Sensing and Tactile Artificial Muscles from Reactive Materials
Abstract
:1. Introduction
2. Conducting Polymers: Classification
- Polymer/macro-ion blends. The synthesized oxidized material contains macro-ions, which are not interchanged during redox processes:
3. Electrochemical Behavior of Conducting Polymers in Solution
4. Electrochemical Properties
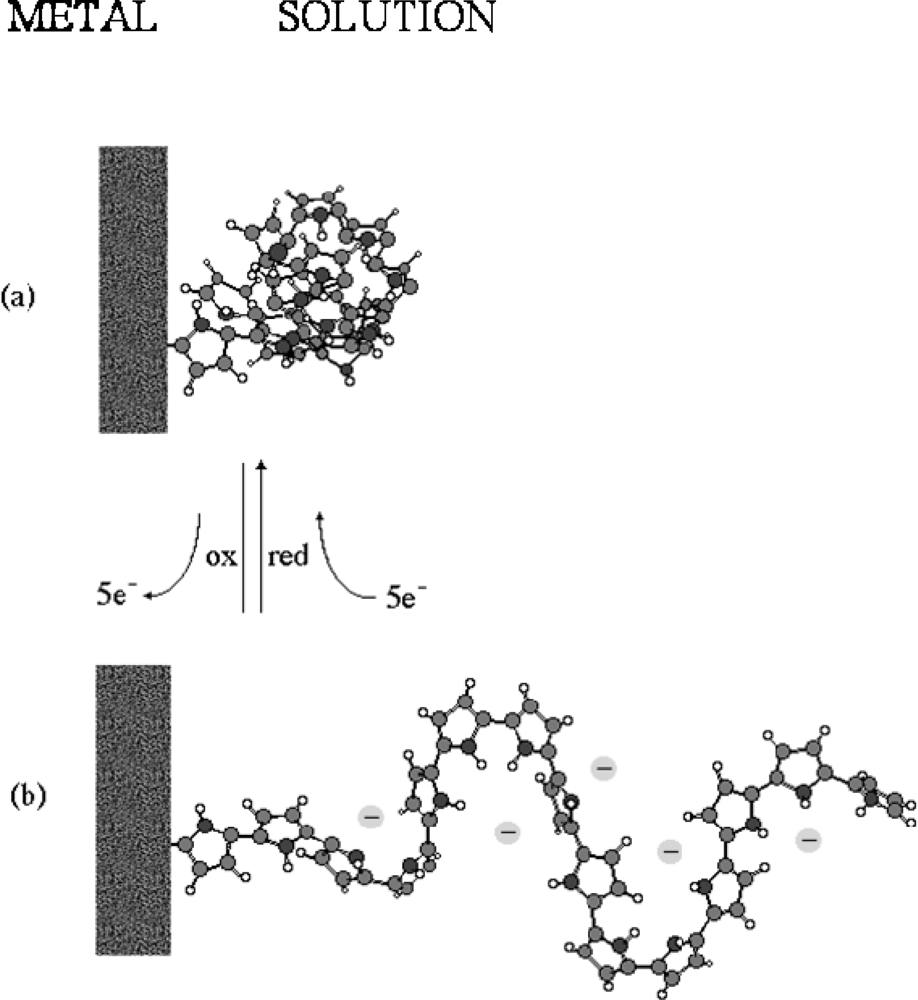
- - Electrochemo-mechanical properties: the entrance and expulsion of counterions and solvent from the solution, driven by the electrochemical reaction, promotes reversible changes on the material volume, (see Figure 4), which can be applied to generate macroscopic movement and mechanical energy [67–70].
- - Electrochromic properties: the reversible reorganization of the double bonds along the polymeric chains generates and destroys chromophores (polarons and bipolarons) adsorbing light along the UV-vis and near IR regions of the spectra. The color of the material can be changed reversibly, by controlling the concentration of chromophores, under electrochemical reaction in a continuous and reversible manner [71–75].
- - Charge storage: transition from neutral to oxidized polymers implies the storage of positive charges along the polymeric chains. Transition from neutral to reduced polymer implies the storage of negative charges along the polymeric chains. Therefore, CPs can be used as electrodic materials for polymeric batteries [76–78].
- - Porosity: a film of a basic conducting polymer in a neutral state is a porous compacted structure, in which average distances between chains is short. During oxidation, coulombic repulsions among the emerging positive charges in neighboring chains increase the average distance between chains allowing counterions entrance [54,79–81].
- - Electron/Chemical transduction: reverse electrochemical reactions are linked to the simultaneous interchange of chemical ions between the CP and the solution. This must be a univoque relationship; each injected electron forces the interchange of an one-valence chemical ion with the ambient electrolyte, suitable for a reversible storage and release of chemical and pharmacological compounds [82–84].
5. Multifunctional and Biomimicking Properties
6. Unparalleled Simultaneous Sensing Possibilities
7. Film preparation of Polypyrrole in Dodecyl Benzene Sulfonate (DBSA) Aqueous Solutions
7.1. Voltammetric Response
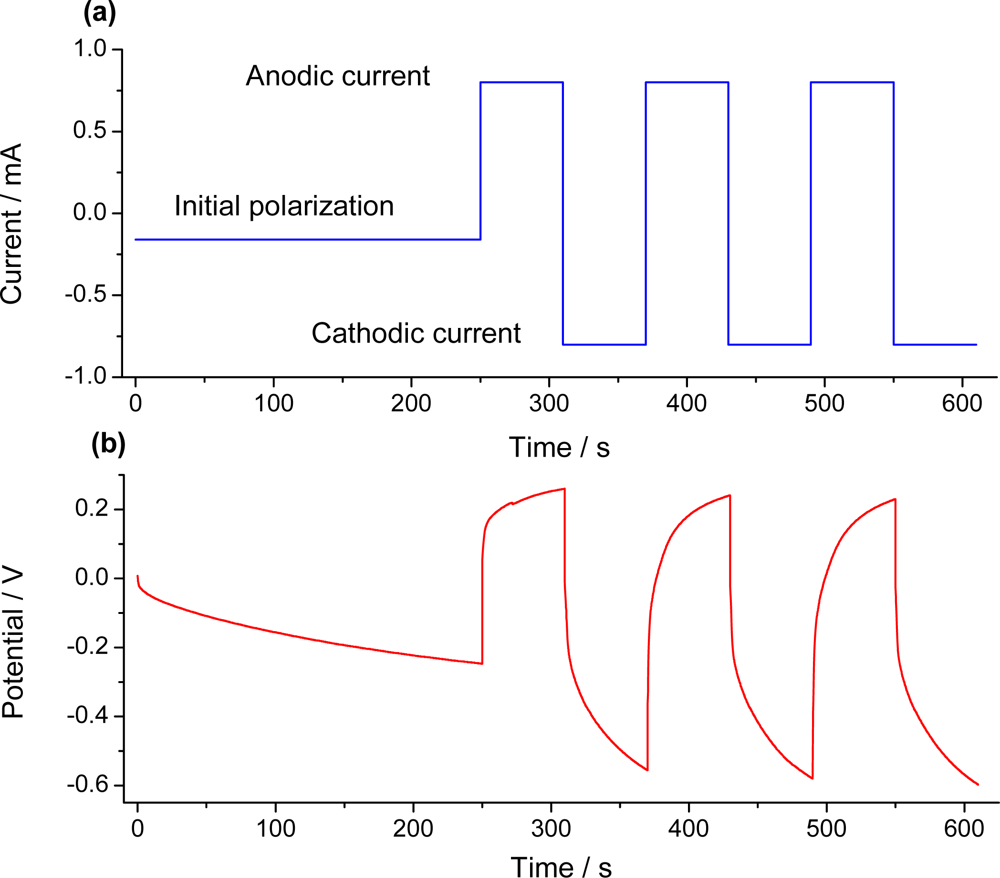
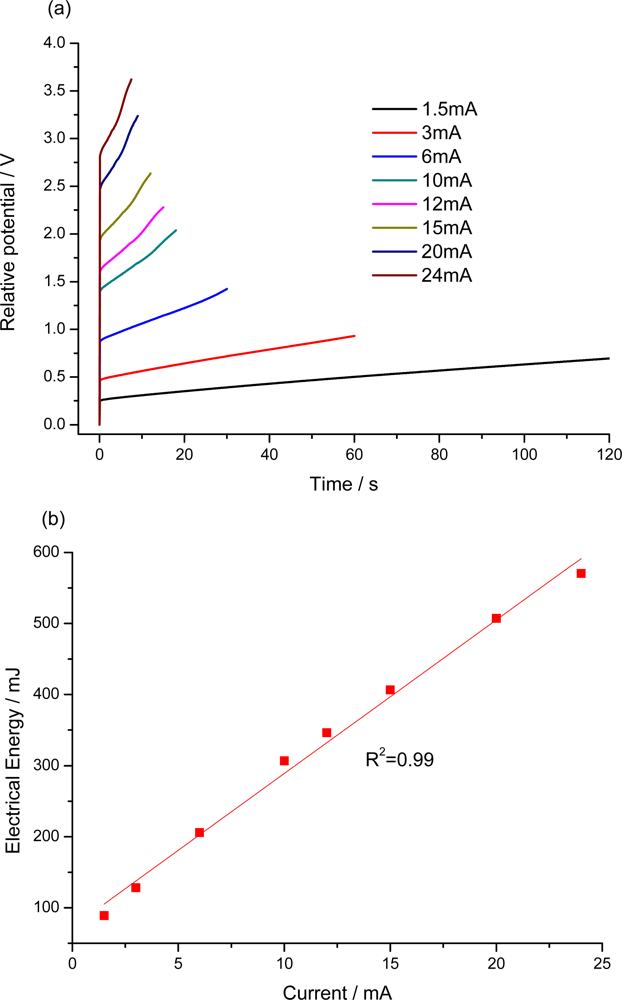
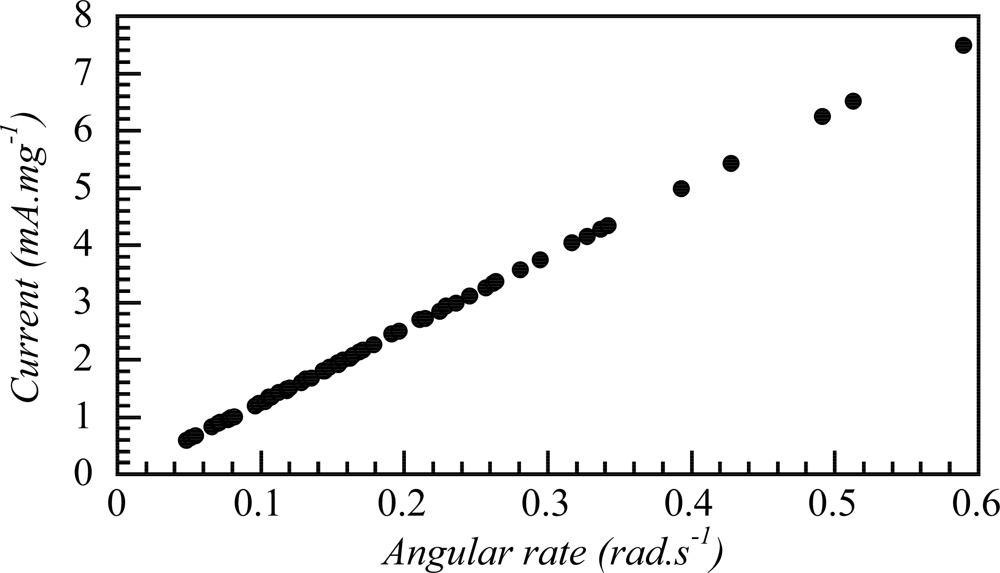
7.2. Sensing Abilities under Constant Current
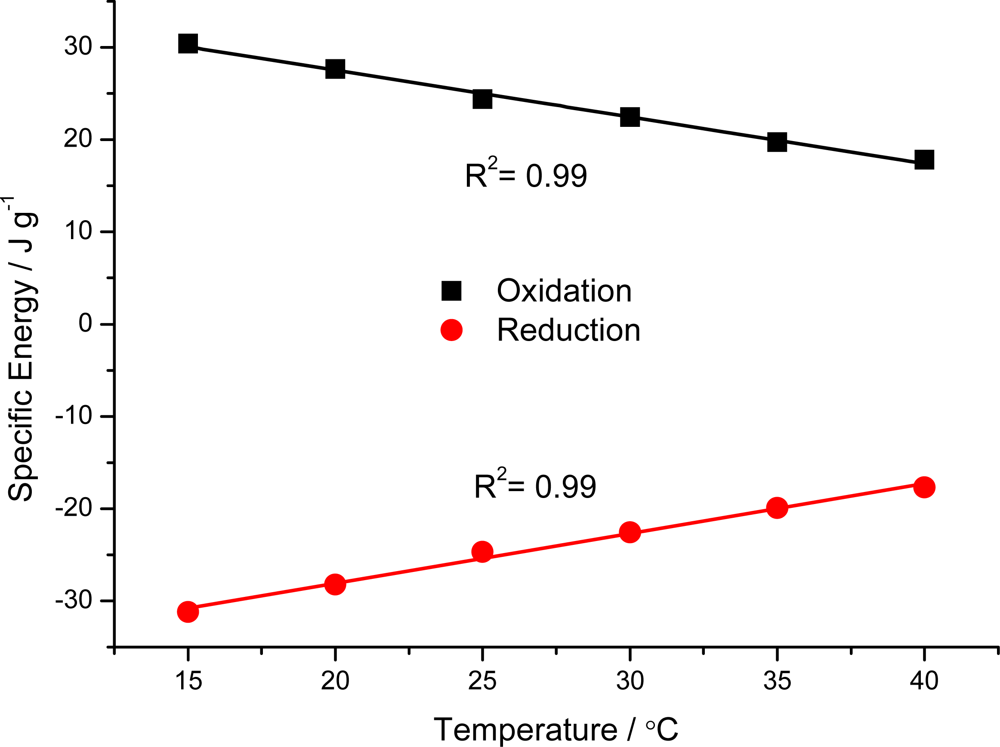
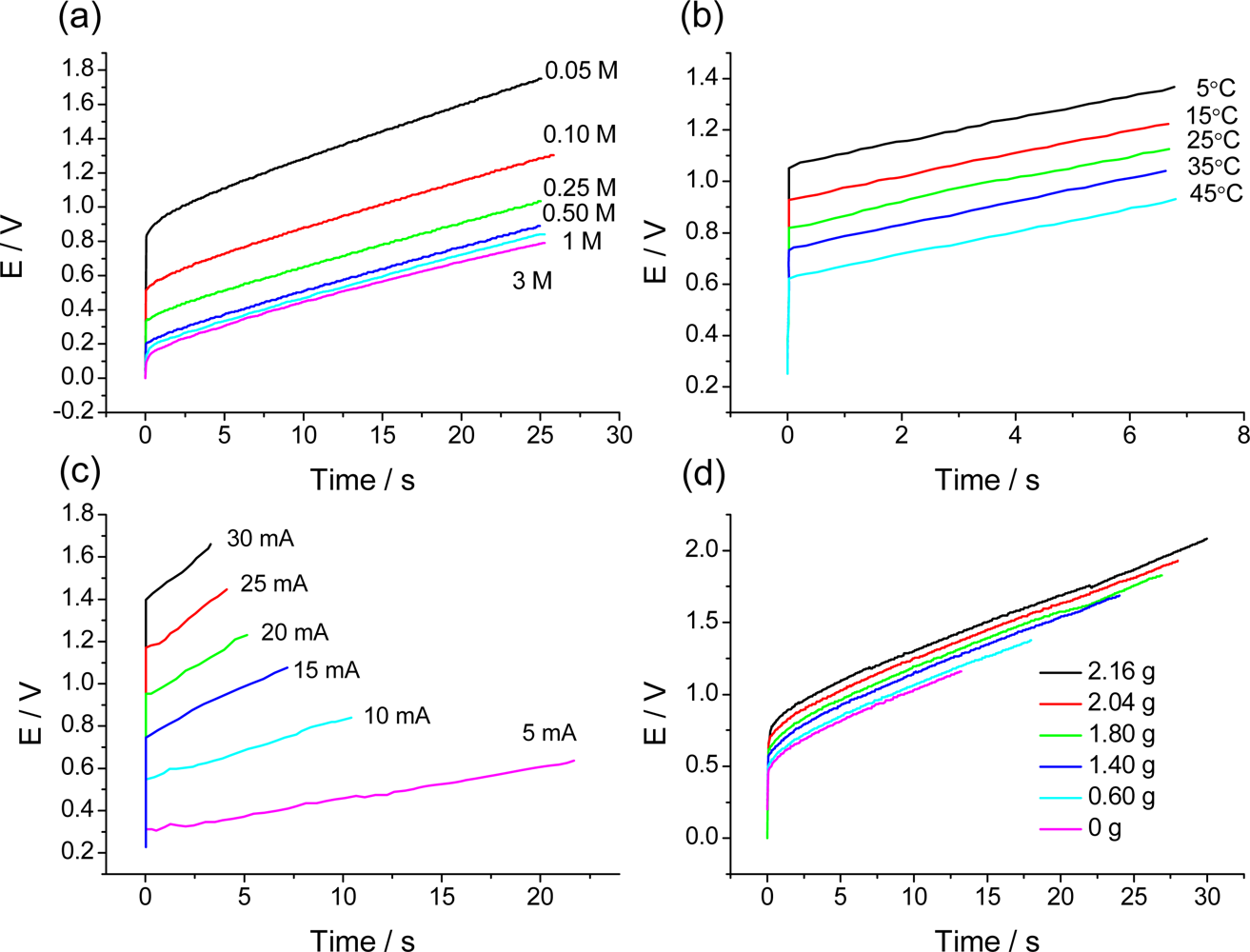
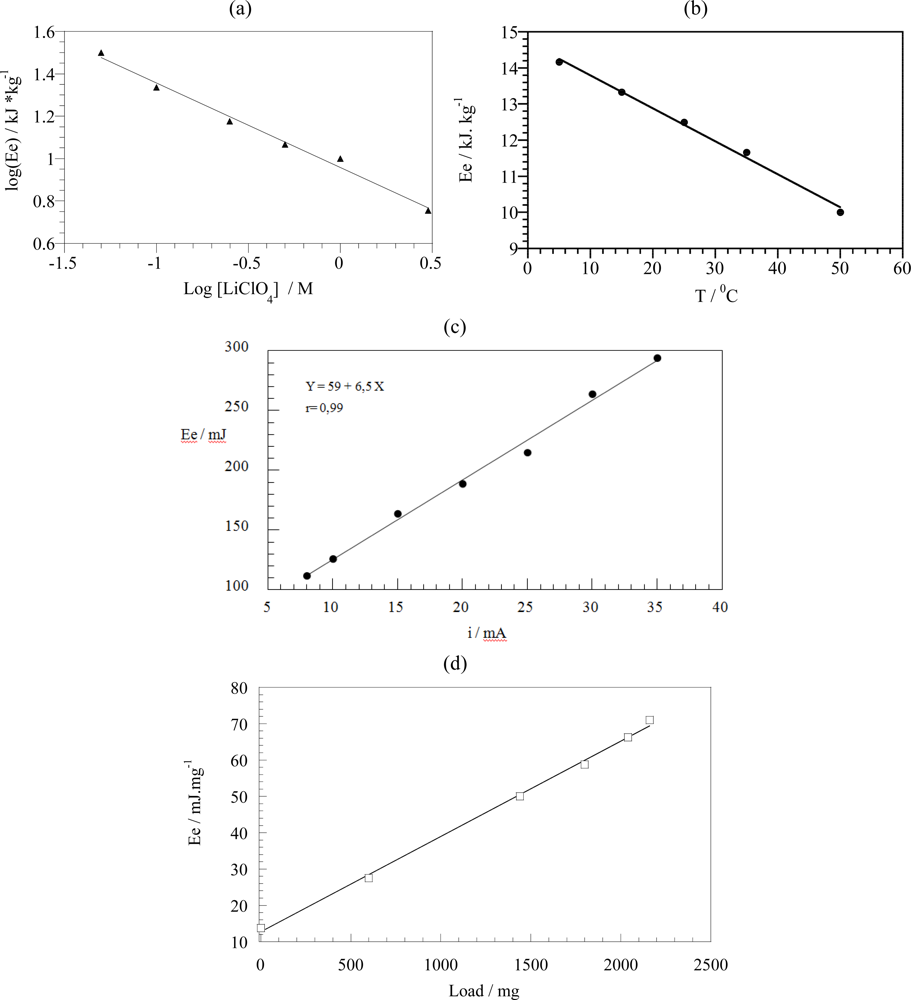
7.3. Temperature Sensor
7.4. Concentration Sensor
7.5. Expected Sensing Electrochemical Devices
8. Muscles and Artificial Muscles
8.1. Artificial Muscles
8.2. Classification of the Polymeric Artificial Muscles
- - Electromechanical actuators: Artificial muscles responding mainly to electric fields, E (V), being the dimensions variation of the electroactive polymer proportional to:
- E2: Electrostrictive actuators
- E: Piezoelectric actuatorsFerroelectric actuatorsElectrostatic actuatorsElectrokinetic actuators (electroosmotic)
- - Electrochemomechanical devices: Artificial muscles responding mainly to electric charges, Q (mC), being the dimensions variation under control of the electrochemical reaction, and proportional to:
- Q: Electrochemical actuators
8.3. Electrochemomechanical Muscles: Volume Variation
8.4. Electrochemical Basic Molecular Motors and Muscle Similitude
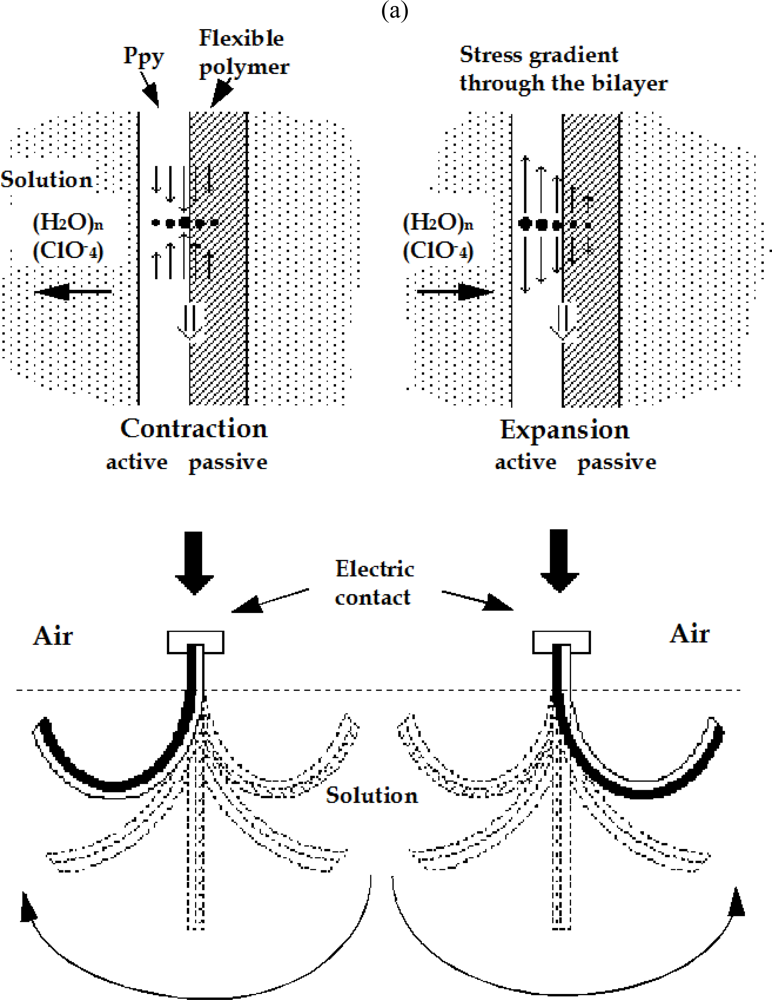
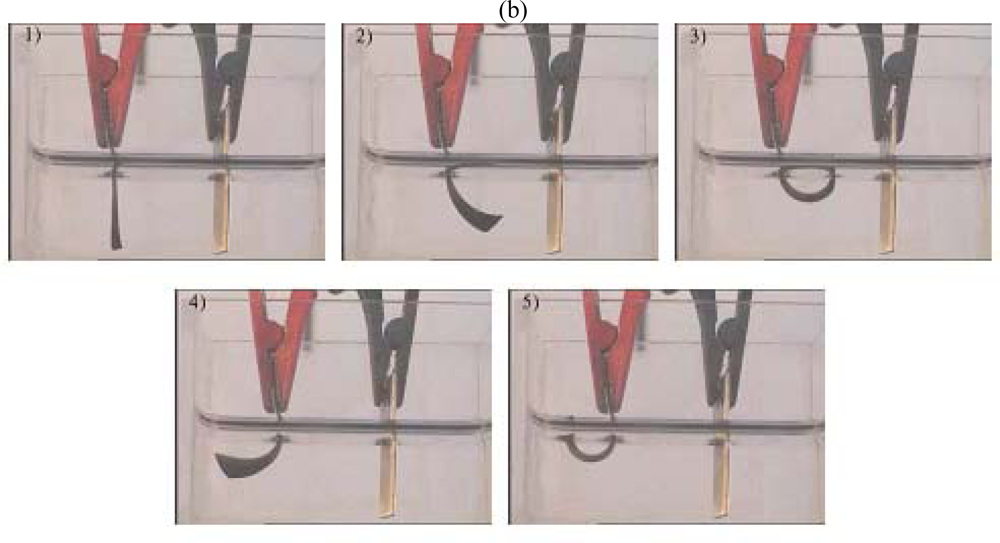
8.5. Devices Giving Macroscopic Movements
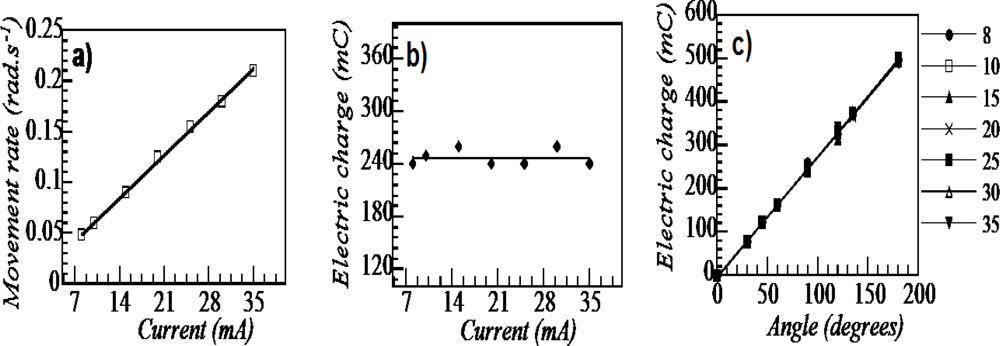
8.6. Electrochemical Nature of the Movement
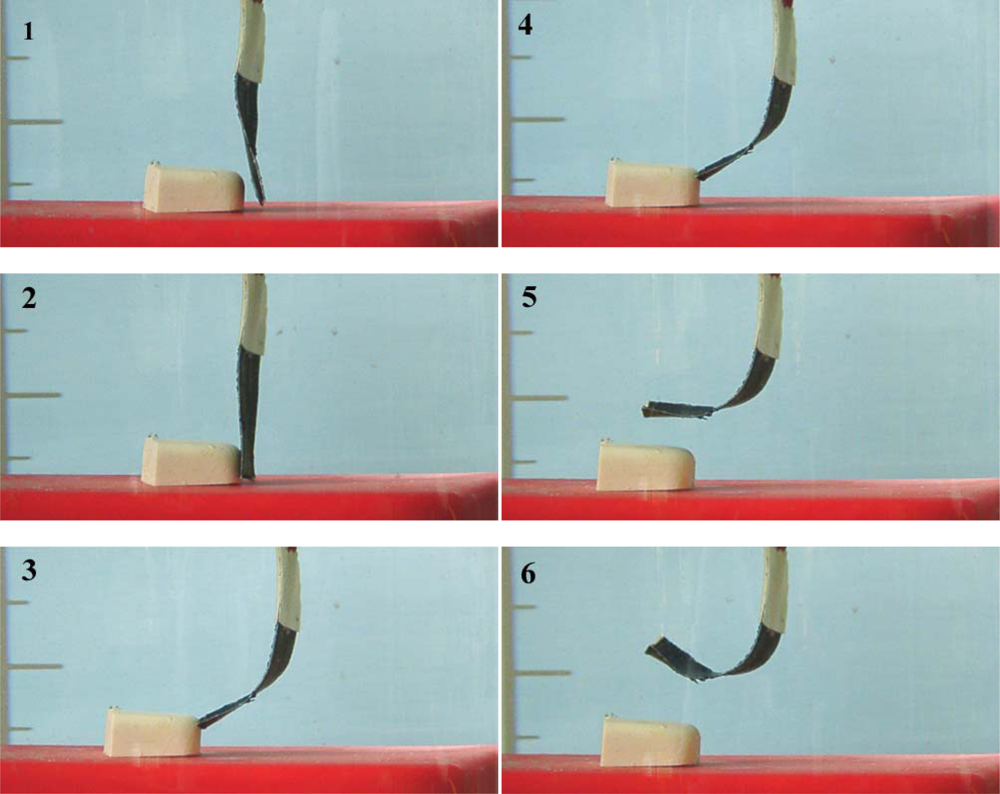
8.7. Sensing Muscles
8.8. Tactile Muscles
9. Limitations and Challenges
Acknowledgments
References and Notes
- Stryer, L.; Berg, J.M.; Tymoczko, J.L. Biochemistry, 4th ed.; W.H. Freeman & Company: New York, NY, USA, 1995. [Google Scholar]
- Otero, T.F.; Rodriguez, J. Electrochemomechanical and electrochemopositioning devices: artifcial muscles. In Intrinsically Conducting Polymers: An Emerging Technology; Aldissi, M., Ed.; Kluwer: Dordrecht, The Netherlands, 1993; pp. 179–190. [Google Scholar]
- Otero, T.F. Conducting polymers, electrochemistry, and biomimicking processes. In Modern Aspects of Electrochemistry; Bockris, J.O.M., White, R.E., Conway, B.E., Eds.; Kluwer Academic/Plenum Publisher: New York, NY, USA, 1999; pp. 307–434. [Google Scholar]
- Otero, T.F. Electrochemomechanical devices based on conducting polymers. In Polymer Sensors and Actuators; de Rossi, D., Osada, Y., Eds.; Springer: Berlin, Germany, 2000; pp. 295–323. [Google Scholar]
- Otero, T.F. Biomimicking materials with smart polymers. In Structural Biological Materials. Design and Structure-Properties Relationships; Elices, M., Cahn, R.W., Eds.; Pergamon Materials Series: Amsterdam, the Netherlands, 2000; pp. 187–220. [Google Scholar]
- Otero, T.F. Artificial muscles, sensing and multifunctionality. In Intelligent Materials; Shahinpoor, M., Schenider, H.-J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2008; pp. 142–190. [Google Scholar]
- Dallolio, A.; Dascola, G.; Varacca, V.; Bocchi, V. Electronic paramagnetic resonance and conductivity of a black electrolytic oxypyrrole. Cr. Acad. Sci. C. Chim 1968, 267, 433. [Google Scholar]
- Diaz, A.F.; Kanazawa, K.K.; Gardini, G.P. Electrochemical polymerization of pyrrole. J. Chem. Soc. Chem. Commun 1979, 14, 635–636. [Google Scholar]
- Diaz, A.F.; Lee, W.Y.; Logan, A.; Green, D.C. Chemical modification of a polypyrrole electrode surface. J. Electroanal. Chem 1980, 108, 377–380. [Google Scholar]
- Salmon, M.; Kanazawa, K.K.; Diaz, A.F.; Krounbi, M. A Chemical route to pyrrole polymer-films. J. Polym. Sci. Part C: Polym. Lett 1982, 20, 187–193. [Google Scholar]
- Rapi, S.; Bocchi, V.; Gardini, G.P. Conducting polypyrrole by chemical synthesis in water. Synth. Met 1988, 24, 217–221. [Google Scholar]
- Rodriguez, J.; Grande, H.J.; Otero, T.F. Polypyrroles: from basic research to technological applications. In Handbook of Organic Conductive Molecules and Polymers; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 415–468. [Google Scholar]
- Wallace, G.G.; Spinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Assembly of polypyrroles. In Conductive Electroactive Polymers: Intelligent Materials Systems; CRC Press: Boca Raton, FL, USA, 2003; pp. 51–88. [Google Scholar]
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, A Novel conducting polymer—morphology and chemistry of its oxidation and reduction in aqueous-electrolytes. J. Chem. Soc. Faraday Trans. I 1986, 82, 2385–2400. [Google Scholar]
- Letheby, H. XXIX. On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc 1862, 15, 161–163. [Google Scholar]
- Mohilner, D.M.; Argersinger, W.J.; Adams, R.N. Investigation of kinetics and mechanism of anodic oxidation of aniline in aqueous sulfuric acid solution at a platinum electrode. J. Am. Chem. Soc 1962, 84, 3618–3622. [Google Scholar]
- Kitani, A.; Kaya, M.; Sasaki, K. Performance study of aqueous polyaniline batteries. J. Electrochem. Soc 1986, 133, 1069–1073. [Google Scholar]
- Trivedi, D.C. Polyanilines. In Handbook of Organic Conductive Molecules and Polymers, 2nd ed.; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 505–572. [Google Scholar]
- Wallace, G.G.; Spinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Synthesis of polyanilines. In Conductive Electroactive Polymers: Intelligent Materials Systems; CRC Press: Boca Raton, FL, USA, 2003; pp. 121–160. [Google Scholar]
- Hotta, S.; Soga, M.; Sonoda, N. Novel organosynthetic routes to polythiophene and its derivatives. Synth. Met 1988, 26, 267–279. [Google Scholar]
- Yamamoto, T.; Sanechika, K.; Yamamoto, A. Preparation of thermostable and electric-conducting poly(2,5-thienylene). J. Polym. Sci. Polym. Lett. Ed 1980, 18, 9–12. [Google Scholar]
- Diaz, A.F.; Crowley, J.; Bargon, J.; Gardini, G.P.; Torrance, J. B. Electrooxidation of aromatic oligomers and conducting polymers. J. Electroanal. Chem 1981, 121, 355–361. [Google Scholar]
- Wallace, G.G.; Spinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Synthesis and properties of polythiophenes. In Conductive Electroactive Polymers: Intelligent Materials Systems; CRC Press: Boca Raton, FL, USA, 2003; pp. 179–206. [Google Scholar]
- Tourillon, G.; Garnier, F. New electrochemically generated organic conducting polymers. J. Electroanal. Chem 1982, 135, 173–178. [Google Scholar]
- Ohsawa, T.; Kaneto, K.; Yoshino, K. Electrical and optical-properties of electrochemically prepared polyfuran. Jpn. J. Appl. Phys 1984, 23, L663–L665. [Google Scholar]
- Zotti, G.; Schiavon, G.; Comisso, N.; Berlin, A.; Pagani, G. Electrochemical synthesis and characterization of polyconjugated polyfuran. Synth. Met 1990, 36, 337–351. [Google Scholar]
- Waltman, R.J.; Diaz, A.F.; Bargon, J. Substituent effects in the electropolymerization of aromatic heterocyclic-compounds. J. Phys. Chem 1984, 88, 4343–4346. [Google Scholar]
- Pandey, P.C. A new conducting polymer-coated glucose sensor. J. Chem. Soc. Faraday Trans. I 1988, 84, 2259–2265. [Google Scholar]
- Diaz, A.F.; Castillo, J.; Kanazawa, K.K.; Logan, J.A.; Salmon, M.; Fajardo, O. Conducting poly-n-alkylpyrrole polymer-films. J. Electroanal. Chem 1982, 133, 233–239. [Google Scholar]
- Macinnes, D.; Funt, B. L. Poly-ortho-methoxyaniline—a new soluble conducting polymer. Synth. Met 1988, 25, 235–242. [Google Scholar]
- Somanathan, N.; Radhakrishnan, S. Optical properties of functionalized polythiophenes. Int. J. Mod. Phys. B 2005, 19, 4645–4676. [Google Scholar]
- Abdou, M.S.A.; Holdcroft, S. Self-doped conductive polymers. In Handbook of Organic Conductive Molecules and Polymers, 2nd ed.; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 833–858. [Google Scholar]
- Freund, M.S.; Deore, B.A. Self-Doped Conducting Polymers; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Nazzal, A.I.; Street, G.B. Pyrrole styrene graft-copolymers. J. Chem. Soc. Chem. Commun 1985, 6, 375–376. [Google Scholar]
- Wei, Y.; Hariharan, R.; Patel, S.A. Chemical and electrochemical copolymerization of aniline with alkyl ring-substituted anilines. Macromolecules 1990, 23, 758–764. [Google Scholar]
- Carrasco, J.; Figueras, A.; Otero, T.F.; Brillas, E. Anodic electrosynthesis and cathodic electrodissolution of poly(2,5-di-(2-thienyl)pyrrole). A new way of processibility. Synth. Met 1993, 61, 253–258. [Google Scholar]
- Gumbs, R.W. Polythiophene and polypyrrole copolymers. In Handbook of Organic Conductive Molecules and Polymers, 2nd ed.; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 469–504. [Google Scholar]
- Ferraris, J.P.; Guerrero, D.J. Recent advances in heteroatomic copolymers. In Handbook of Conducting Polymers; Skotheim, T., Elsenbaumer, R.L., Reynolds, J.R., Eds.; Marcel Dekker: New York, NY, USA, 1997; pp. 259–276. [Google Scholar]
- Otero, T.F.; Vázquez, M.V. Electrogeneration of a composite polypyrrole-carboxymethylcellulose: kinetic study. J. Electroanal. Chem 1995, 397, 171–176. [Google Scholar]
- Otero, T.F.; Olazábal, V. Electrogeneration of polypyrrole in presence of polyvinylsulphonate. Kinetic study. Electrochim. Acta 1996, 41, 213–220. [Google Scholar]
- Otero, T.F.; González-Tejera, M.J. Polypyrrole + polyacrylate composites: electrogeneration. J. Electroanal. Chem 1996, 410, 69–77. [Google Scholar]
- Otero, T.F.; Sansiñena, J.M. Influence of synthesis conditions on polypyrrole-poly(styrenesulphonate) composite electroactivity. J. Electroanal. Chem 1996, 412, 109–116. [Google Scholar]
- Gómez-Romero, P.; Lira-Cantú, M. Hybrid organic-inorganic electrodes: the molecular material formed between polypyrrole and the phosphomolybdate anion. Adv. Mater 1997, 9, 144–147. [Google Scholar]
- Saunders, R.; Murray, K.S.; Flemming, R.J.; Cervini, R.; Allen, N. S. Handbook of Organic Conductive Molecules and Polymers, 3rd ed.; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 634–676. [Google Scholar]
- Clemente-Leon, M.; Coronado, E.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gomez-Garcia, C.J.; Fernandez-Otero, T. Hybrid molecular materials based on organic molecules and the inorganic magnetic cluster [M-4(H2O)(2)(PW9O34)(2)](10-) (M2+ = Co, Mn). J. Mater. Chem 1998, 8, 309–312. [Google Scholar]
- Otero, T.F.; Herrasti, P.; Ocon, P.; Alves, C.R. Electrogeneration of polypyrrole-carboxymethylcellulose composites: electrochemical, microgravimetric and morphological studies. Electrochim. Acta 1998, 43, 1089–1100. [Google Scholar]
- Ruckenstein, E.; Chen, J.H. Polypyrrole conductive composites prepared by coprecipitation. Polymer 1991, 32, 1230–1235. [Google Scholar]
- De Paoli, M.A. Conductive polymer blends and composites. In Handbook of Organic Conductive Molecules and Polymers, 2nd ed.; Nalwa, H.S., Ed.; John Wiley & Sons: Chichester, UK, 1997; pp. 773–798. [Google Scholar]
- Huang, J.C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol 2002, 21, 299–313. [Google Scholar]
- Otero, T.F.; GonzalezTejera, M.J. Polypyrrole plus polyacrylate composites, kinetic study. J. Electroanal. Chem 1997, 429, 19–25. [Google Scholar]
- Otero, T.F. Artificial muscles, electrodissolution and redox processes in conducting polymers. In Handbook of Organic Conductive Molecules and Polymers; Nalwa, H.S., Ed.; John Wiley & Sons: New York, NY, USA, 1997; pp. 517–594. [Google Scholar]
- Otero, T.F. Electrochemomechanical devices: artificial muscles based on conducting polymers. In Handbook of Conducting Polymers, 3rd ed.; Skotheim, T.A., Elsenhaumer, R.L., Reynolds, J.R., Eds.; Marcel Dekker Inc: New York, NY, USA, 1998; pp. 1015–1028. [Google Scholar]
- Inzelt, G.; Horányi, G.; Chambers, J.Q. Radiotracer study of the sorption of counter- and co-ions in tetracyanoquinodimethane and poly(vinyl ferrocene) modified electrodes. Electrochim. Acta 1987, 32, 757–763. [Google Scholar]
- Tsai, E.W.; Pajkossy, T.; Rajeshwar, K.; Reynolds, J.R. Anion-exchange behavior of polypyrrole membranes. J. Phys. Chem 1988, 92, 3560–3565. [Google Scholar]
- Shimidzu, T.; Ohtani, A.; Iyoda, T.; Honda, K. Effective adsorption desorption of cations on a polypyrrole polymer anion composite electrode. J. Chem. Soc. Chem. Commun 1986, 18, 1415–1417. [Google Scholar]
- Shimidzu, T.; Ohtani, A.; Iyoda, T.; Honda, K. Charge-controllable polypyrrole polyelectrolyte composite membranes. II. Effect of incorporated anion size on the electrochemical oxidation reduction process. J. Electroanal. Chem 1987, 224, 123–135. [Google Scholar]
- Li, F.B.; Albery, W.J. Electrochemical and in situ electron-paramagnetic resonance studies of polypyrrole doped with polystyrene sulfonate. J. Chem. Soc. Faraday Trans. I 1991, 87, 2949–2954. [Google Scholar]
- Yue, J.; Wang, Z.H.; Cromack, K.R.; Epstein, A.J.; Macdiarmid, A.G. Effect of sulfonic-acid group on polyaniline backbone. J. Am. Chem. Soc 1991, 113, 2665–2671. [Google Scholar]
- Otero, T.F.; Santos, F. Polythiophene oxidation: rate coefficients, activation energy and conformational energies. Electrochim. Acta 2008, 53, 3166–3174. [Google Scholar]
- Otero, T.F.; Abadias, R. Potentiostatic oxidation of poly(3-methylthiophene): influence of the prepolarization time at cathodic potentials on the kinetics. J. Electroanal. Chem 2008, 618, 39–44. [Google Scholar]
- Hillman, A.R.; Loveday, D.C.; Swann, M.J.; Eales, R.M.; Hamnett, A.; Higgins, S.J.; Bruckenstein, S.; Wilde, C.P. Charge transport in electroactive polymer-films. Faraday Discuss. Chem. Soc 1989, 88, 151–163. [Google Scholar]
- Inzelt, G. Mechanism of charge transport in polymer-modified electrodes. In Electroanalytical Chemistry: A Series of Advances; Bard, A.J., Ed.; Marcel Dekker: New York, NY, USA, 1994; pp. 89–243. [Google Scholar]
- Inzelt, G. Redox Transformations and Transport Processes. In Conducting Polymers; Scholz, F., Ed.; Springer-Verlag: Berlin: Heidelberg, Germany, 2008; pp. 169–224. [Google Scholar]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation behaviour of polypyrrole. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar]
- Fermín, D.J.; Teruel, H.; Scharifker, B.R. Changes in the population of neutral species and charge carriers during electrochemical oxidation of polypyrrole. J. Electroanal. Chem 1996, 401, 207–214. [Google Scholar]
- Sadki, S.; Schottland, P.; Brodie, N.; Sabouraud, G. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev 2000, 29, 283–293. [Google Scholar]
- Otero, T.F.; Angulo, E.; Rodriguez, J.; Santamaria, C. Electrochemomechanical properties from a bilayer—polypyrrole nonconducting and flexible material artificial muscle. J. Electroanal. Chem 1992, 341, 369–375. [Google Scholar]
- Okuzaki, H.; Funasaka, K. Electromechanical properties of a humido-sensitive conducting polymer film. Macromolecules 2000, 33, 8307–8311. [Google Scholar]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater 2003, 15, 481–494. [Google Scholar]
- Cortes, M.T.; Moreno, J.C. Artificial muscles based on conducting polymers. E-Polymers 2003, 41, 1–42. [Google Scholar]
- Kaufman, F.B.; Schroeder, A.H.; Engler, E.M.; Patel, V.V. Polymer-modified electrodes—a new class of electrochromic materials. Appl. Phys. Lett 1980, 36, 422–425. [Google Scholar]
- Heuer, H.W.; Wehrmann, R.; Kirchmeyer, S. Electrochromic window based on conducting poly (3,4-ethylenedioxythiophene)poly(styrene sulfonate). Adv. Funct. Mater 2002, 12, 89–94. [Google Scholar]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar]
- Dyer, A.L.; Reynolds, J.R. Electrochromism of conjugated conducting polymers. In Handbook of Conducting Polymers, 3rd ed.; Skotheim, T.A., Elsenbaumer, R.L., Reynolds, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Seshadri, V.; Padilla, J.; Bircan, H.; Radmard, B.; Draper, R.; Wood, M.; Otero, T.F.; Sotzing, G.A. Optimization, preparation, and electrical short evaluation for 30 cm2 active area dual conjugated polymer electrochromic windows. Org. Electron 2007, 8, 367–381. [Google Scholar]
- Feldman, B.J.; Burgmayer, P.; Murray, R.W. The Potential Dependence of electrical-conductivity and chemical charge storage of poly(pyrrole) films on electrodes. J. Am. Chem. Soc 1985, 107, 872–878. [Google Scholar]
- Irvin, J.A.; Irvin, D.J.; Stenger-Smith, J.D. Electroactive polymers for batteries and supercapacitors. In Handbook of Conducting Polymers, 3rd ed.; Skotheim, T.A., Elsenbaumer, R.L., Reynolds, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Peng, C.; Zhang, S.W.; Jewell, D.; Chen, G.Z. Carbon nanotube and conducting polymer composites for supercapacitors. Prog. Nat. Sci 2008, 18, 777–788. [Google Scholar]
- Burgmayer, P.; Murray, R.W. An ion gate membrane—electrochemical control of ion permeability through a membrane with an embedded electrode. J. Am. Chem. Soc 1982, 104, 6139–6140. [Google Scholar]
- Ehrenbeck, C.; Juttner, K. Development of an anion cation permeable free-standing membrane based on electrochemical switching of polypyrrole. Electrochim. Acta 1996, 41, 511–518. [Google Scholar]
- Chan, C.; Chang, S.; Naguib, H.E. Development and characterization of polypyrrole bi-layer and tri-layer thin porous films. Smart. Mater. Struct 2009, 18, 104022. [Google Scholar]
- Zinger, B.; Miller, L.L. Timed release of chemicals from polypyrrole films. J. Am. Chem. Soc 1984, 106, 6861–6863. [Google Scholar]
- Miller, L.L.; Zinger, B.; Zhou, Q.X. Electrically controlled release of hexacyanoferrate(4-) from polypyrrole. J. Am. Chem. Soc 1987, 109, 2267–2272. [Google Scholar]
- Zhong, Y.; Bellamkonda, R.V. Controlled release of anti-inflammatory agent [alpha]-MSH from neural implants. J. Controlled Release 2005, 106, 309–318. [Google Scholar]
- Otero, T.F. Biomimetics, artificial muSCLES & NANO-BIO 2007: scientists meet doctors. J. Phys. Conf. Ser 2008, 127, 011001. [Google Scholar]
- Binh, T.T.N. Development of a biomimetic nanoporous membrane for the selective transport of charged proteins. Bioinspir. Biomim 2008, 3, 035008. [Google Scholar]
- Otero, T.F. Soft, wet, and reactive polymers. Sensing artificial muscles and conformational energy. J. Mater. Chem 2009, 19, 681–689. [Google Scholar]
- Li, C.; Bai, H.; Shi, G.Q. Conducting polymer nanomaterials: electrosynthesis and applications. Chem. Soc. Rev 2009, 38, 2397–2409. [Google Scholar]
- Otero, T.F. Electro-chemo-mechanical actuators touching and sensing both, physical and chemical ambient. Adv. Sci. Tech 2008, 61, 112–121. [Google Scholar]
- Sansiñena, J.M. Músculos artificiales: dispositivos electroquimiomecánicos basados en polímeros conductores. University of the Basque Country (UPV/EHU): San Sebastian, Spain, 1998. [Google Scholar]
- Otero, T.F.; Sansiñena, J.M. Artificial muscles based on conducting polymers. Bioelectroch. Bioener 1995, 38, 411–414. [Google Scholar]
- Otero, T.F.; Cortes, M.T. A sensing muscle. Sens. Actuators B 2003, 96, 152–156. [Google Scholar]
- Otero, T.F. Sensing muscles based on conducting polymers: conformational energy. Proceedings of Actuator 08, Bremen, Germany; 2008; pp. 248–251. [Google Scholar]
- Shimoda, S.; Smela, E. The effect of pH on polymerization and volume change in PPy(DBS). Electrochim. Acta 1998, 44, 219–238. [Google Scholar]
- Maw, S.; Smela, E.; Yoshida, K.; Sommer-Larsen, P.; Stein, R.B. The effects of varying deposition current density on bending behaviour in PPy(DBS)-actuated bending beams. Sens. Actuators A 2001, 89, 175–184. [Google Scholar]
- Takashima, W.; Pandey, S.S.; Kaneto, K. Cyclic voltammetric and electrochemomechanical characteristics of freestanding polypyrrole films in diluted media. Thin Solid Films 2003, 438–439, 339–345. [Google Scholar]
- Otero, T.F.; Ariza, M.J. Revisiting the electrochemical and polymeric behavior of a polypyrrole free-standing electrode in aqueous solution. J. Phys. Chem. B 2003, 107, 13954–13961. [Google Scholar]
- John, M.H.; Ian, W.H.; John, B. A comparative analysis of actuator technologies for robotics. In The Robotics Review, 2nd ed.; MIT Press: Cambridge, MA, USA, 1992; pp. 299–342. [Google Scholar]
- Madden, J.D.W.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.A.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial muscle technology: physical principles and naval prospects. IEEE J. Oceanic. Eng 2004, 29, 706–728. [Google Scholar]
- Dunn-Rankin, D.; Leal, E.M.; Walther, D.C. Personal power systems. Progt. Energ. Combust 2005, 31, 422–465. [Google Scholar]
- Katchalsky, A. Rapid swelling and deswelling of reversible gels of polymeric acids by ionization. Experientia 1949, 5, 319–320. [Google Scholar]
- Katchalsky, A.; Eisenberg, H. Polyvinylphosphate contractile systems. Nature 1950, 166, 267–267. [Google Scholar]
- Kuhn, W.; Hargitay, B.; Katchalsky, A.; Eisenberg, H. Reversible dilation and contraction by changing the state of ionization of high-polymer acid networks. Nature 1950, 165, 514–516. [Google Scholar]
- Katchalsky, A.; Zwick, M. Mechanochemistry and ion exchange. J. Polym. Sci 1955, 16, 221–234. [Google Scholar]
- Pei, Q. B.; Inganas, O. Conjugated polymers and the bending cantilever method—electrical muscles and smart devices. Adv. Mater 1992, 4, 277–278. [Google Scholar]
- Otero, T.F.; Angulo, E.; Rodriguez, J.; Santamaria, C. Dispositivos laminares que emplean polímeros conductores capaces de provocar movimientos mecánicos. Spanish patent 1992/1/17.
- Otero, T.F.; Rodriguez, J.; Santamaria, C. Músculos artificiales formados por multicapas: polímeros conductores-no conductores. Spanish patent 1992/12/28.
- Mazzoldi, A.; Degl'Innocenti, C.; Michelucci, M.; De Rossi, D. Actuative properties of polyaniline fibers under electrochemical stimulation. Mat. Sci. Eng. C-Bio 1998, 6, 65–72. [Google Scholar]
- Madden, J.; Hunter, I.W.; Gilbert, R.J. Development of an artificial muscle fiber composed of the conducting polymer actuator polypyrrole. Gastroenterology 2002, 122, A164. [Google Scholar]
- Ding, J.; Liu, L.; Spinks, G.M.; Zhou, D.; Wallace, G.G.; Gillespie, J. High performance conducting polymer actuators utilising a tubular geometry and helical wire interconnects. Synth. Met 2003, 138, 391–398. [Google Scholar]
- Yamato, K.; Kaneto, K. Tubular linear actuators using conducting polymer, polypyrrole. Anal. Chim. Acta 2006, 568, 133–137. [Google Scholar]
- Foroughi, J.; Spinks, G.M.; Wallace, G.G. Effect of synthesis conditions on the properties of wet spun polypyrrole fibres. Synth. Met 2009, 159, 1837–1843. [Google Scholar]
- Spinks, G.M.; Zhou, D.Z.; Liu, L.; Wallace, G.G. The amounts per cycle of polypyrrole electromechanical actuators. Smart. Mater. Struct 2003, 12, 468–472. [Google Scholar]
- Hara, S.; Zama, T.; Sewa, S.; Takashima, W.; Kaneto, K. Polypyrrole-metal coil composites as fibrous artificial muscles. Chem. Lett 2003, 32, 800–801. [Google Scholar]
- Hara, S.; Zama, T.; Ametani, A.; Takashima, W.; Kaneto, K. Enhancement in electrochemical strain of a polypyrrole-metal composite film actuator. J. Mater. Chem 2004, 14, 2724–2725. [Google Scholar]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Polypyrrole-metal coil composite actuators as artificial muscle fibres. Synth. Met 2004, 146, 47–55. [Google Scholar]
- Bay, L.; West, K.; Sommer-Larsen, P.; Skaarup, S.; Benslimane, M. A conducting polymer artificial muscle with 12% linear strain. Adv. Mater 2003, 15, 310–313. [Google Scholar]
- Lu, W.; Fadeev, A.G.; Qi, B.H.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.Z.; Wallace, G.G.; MacFarlane, D.R.; Forsyth, S.A.; Forsyth, M. Use of ionic liquids for pi-conjugated polymer electrochemical devices. Science 2002, 297, 983–987. [Google Scholar]
- Okuzaki, H. A biomorphic origami actuator fabricated by folding a conducting paper. J. Phys. Conf. Ser 2008, 127, 012001. [Google Scholar]
- Kaneto, K.; Sonoda, Y.; Takashima, W. Direct measurement and mechanism of electro-chemomechanical expansion and contraction in polypyrrole films. Jpn. J. Appl. Phys. 2000, 5918–5922. [Google Scholar]
- Pandey, S.S.; Takashima, W.; Kaneto, K. Structure property correlation: electrochemomechanical deformation in polypyrrole films. Thin Solid Films 2003, 438, 206–211. [Google Scholar]
- Zama, T.; Hara, S.; Takashima, W.; Kaneto, K. The correlation between electrically induced stress and mechanical tensile strength of polypyrrole actuators. Bull. Chem. Soc. Jpn 2004, 77, 1425–1426. [Google Scholar]
- Baughman, R.H.; Shacklette, L.W. Application of dopant-induced structure-property changes of conducting polymers. In Science and Application of Conducting Polymers; Salaneck, W.R., Clark, D.T., Samuelsen, E.J., Eds.; IOP Publishing Ltd: Bristol, UK, 1991; pp. 47–61. [Google Scholar]
- Pei, Q.B.; Inganas, O. Electrochemical applications of the bending beam method. I. Mass-transport and volume changes in polypyrrole during redox. J. Phys. Chem 1992, 96, 10507–10514. [Google Scholar]
- Suarez, M.F.; Compton, R.G. In situ atomic force microscopy study of polypyrrole synthesis and the volume changes induced by oxidation and reduction of the polymer. J. Electroanal. Chem 1999, 462, 211–221. [Google Scholar]
- Smela, E.; Gadegaard, N. Surprising volume change in PPy(DBS): an atomic force microscopy study. Adv. Mater 1999, 11, 953–957. [Google Scholar]
- Smela, E.; Gadegaard, N. Volume change in polypyrrole studied by atomic force microscopy. J. Phys. Chem. B 2001, 105, 9395–9405. [Google Scholar]
- Barbero, C.; Kotz, R. Nanoscale Dimensional changes and optical-properties of polyaniline measured by in-situ spectroscopic ellipsometry. J. Electrochem. Soc 1994, 141, 859–865. [Google Scholar]
- Ehrenbeck, C.; Juttner, K. Ion conductivity and permselectivity measurements of polypyrrole membranes at variable states of oxidation. Electrochim. Acta 1996, 41, 1815–1823. [Google Scholar]
- Juttner, K.; Ehrenbeck, C. Electrochemical measurements of the ion conductivity, permselectivity and transference numbers of polypyrrole and polypyrrole derivatives. J. Solid State Electrochem 1998, 2, 60–66. [Google Scholar]
- Andrade, E. M.; Molina, F.V.; Florit, M.I.; Posadas, D. Volume changes of poly(2-methylaniline) upon redox switching—anion and relaxation effects. Electrochem. Solid-State Lett 2000, 3, 504–507. [Google Scholar]
- Lizarraga, L.; Andrade, E.M.; Molina, F.V. Swelling and volume changes of polyaniline upon redox switching. J. Electroanal. Chem 2004, 561, 127–135. [Google Scholar]
- Davis, A.P. Nanotechnology—synthetic molecular motors. Nature 1999, 401, 120–121. [Google Scholar]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Edit 2007, 46, 72–191. [Google Scholar]
- Pei, Q.; Inganäs, O. Electrochemical muscles: bending strips built from conjugated polymers. Synth. Met 1993, 57, 3718–3723. [Google Scholar]
- Takashima, W.; Kaneko, M.; Kaneto, K.; Macdiarmid, A.G. The electrochemical actuator using electrochemically-deposited poly-aniline film. Synth. Met 1995, 71, 2265–2266. [Google Scholar]
- Smela, E.; Inganäs, O.; Pei, Q.B.; Lundstrom, I. Electrochemical muscles—micromachining fingers and corkscrews. Adv. Mater 1993, 5, 630–632. [Google Scholar]
- Jager, E.W.H.; Smela, E.; Inganäs, O. Microfabricating conjugated polymer actuators. Science 2000, 290, 1540–1545. [Google Scholar]
- Jager, E.W.H.; Inganäs, O.; Lundstrom, I. Microrobots for micrometer-size objects in aqueous media: Potential tools for single-cell manipulation. Science 2000, 288, 2335–2338. [Google Scholar]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met 1996, 78, 339–353. [Google Scholar]
- Takashima, W.; Pandey, S.S.; Kaneto, K. Investigation of bi-ionic contribution for the enhancement of bending actuation in polypyrrole film. Sens. Actuators B 2003, 89, 48–52. [Google Scholar]
- Han, G.; Shi, G. Conducting polymer electrochemical actuator made of high-strength three-layered composite films of polythiophene and polypyrrole. Sensor Actuat. B-Chem 2004, 99, 525–531. [Google Scholar]
- Higgins, S.J.; Lovell, K.V.; Rajapakse, R.M.G.; Walsby, N.M. Grafting and electrochemical characterisation of poly-(3,4-ethylenedioxythiophene) films, on Nafion and on radiation-grafted polystyrenesulfonate-polyvinylidene fluoride composite surfaces. J. Mater. Chem 2003, 13, 2485–2489. [Google Scholar]
- Deshpande, S.D.; Kim, J.; Yun, S.R. New electro-active paper actuator using conducting polypyrrole: actuation behaviour in LiClO4 acetonitrile solution. Synth. Met 2005, 149, 53–58. [Google Scholar]
- Blonsky, P. M.; Meridian, I. Structurally stable gelled electrolytes. 1997. [Google Scholar]
- Song, M.K.; Cho, J.Y.; Cho, B.W.; Rhee, H.W. Characterization of UV-cured gel polymer electrolytes for rechargeable lithium batteries. J. Power Sources 2002, 110, 209–215. [Google Scholar]
- Vidal, F.; Popp, J.F.; Plesse, C.; Chevrot, C.; Teyssie, D. Feasibility of conducting semi-interpenetrating networks based on a poly(ethylene oxide) network and poly(3,4-ethylenedioxythiophene) in actuator design. J. Appl. Polym. Sci 2003, 90, 3569–3577. [Google Scholar]
- Plesse, C.; Vidal, F.; Randriamahazaka, H.; Teyssie, D.; Chevrot, C. Synthesis and characterization of conducting interpenetrating polymer networks for new actuators. Polymer 2005, 46, 7771–7778. [Google Scholar]
- Plesse, C.; Vidal, F.; Gauthier, C.; Pelletier, J.M.; Chevrot, C.; Teyssié, D. Poly(ethylene oxide)/polybutadiene based IPNs synthesis and characterization. Polymer 2007, 48, 696–703. [Google Scholar]
- Cho, M.; Seo, H.; Nam, J.; Choi, H.; Koo, J.; Lee, Y. High ionic conductivity and mechanical strength of solid polymer electrolytes based on NBR/ionic liquid and its application to an electrochemical actuator. Sensor Actuat. B-Chem 2007, 128, 70–74. [Google Scholar]
- Tran-Van, F.; Beouch, L.; Vidal, F.; Yammine, P.; Teyssie, D.; Chevrot, C. Self-supported semi-interpenetrating polymer networks for new design of electrochromic devices. Electrochim. Acta 2008, 53, 4336–4343. [Google Scholar]
- Vidal, F.; Plesse, C.; Palaprat, G.; Juger, J.; Citerin, J.; Kheddar, A.; Chevrot, C.; Teyssie, D. Synthesis and characterization of ipns for electrochemical actuators. In Artificial Muscle Actuators Using Electroactive Polymers; Vincenzini, P., BarCohen, Y., Carpi, F., Eds.; Trans Tech Publications Ltd: Stafa-Zurich, Switzerland, 2009; pp. 8–17. [Google Scholar]
- Otero, T.F.; Cortes, M.T.; Boyano, I. Macroscopic devices and complex movements developed with artificial muscles. In Smart Structures and Materials 2002: Electroactive Polymer Actuators and Devices (Eapad); Bellingham, WA, USA, 2002; pp. 395–402. [Google Scholar]
- Otero, T.F.; Cortes, M.T.; Arenas, G.V. Linear movements from two bending triple-layers. Electrochim. Acta 2007, 53, 1252–1258. [Google Scholar]
- Otero, T.F.; Broschart, M. Polypyrrole artificial muscles: a new rhombic element. Construction and electrochemomechanical characterization. J. Appl. Electrochem 2006, 36, 205–214. [Google Scholar]
- Vozzi, G.; Carpi, F.; Mazzoldi, A. Realization of conducting polymer actuators using a controlled volume microsyringe system. Smart. Mater. Struct 2006, 15, 279–287. [Google Scholar]
- Alici, G.; Huynh, N. N. Performance quantification of conducting polymer actuators for real applications: a microgripping system. IEEE ASME Trans. Mechatronics 2007, 12, 73–84. [Google Scholar]
- Smela, E.; Inganäs, O.; Lundstrom, I. Controlled folding of micrometer-size structures. Science 1995, 268, 1735–1738. [Google Scholar]
- Pede, D.; Smela, E.; Johansson, T.; Johansson, M.; Inganäs, O. A general-purpose conjugated-polymer device array for imaging. Adv. Mater 1998, 10, 233–237. [Google Scholar]
- Smela, E.; Kallenbach, M.; Holdenried, J. Electrochemically driven polypyrrole bilayers for moving and positioning bulk micromachined silicon plates. J. Microelectromech. S 1999, 8, 373–383. [Google Scholar]
- Smela, E. A microfabricated movable electrochromic “pixel” based on polypyrrole. Adv. Mater 1999, 11, 1343–1345. [Google Scholar]
- Jager, E.W.H.; Smela, E.; Inganäs, O.; Lundstrom, I. Polypyrrole microactuators. Synth. Met 1999, 102, 1309–1310. [Google Scholar]
- Jager, E.W.H.; Inganäs, O.; Lundstrom, I. Perpendicular actuation with individually controlled polymer microactuators. Adv. Mater 2001, 13, 76–79. [Google Scholar]
- Roemer, M.; Kurzenknabe, T.; Oesterschulze, E.; Nicoloso, N. Microactuators based on conducting polymers. Anal. Bioanal. Chem 2002, 373, 754–757. [Google Scholar]
- He, X.M.; Li, C.; Chen, F.G.; Shi, G.Q. Polypyrrole microtubule actuators for seizing and transferring microparticles. Adv. Funct. Mater 2007, 17, 2911–2917. [Google Scholar]
- Kiefer, R.; Mandviwalla, X.; Archer, R.; Tjahyono, S.S.; Wang, H.; MacDonald, B.; Bowmaker, G.A.; Kilmartin, P.A.; Travas-Sejdic, J. The application of polypyrrole trilayer actuators in microfluidics and robotics—art. no. 69271E. In Electroactive Polymer Actuators and Devices (Eapad) 2008; Bellingham, WA, USA, 2008; pp. E9271–E9271. [Google Scholar]
- Otero, T.F.; Cortes, M.T. Artificial muscle: movement and position control. Chem. Commun 2004, 3, 284–285. [Google Scholar]
- Otero, T.F.; Sansinena, J.M. Bilayer dimensions and movement in artificial muscles. Bioelectroch. Bioener 1997, 42, 117–122. [Google Scholar]
- Otero, T.F.; Sansinena, J.M. Soft and wet conducting polymers for artificial muscles. Adv. Mater 1998, 10, 491–494. [Google Scholar]
- Otero, T.F.; Cortes, M.T. Electrochemical characterization and control triple-layer muscles. In Smart Structures and Materials 2000: Electroactive Polymer Actuators and Devices (Eapad); Bellingham, WA, USA, 2000; pp. 252–260. [Google Scholar]
- Otero, T.F.; Cortes, M.T. Characterization of triple layers. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; Bellingham, WA, USA, 2001; pp. 93–100. [Google Scholar]
- Otero, T.F.; Boyano, I.; Cortes, M.T.; Vazquez, G. Nucleation, non-stoiquiometry and sensing muscles from conducting polymers. Electrochim. Acta 2004, 49, 3719–3726. [Google Scholar]
- Otero, T.F.; Cortes, M.T. Artificial muscles with tactile sensitivity. Adv. Mater 2003, 15, 279–282. [Google Scholar]
- Otero, T.F.; Cortes, M.T.; Boyano, L.; Vazquez, G. Nucleation, non-stoiquiometry, and tactile muscles with conducting polymers. In Smart Structures and Materials 2004: Electroactive Polymer Actuators and Devices (Eapad); Bellingham, WA, USA, 2004; pp. 425–432. [Google Scholar]
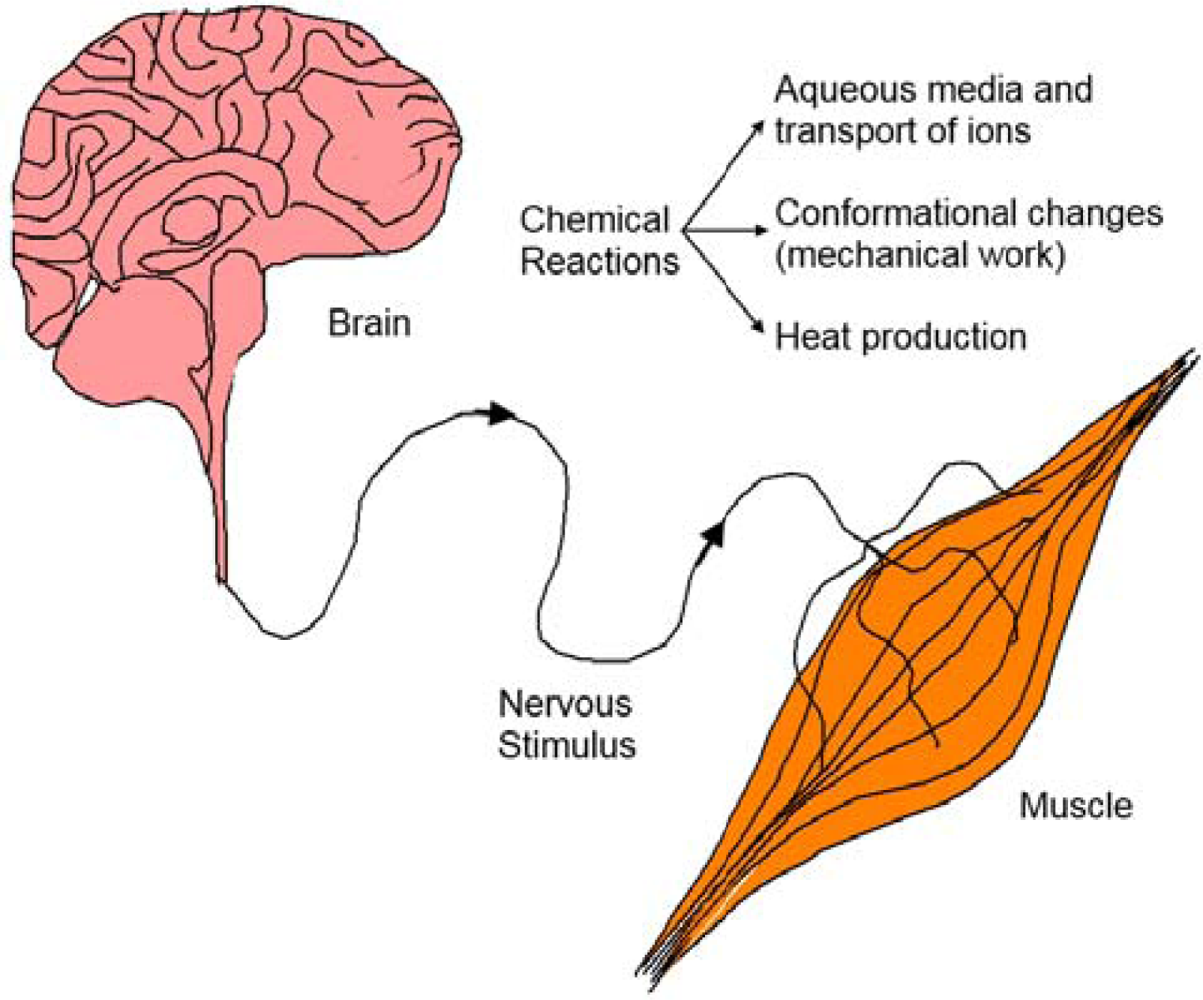

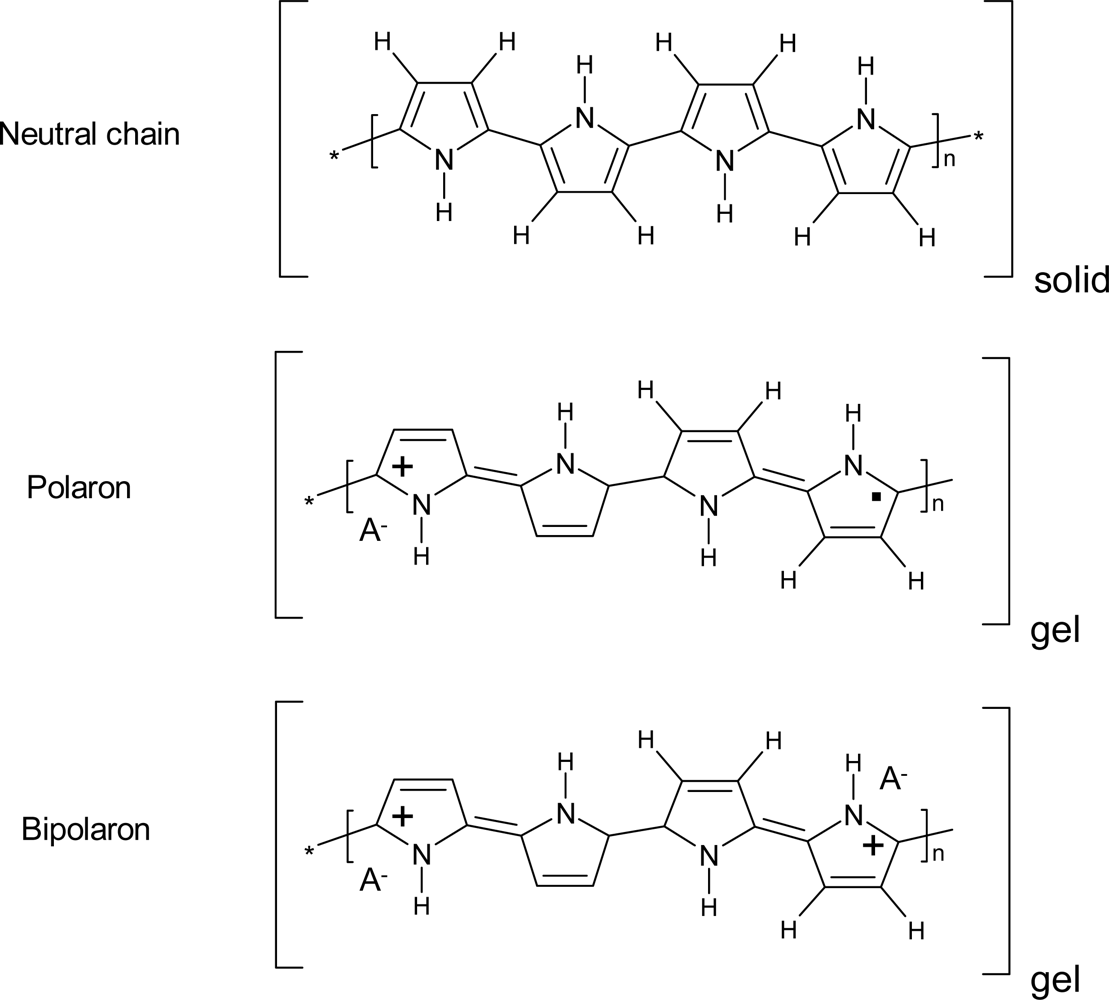
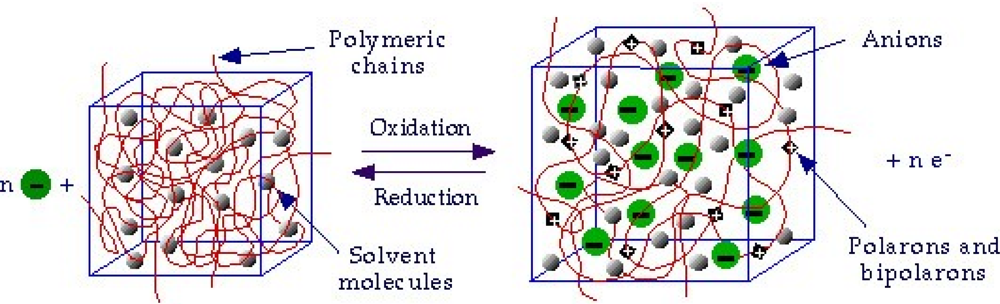
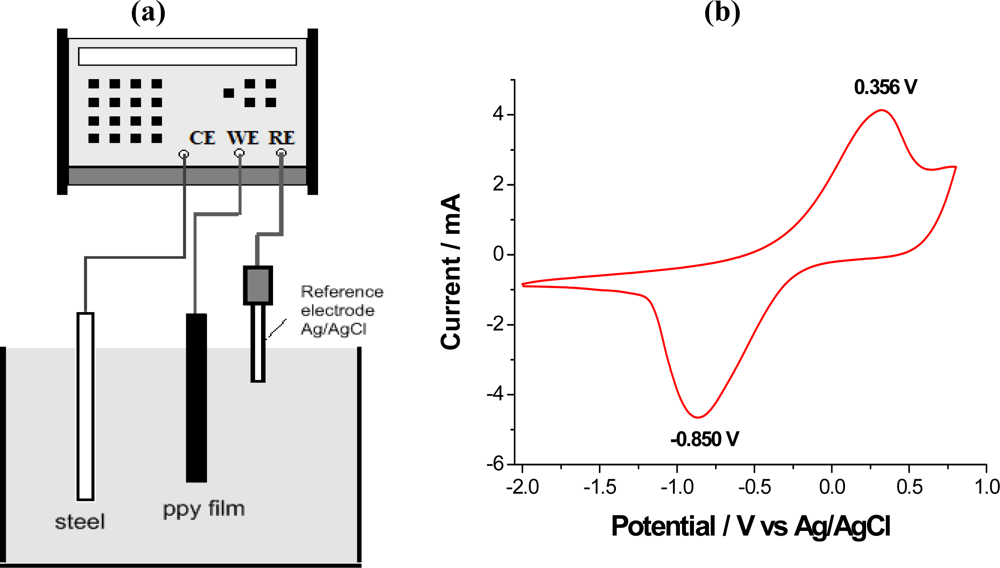



| Property | Action | Inspired organ |
|---|---|---|
| Electrochemomechanical | Change of volume | Muscles |
| Electrochromic | Change of color | Mimetic skins |
| Charge storage | Current generation | Electric organs |
| Electroporosity | Transversal ionic flow | Membrane |
| Chemical or pharmacological storage | Chemical modulation or chemical dosage | Glands |
| Electron/ion transduction | ΔV (Chem/Phys. properties) | Bio-sensors |
| Electron/neurotransmitter | Channel V action | Nervous interface |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Conzuelo, L.V.; Arias-Pardilla, J.; Cauich-Rodríguez, J.V.; Smit, M.A.; Otero, T.F. Sensing and Tactile Artificial Muscles from Reactive Materials. Sensors 2010, 10, 2638-2674. https://doi.org/10.3390/s100402638
Conzuelo LV, Arias-Pardilla J, Cauich-Rodríguez JV, Smit MA, Otero TF. Sensing and Tactile Artificial Muscles from Reactive Materials. Sensors. 2010; 10(4):2638-2674. https://doi.org/10.3390/s100402638
Chicago/Turabian StyleConzuelo, Laura Valero, Joaquín Arias-Pardilla, Juan V. Cauich-Rodríguez, Mascha Afra Smit, and Toribio Fernández Otero. 2010. "Sensing and Tactile Artificial Muscles from Reactive Materials" Sensors 10, no. 4: 2638-2674. https://doi.org/10.3390/s100402638
APA StyleConzuelo, L. V., Arias-Pardilla, J., Cauich-Rodríguez, J. V., Smit, M. A., & Otero, T. F. (2010). Sensing and Tactile Artificial Muscles from Reactive Materials. Sensors, 10(4), 2638-2674. https://doi.org/10.3390/s100402638




