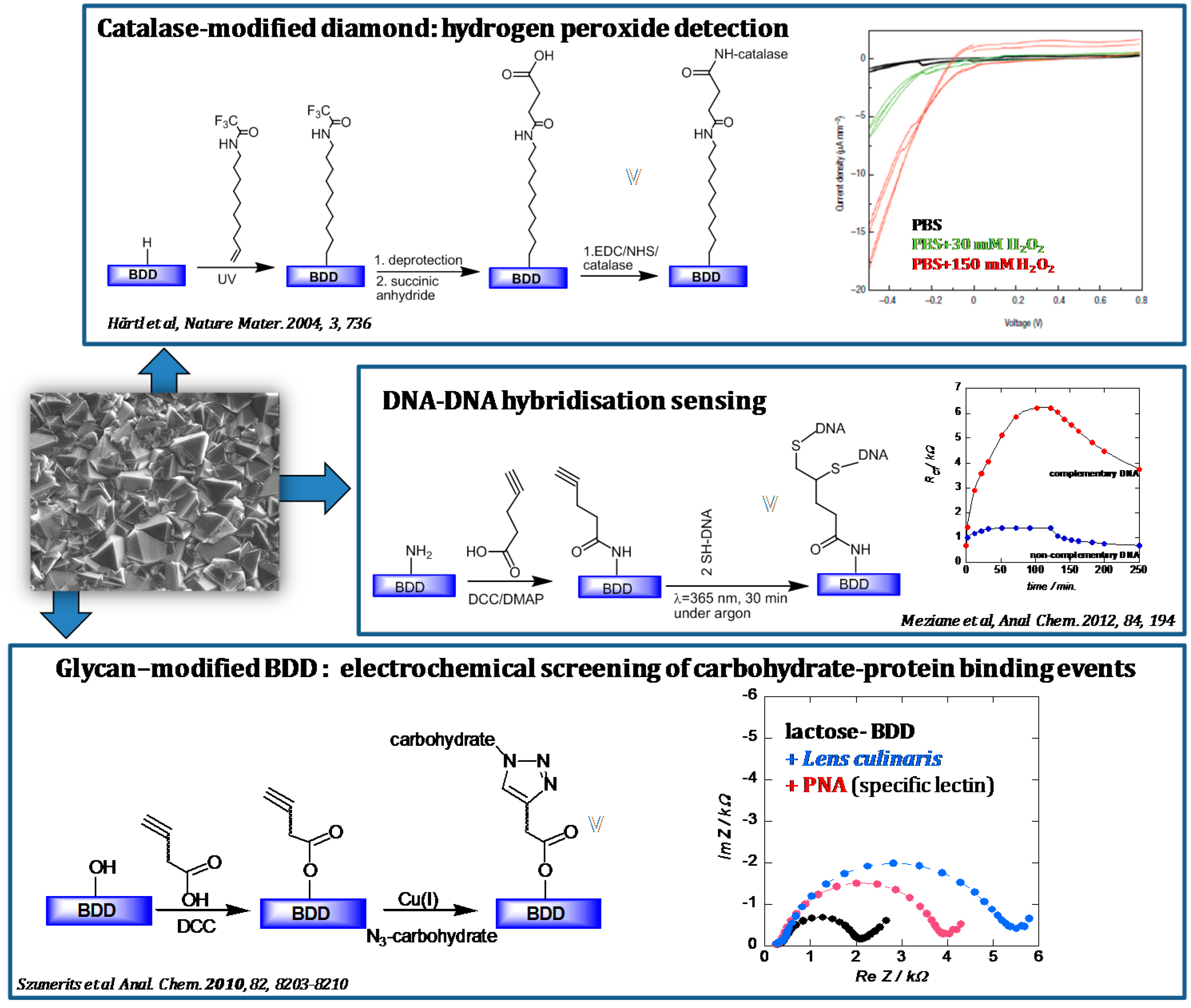
- Hartl, A.; Schmich, E.; Garrido, J.A.; Hernando, J.; Catharino, S.C.R.; Walter, S.; Feulber, P.; Kromka, A.; Steinmuller, D.; Stutzmann, M. Protein-modified nanocrystalline diamond thin films for biosensor applications. Nat. Mater. 2004, 3, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Ferro, S.; de Battist, A. The 5-V window of polarizability of fluorinated diamond electrodes in aqueous solutions. Anal. Chem. 2003, 75, 7040–7042. [Google Scholar] [CrossRef] [PubMed]
- Meziane, D.; Barras, A.; Kromka, A.; Houdkova, J.; Boukherroub, R.; Szunerits, S. Thiol-yne reaction on boron-doped diamond electrodes. Anal. Chem. 2012, 84, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Niedziółka-Jönsson, J.; Boukherroub, R.; Woisel, P.; Baumann, J.S.; Siriwardena, A. Label-free detection of lectins on carbohydrate-modified boron-doped diamond Surfaces. Anal. Chem. 2010, 82, 8203–8210. [Google Scholar] [CrossRef] [PubMed]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Szunerits, S.; Coffinier, Y.; Galopin, E.; Brenner, J.; Boukherroub, R. Preparation of boron-doped diamond nanowires and their application for sensitive electrochemical detection of tryptophan. Electrochem. Commun. 2010, 12, 438–441. [Google Scholar] [CrossRef]
- Luo, D.; Wu, L.; Zhi, J. Fabrication of boron-doped diamond nanorod forest electrodes and their application in nonenzymatic amperometric glucose sensing. ACS Nano 2009, 3, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Uetsuka, H.; Osawa, E.; Nebel, C.E. Vertically aligned diamond nanowires for DNA sensing. Angew. Chem. Int. Ed. 2008, 47, 5183–5185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Subramanian, P.; Li, M.; Yeap, W.S.; Haenen, K.; Coffinier, Y.; Boukherroub, R.; Szunerits, S. Non-enzymatic glucose sensing on long and short diamond nanowires electrodes. Electrochem. Commun. 2013, 34, 286–290. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Fedoseev, D.V.; Lukyanovich, V.M.; Spitzin, B.V.; Ryabov, V.A.; Lavrentyev, A.V. Filamentary diamond crystals. J. Cryst. Growth 1968, 2, 380–384. [Google Scholar] [CrossRef]
- Shiomi, H. Reactive ion etching of diamond in O2 and CF4 plasma and fabrication of porous diamond for field emitter cathodes. Jpn. J. Appl. Phys. 1997, 36, 7745–7748. [Google Scholar] [CrossRef]
- Masuda, H.; Watanaba, M.; Yasui, K.; Tryk, D.; Rao, T.; Fujishima, A. Fabrication of a nanostructured diamond honeycomb film. Adv. Mater. 2000, 12, 444–447. [Google Scholar] [CrossRef]
- Baik, E.S.; Baik, Y.J.; Jeaon, D. Aligned diamond nanowhiskers. J. Mater. Res. 2000, 15, 923–926. [Google Scholar] [CrossRef]
- Ando, Y.; Nishibayashi, Y.; Sawaben, A. ‘Nano-rods’ of single crystalline diamond. Diamond Relat. Mater. 2004, 13, 633–637. [Google Scholar] [CrossRef]
- Okuyama, S.; Matsushita, S.I.; Fujishima, A. Periodic submicrocylinder diamond surfaces using two-dimensional fine particle arrays. Langmuir 2002, 18, 8282–8287. [Google Scholar] [CrossRef]
- Zou, Y.S.; Yang, T.; Zhang, W.J.; Chong, Y.M.; He, B.; Bello, I.; Lee, S.T. Fabrication of diamond nanopillar and their arrays. Appl. Phys. Lett. 2008, 92, 053105. [Google Scholar] [CrossRef]
- Yang, N.; Uetsuka, H.; Osawa, E.; Nebel, C.E. Vertically aligned nanowires from boron-doped diamond. Nano Lett. 2008, 8, 3572–3576. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, B.J.M.; Khan, M.; Zhang, Y.; Bainec, T.M.; Martinick, K.; McCutcheon, M.; Hemmer, P.; Loncar, M. Fabrication of diamond nanowires for quantum information processing applications. Diamond Relat. Mater. 2010, 19, 621–629. [Google Scholar] [CrossRef]
- Smirnov, W.; Kriele, A.; Yang, N.; Nebel, C.F. Aligned diamond nano-wires: Fabrication and characterisation for advanced applications in bio and electrochemistry. Diamond Relat. Mater. 2009, 18, 186–189. [Google Scholar] [CrossRef]
- Nebel, C.E.; Yang, N.; Uetsuka, H.; Osawa, E.; Tokuda, N.; Williams, O. Diamond nano-wires, a new approach towards next generation electrochemical gene sensor platforms. Diamond Relat. Mater. 2009, 18, 910–917. [Google Scholar] [CrossRef]
- Zheng, W.W.; Hsieh, Y.H.; Chiu, Y.C.; Cai, S.J.; Cheng, C.L.; Chen, C. Organic functionalization of ultradispersed nanodiamond: Synthesis and applications. J. Mater. Chem. 2009, 19, 8432–8441. [Google Scholar] [CrossRef]
- Marcon, L.; Riquet, F.; Vicogne, D.; Szunerits, S.; Bodart, J.F.; Boukherroub, R. Cellular and in vivo toxicity of functionalized nanodiamond in Xenopus embryos. J. Mater. Chem. 2010, 20, 8064–8069. [Google Scholar] [CrossRef]
- Coffinier, Y.; Galopin, E.; Szunerits, S.; Boukherroub, R. Preparation of superhydrophobic and oleophobic diamond nanograss array. J. Mater. Chem. 2010, 20, 10671–10675. [Google Scholar] [CrossRef]
- Coffinier, Y.; Szunerits, S.; Drobecq, H.; Melnyk, O.; Boukherroub, R. Diamond nanowires for highly sensitive matrix-free mass spectrometry analysis of small molecules. Nanoscale 2012, 4, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Yanagishita, T.; Yasui, K.; Nishio, K.; Yagi, I.; Rao, N.; Fujishima, A. Synthesis of well-aligned diamond nanocylinders. Adv. Mater. 2001, 13, 247–249. [Google Scholar] [CrossRef]
- Sun, L.T.; Gond, J.; Zhu, D.Z.; Zhu, Z.Y.; He, S. Diamond nanorods from carbon nanotubes. Adv. Mater. 2004, 16, 1849–1853. [Google Scholar] [CrossRef]
- Vlasov, I.I.; Lebedev, O.I.; Ralchenko, V.G.; Goovaerts, E.; Bertoni, G.; Tendeloo, G.V.; Konov, V.I. Hybrid diamond-graphite nanowires produced by microwave plasma chemical vapor deposition. Adv. Mater. 2007, 19, 4058–4062. [Google Scholar] [CrossRef]
- Shang, N.; Papakonstantinou, P.; Wang, P.; Zakharov, A.; Palnitkar, U.; Lin, I.N.; Chu, M.; Stamboulis, A. Self-assembled growth, microstructure, and field-emission high-performance of ultrathin diamond nanorods. ACS Nano 2009, 3, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Cloutier, S.G.; Palefsky, S.; Xu, J. Synthesis of diamond nanowires using atmopsheric-pressure chemical vapor deposition. Nano Lett. 2010, 10, 3272–3276. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Xu, J. Diamond nanowire-a challenge from extremes. Nanoscale 2012, 4, 5293–5299. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Lewes-Malandrakis, G.; Wolfer, M.T.; Muller-Sebert, W.; Gentile, P.; Aradilla, D.; Schubert, T.; Nebel, C.E. Diamond-coated silicon wires for supercapacitor applications in ionic liquids. Diamond Relat. Mater. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Girard, H.A.; Scorsone, E.; Saada, S.; Gesset, C.; Arnault, J.C.; Perruchas, S.; Rousseau, L.; David, S.; Pichot, V.; Spitzer, D.; Berganzo, P. Electrostatic grafting of diamond nanoparticles towards 3D diamond nanostructures. Diamond Relat. Mater. 2012, 23, 83–87. [Google Scholar] [CrossRef]
- Peng, K.Q.; Yan, Y.J.; Gao, S.P.; Zhu, J. Synthesis of large-area silicon nanowire arrays via self-assembling nanoelectrochemistry. Adv. Mater. 2002, 14, 1164–1167. [Google Scholar] [CrossRef]
- Babinec, T.M.; Hausmann, B.J.M.; Khan, M.; Zhang, Y.; Maze, J.R.; Hemmer, P.R.; Loncar, M. A diamond nanowire single-photon source. Nature Nanotechnol. 2010, 5, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Foord, J.; Steinmueller, D.; Coffinier, Y.; Boukherroub, R.; Szunerits, S. Diamond nanowires decorated with metallic nanoparticles: A novel electrical interface for the immobilization of histidinylated biomolecules. Electrochim. Acta 2013, 110, 4–8. [Google Scholar] [CrossRef]
- Subramanian, P.; Mazurenko, I.; Zaitsev, V.; Coffinier, Y.; Boukherroub, R.; Szunerits, S. Diamond nanowires modified with poly[3-(pyrrolyl)carboxylic acid] for the immobilization of histidine-tagged peptides. Analyst 2014, 139, 4343–4349. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Motorina, A.; Yeap, W.S.; Haenen, K.; Coffinier, Y.; Zaitsev, V.; Niedziolka-Jonsson, J.; Boukherroub, R.; Szunerits, S. Impedimetric immunosensor based on diamond nanowires decorated with nickel nanoparticles. Analyst 2014, 139, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Vasilescu, A.; Subramanian, P.; Vezeanu, A.; Andrei, V.; Coffinier, Y.; Li, M.; Boukherroub, R.; Szunerits, S. Simultaneous electrochemical detection of tryptophan and tyrosine using boron-doped diamond and diamond nanowires electrodes. Electrochem. Commun. 2013, 35, 84–87. [Google Scholar] [CrossRef]
- Yang, N.; Uetsuka, H.; Nebel, C.E. Biofunctionalization of vertically aligned diamond nanowires. Adv. Funct. Mater. 2009, 19, 887–893. [Google Scholar] [CrossRef]
- Yang, N.; Uetsuka, H.; Williams, O.A.; Osawa, E.; Tokuda, N.; Nebel, C.E. Vertically aligned diamond nanowires: Fabrication, characterization, and application for DNA sensing. Phys. Stat. Sol. A 2009, 206, 2048–2056. [Google Scholar] [CrossRef]
- Uetsuka, H.; Shin, D.; Tokuda, N.; Saeki, K.; Nebel, C.E. Electrochemical grafting of boron-doped single-crystalline chemical vapor deposition diamond with nitrophenyl molecules. Langmuir 2007, 23, 3466–3472. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Smirnov, W.; Nebel, C.E. Three-dimensional electrochemical reactions on tip-coated diamond nanowires with nickel nanoparticles. Electrochem. Commun. 2013, 27, 89–91. [Google Scholar] [CrossRef]
- Zhao, G.; Qi, Y.; Tian, Y. Simultaneous and direct determination of tryptophan and tyrosine at boron-doped diamond electrode. Electroanalysis 2006, 18, 830–834. [Google Scholar] [CrossRef]
- Shalini, J.; Sankaran, K.J.; Dong, C.L.; Lee, C.Y.; Tai, N.H.; Lin, I.N. In situ detection of dopamine using nitrogen incorporated diamond nanowire electrode. Nanoscale 2013, 5, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Toghill, K.E.; Xiao, L.; Stradiotto, N.R.; Compton, R.G. The determination of methanol using an electrolytically fabricated nickel microparticle modified boron-doped diamond electrode. Electroanalysis 2010, 22, 491–500. [Google Scholar] [CrossRef]
- Karas, M.; Hillenkamp, F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988, 60, 259–280. [Google Scholar] [CrossRef]
- Hanton, S.D. Mass spectrometry of polymers and polymer surfaces. Chem. Rev. 2001, 101, 527–569. [Google Scholar] [CrossRef] [PubMed]
- Knochenmuss, R.; Zenobi, R. MALDI ionization: The role of in-plume processes. Chem. Rev. 2003, 103, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Li, L. MALDI Mass Spectrometry for Synthetic Polymer Analysis (Chemical Analysis: A Series of Monographs on Analytical Chemistry and Its Applications); Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Arakawa, R.; Kawasaki, H. Functionalized nanoparticles and nanostructured surfaces for surface-assisted laser desorption/ionization mass spectrometry. Anal. Sci. 2010, 26, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Sunner, J.; Dratz, E.; Chen, Y.C. Graphite surface-assisted laser desorption/ionization time-of-flight mass spectrometry of peptides and proteins from liquid solutions. Anal. Chem. 1995, 67, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Liu, B.H.; Girault, H.H. Nanomaterial-assisted laser desorption ionization for mass spectrometry-based biomedical analysis. Nanomedicine 2010, 5, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Stecher, G.; Feuerstein, I.; Steinmueller, D.E.A. Chemically modified nano crystalline diamond layer as material enhanced laser desorption ionisation (MELDI) surface in protein profiling. Curr. Nanosci. 2006, 2, 1–7. [Google Scholar] [CrossRef]
- Wei, L.M.; Xue, Y.; Zhou, X.W.; Jin, H.; Shi, Q.; Lu, H.L.; Yang, P.Y. Nanodiamond MALDI support for enhancing the credibility of identifying proteins. Talanta 2008, 74, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Piret, G.; Drobecq, H.; Coffinier, Y.; Melnyk, O.; Boukherroub, R. Matrix-free laser desorption/ionization mass spectrometry on silicon nanowire arrays prepared by chemical etching of crystalline silicon. Langmuir 2010, 26, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.N.; Stolee, J.A.; Vertes, A. Nanophotonic ionization for ultratrace and single-cell analysis by mass spectrometry. Anal. Chem. 2012, 84, 7756–7762. [Google Scholar] [CrossRef] [PubMed]
- Northen, T.R.; Yanes, O.; Northen, M.T.; Marrinucci, D.; Uritboonthai, W.; Apon, J.; Golledge, S.L.; Nordstrom, A.; Siuzdak, G. Clathrate nanostructures for mass spectrometry. Nature 2007, 449, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Lu, M.; Zheng, Q.; Zhang, Y.; Dewald, H.D.; Chen, H. Recent advances of electrochemical mass spectrometry. Analyst 2013, 128, 5519–5539. [Google Scholar] [CrossRef] [PubMed]
- Abonnenc, M.; Qiao, L.; Liu, B.H.; Girault, H.H. Electrocheical aspects of electrosptray and laser desorption/ionization for mass spectometry. Annu. Rev. Anal. Chem. 2010, 3, 231–254. [Google Scholar] [CrossRef] [PubMed]
- Bruckenstein, S.; Gadde, R.R. Use of a porous electrode for in situ mass spectrometric determination of volatile electrode reaction products. J. Am. Chem. Soc. 1971, 93, 793–794. [Google Scholar] [CrossRef]
- Jahn, S.; Karst, U. Electrochemistry coupled to (liquid chromatography/) mass spectrometry—Current state and future perspectives. J. Chromatogr. A 2012, 1259, 16–49. [Google Scholar] [CrossRef] [PubMed]
- Gutkin, V.; Gunand, J.; Lev, O. Electrochemical deposition-stripping analysis of molecules and proteins by online electrochemical flow cell/mass spectrometry. Anal. Chem. 2009, 81, 8396–8404. [Google Scholar] [CrossRef] [PubMed]
- Van Berkel, G.J.; Kertesz, V. Electrochemically initiated tagging of thiols using an electrospray ionization based liquid microjunction surface sampling probe two-electrode cell. Rapid Commun. Mass Spectrom. 2009, 23, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Wolter, O.; Heitbaum, J. Differentiel electrochemical mass spectroscopy (DEMS)—A new method for the study of electrode processes. Ber. Bunsenges. Phys. Chem. 1984, 88, 2–6. [Google Scholar] [CrossRef]
- Takats, Z.; Wiseman, J.M.; Gologan, B.; Cooks, R.G. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 2004, 306, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Jurva, U.; Bissel, P.; Isin, E.M.; Igarashi, K.; Kuttab, S.; Castagnoli, N. Model electrochemical-mass spectrometric studies of the cytochrome P450-catalyzed oxidations of cyclic tertiary allylamines. J. Am. Chem. Soc. 2005, 127, 12368–12377. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xu, X.; Cole, R.B. On-line linear sweep voltammetry−electrospray mass spectrometry. Anal. Chem. 1997, 69, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Palii, S.P.; Eyler, J.R.; Brajter-Toth, A. Enhancement of ionization efficiency by electrochemical reaction products in on-line electrochemistry/electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Anal. Chem. 2002, 74, 1097–1103. [Google Scholar]
- Zettersten, C.; Sjöberg, P.J.R.; Nyholm, L. Oxidation of 4-chloroaniline studied by on-line electrochemistry electrospray ionization mass spectrometry. Anal.Chem. 2009, 81, 5180–5187. [Google Scholar] [CrossRef] [PubMed]
- Roussel, C.; Rohner, T.C.; Jensen, H.; Girault, H.H. Mechanistic aspects of on-line electrochemical tagging of free L-cysteine residues during electrospray ionisation for mass spectrometry in protein analysis. ChemPhysChem 2003, 4, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Rohner, T.C.; Rossier, J.S.; Girault, H.H. On-line electrochemcial tagging of cysteines in proteins during nanospray. Electrochem. Commun. 2002, 74, 695–700. [Google Scholar] [CrossRef]
- Roeser, J.; Permentier, H.P.; Bruins, A.P.; Bischoff, R. Electrochemical oxidation and cleavage of tyrosine- and tryptophan-containing tripeptides. Anal. Chem. 2010, 820, 7556–7565. [Google Scholar] [CrossRef] [PubMed]
- Roeser, J.; Permentier, H.P.; Bruins, A.P.; Bischoff, R. Oxidative protein labeling in mass-spectrometry-based proteomics. Anal. Bioanal. Chem. 2010, 82, 7556–7565. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szunerits, S.; Coffinier, Y.; Boukherroub, R. Diamond Nanowires: A Novel Platform for Electrochemistry and Matrix-Free Mass Spectrometry. Sensors 2015, 15, 12573-12593. https://doi.org/10.3390/s150612573
Szunerits S, Coffinier Y, Boukherroub R. Diamond Nanowires: A Novel Platform for Electrochemistry and Matrix-Free Mass Spectrometry. Sensors. 2015; 15(6):12573-12593. https://doi.org/10.3390/s150612573
Chicago/Turabian StyleSzunerits, Sabine, Yannick Coffinier, and Rabah Boukherroub. 2015. "Diamond Nanowires: A Novel Platform for Electrochemistry and Matrix-Free Mass Spectrometry" Sensors 15, no. 6: 12573-12593. https://doi.org/10.3390/s150612573




