Clinical Correlates and Prognostic Value of Plasma Galectin-3 Levels in Degenerative Aortic Stenosis: A Single-Center Prospective Study of Patients Referred for Invasive Treatment
Abstract
:1. Introduction
2. Results
2.1. Patients’ Characteristics
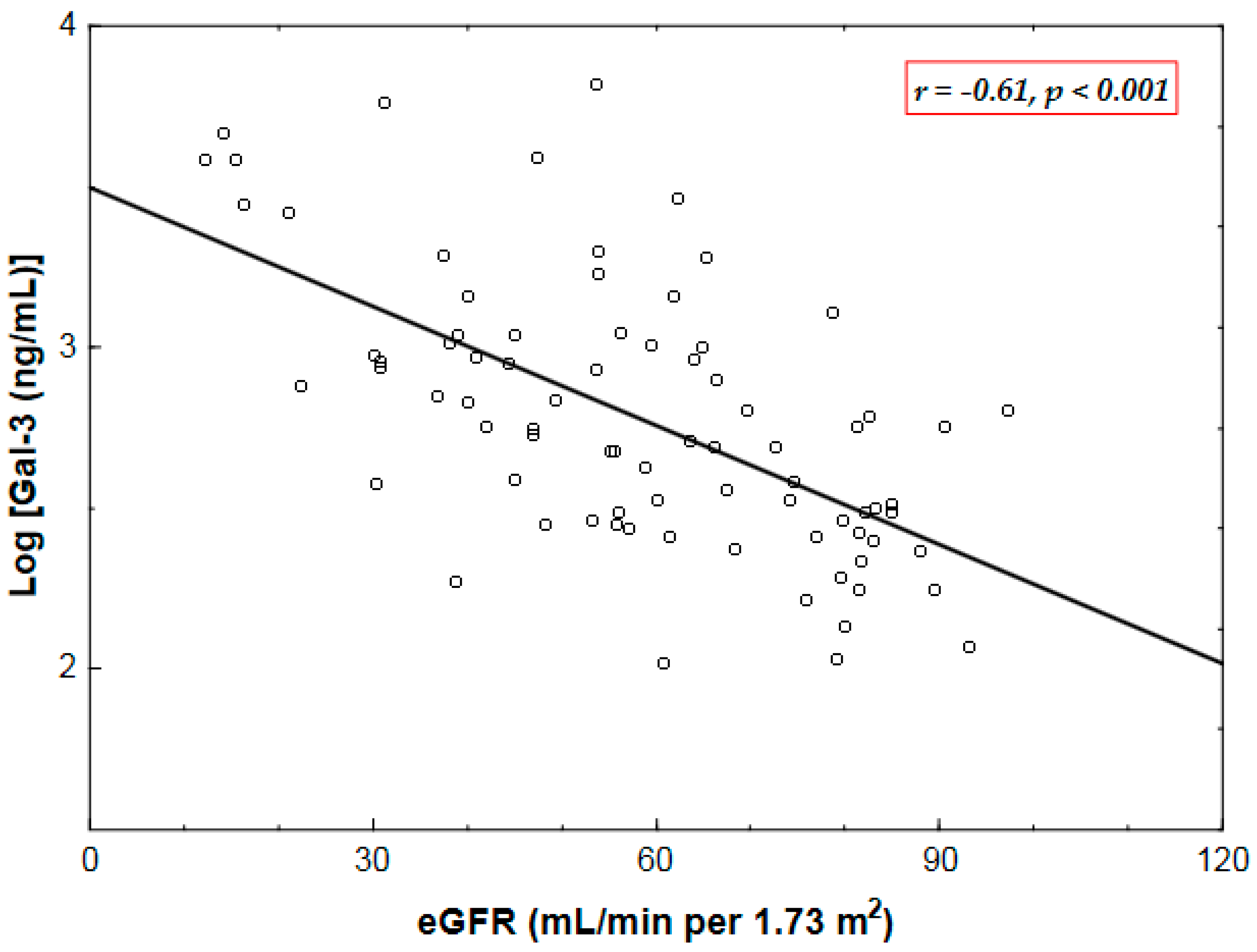
2.2. Correlates of Plasma Gal-3 Levels
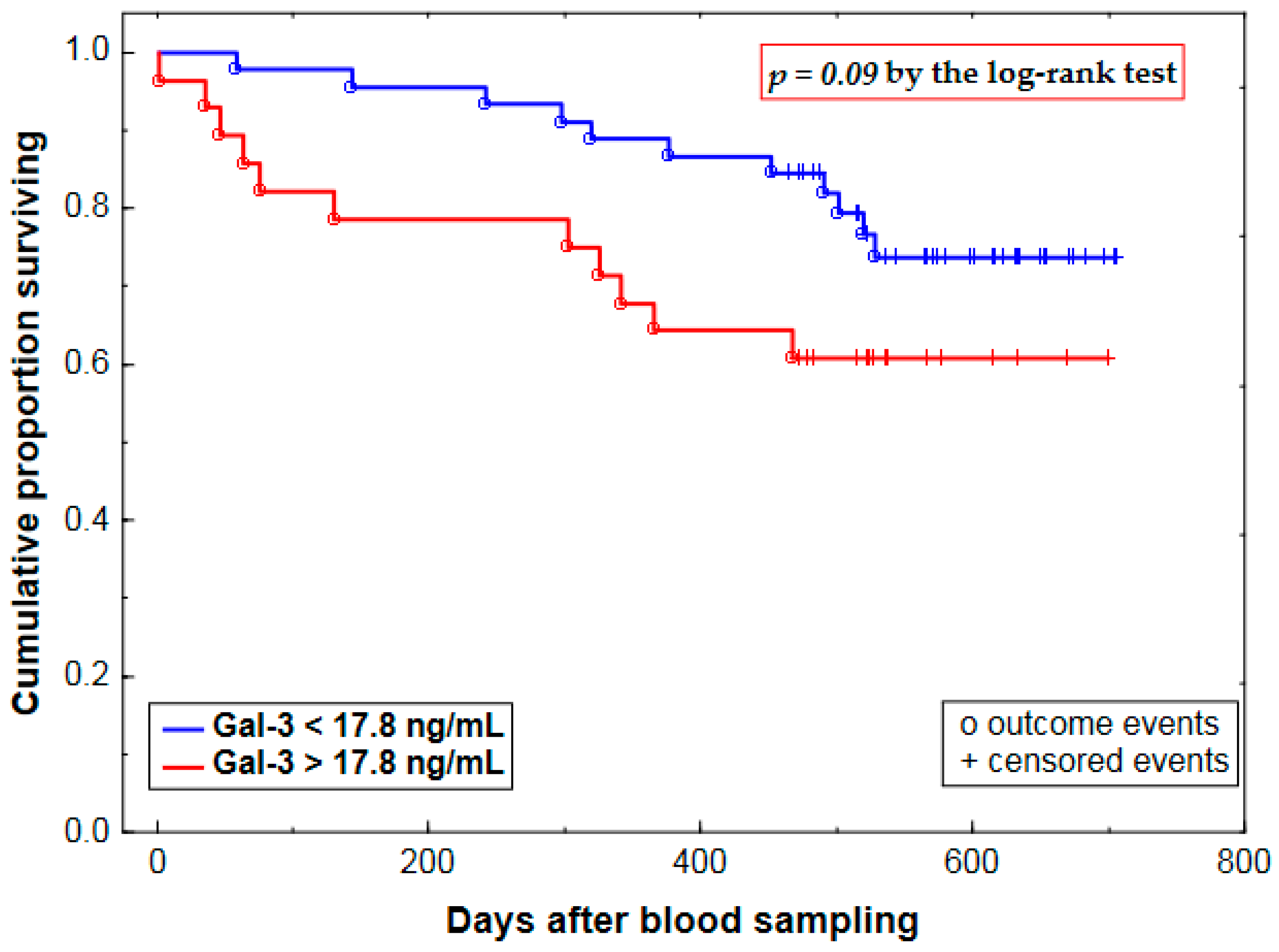
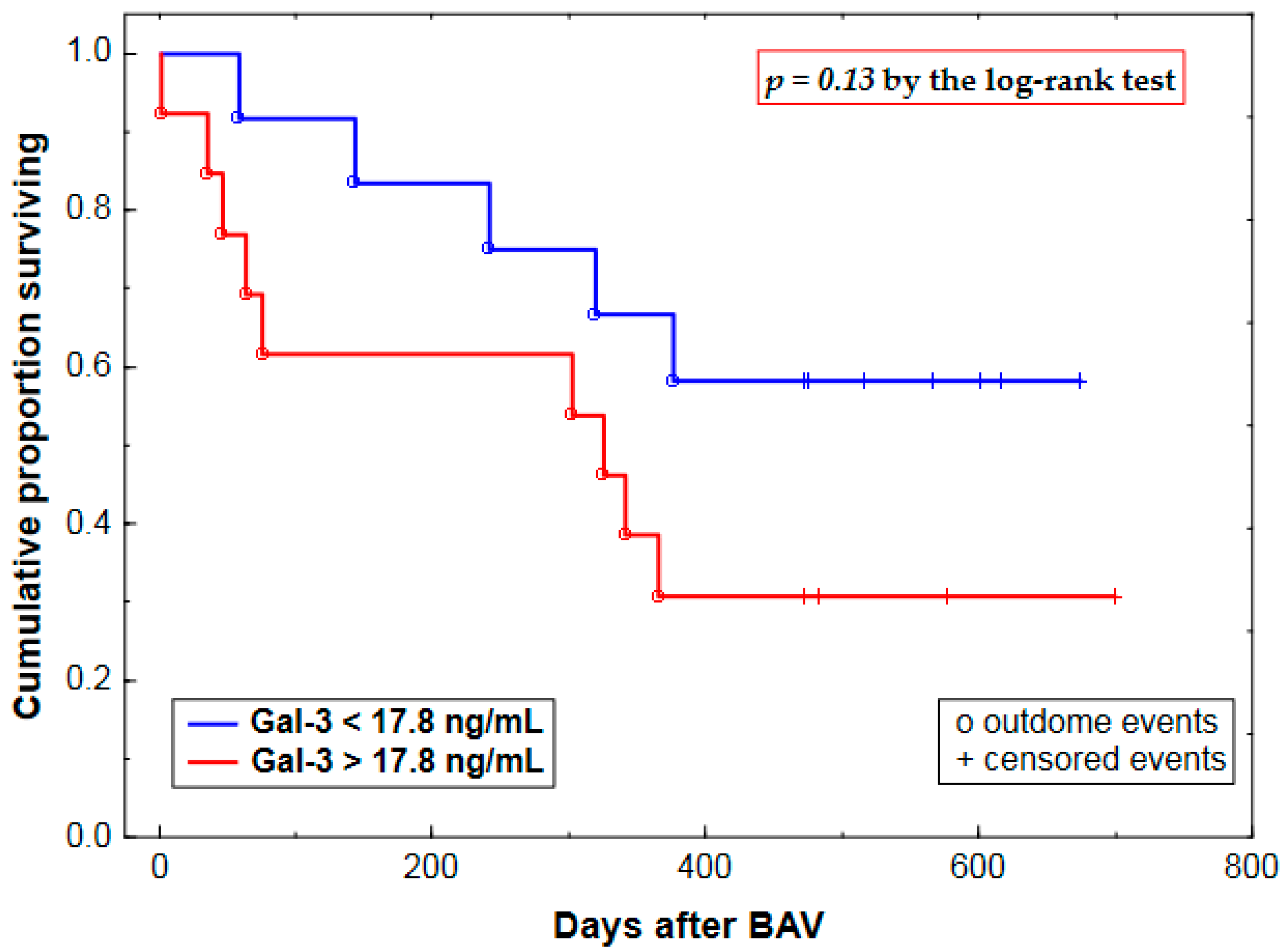
2.3. Prognostic Value of Plasma Gal-3 Levels
3. Discussion
3.1. Relations between Plasma Gal-3 and Renal Function—Mechanisms and Prognostic Implications
3.2. Mechanistic Considerations—Valvular, Myocardial and Vascular Effects of Gal-3 as Potential Contributors to the Prognostic Ability of Pre-procedural Plasma Gal-3 in AS Patients Undergoing BAV
3.3. Mechanistic Considerations—Summary
4. Materials and Methods
4.1. Patients
4.2. Study Protocol
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AS | Aortic stenosis |
| AVA | Aortic valve area |
| BAV | Balloon aortic valvuloplasty |
| CKD | Chronic kidney disease |
| CI | Confidence interval |
| EF | Left ventricular ejection fraction |
| eGFR | Estimated glomerular filtration rate |
| Gal-3 | Galectin-3 |
| HF | Heart failure |
| HR | Hazard ratio |
| LV | Left ventricular |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| NYHA | New York Heart Association |
| MPG | Mean transvalvular aortic pressure gradient |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| SVR | Surgical aortic valve replacement |
| TAVI | Transcatheter aortic valve implantation |
| TGF-β | Tumor growth factor-β |
| Zva | Valvulo-arterial impedance |
References
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.; Schroen, B.; André, S.; Crijns, H.J.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- González, G.E.; Rhaleb, N.E.; D’Ambrosio, M.A.; Nakagawa, P.; Liao, T.D.; Peterson, E.L.; Leung, P.; Dai, X.; Janic, B.; Liu, Y.H.; et al. Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H1287–H1296. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A. Practical experience using galectin-3 in heart failure. Clin. Chem. Lab. Med. 2014, 52, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Lok, D.J.; Lok, S.I.; Bruggink-André de la Porte, P.W.; Badings, E.; Lipsic, E.; van Wijngaarden, J.; de Boer, R.A.; van Veldhuisen, D.J.; van der Meer, P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin. Res. Cardiol. 2013, 102, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Van Kimmenade, R.R.; Januzzi, J.L.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menheere, P.P.; et al. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Chen-Tournoux, A.A.; Picard, M.H.; van Kimmenade, R.R.; Januzzi, J.L. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur. J. Heart Fail. 2010, 12, 826–832. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; Lok, D.J.; Jaarsma, T.; van der Meer, P.; Voors, A.A.; Hillege, H.L.; van Veldhuisen, D.J. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann. Med. 2011, 43, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Shrestha, K.; Shao, Z.; Borowski, A.G.; Troughton, R.W.; Thomas, J.D.; Klein, A.L. Usefulness of plasma galectin-3 levels in systolic heart failure to predict renal insufficiency and survival. Am. J. Cardiol. 2011, 108, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; Januzzi, J.L.; deFilippi, C.; Adourian, A.S.; Shah, S.J.; van Veldhuisen, D.J.; de Boer, R.A. Elevated plasma galectin-3 is associated with near-term rehospitalization in heart failure: A pooled analysis of 3 clinical trials. Am. Heart J. 2014, 167, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Edelmann, F.; Holzendorf, V.; Wachter, R.; Nolte, K.; Schmidt, A.G.; Kraigher-Krainer, E.; Duvinage, A.; Unkelbach, I.; Düngen, H.D.; Tschöpe, C.; et al. Galectin-3 in patients with heart failure with preserved ejection fraction: Results from the Aldo-DHF trial. Eur. J. Heart Fail. 2015, 17, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Liu, C.; Lyass, A.; Courchesne, P.; Pencina, M.J.; Vasan, R.S.; Larson, M.G.; Levy, D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J. Am. Coll. Cardiol. 2012, 60, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- De Boer, R.A.; van Veldhuisen, D.J.; Gansevoort, R.T.; Muller Kobold, A.C.; van Gilst, W.H.; Hillege, H.L.; Bakker, S.J.; van der Harst, P. The fibrosis marker galectin-3 and outcome in the general population. J. Intern. Med. 2012, 272, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Matsumoto, C.; Petrone, A.; Weir, N.L.; Tsai, M.Y.; Gaziano, J.M. Plasma galectin 3 and heart failure risk in the Physicians’ Health Study. Eur. J. Heart Fail. 2014, 16, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.B.; Clopton, P.; Laughlin, G.A.; Maisel, A.S.; Barrett-Connor, E. Galectin-3 is independently associated with cardiovascular mortality in community-dwelling older adults without known cardiovascular disease: The Rancho Bernardo Study. Am. Heart J. 2014, 167, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 2013, 128, e240–e327. [Google Scholar] [CrossRef] [PubMed]
- Milting, H.; Ellinghaus, P.; Seewald, M.; Cakar, H.; Bohms, B.; Kassner, A.; Körfer, R.; Klein, M.; Krahn, T.; Kruska, L.; et al. Plasma biomarkers of myocardial fibrosis and remodeling in terminal heart failure patients supported by mechanical circulatory support devices. J. Heart Lung Transpl. 2008, 27, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Gopal, D.M.; Kommineni, M.; Ayalon, N.; Koelbl, C.; Ayalon, R.; Biolo, A.; Dember, L.M.; Downing, J.; Siwik, D.A.; Liang, C.S.; et al. Relationship of plasma galectin-3 to renal function in patients with heart failure: Effects of clinical status, pathophysiology of heart failure, and presence or absence of heart failure. J. Am. Heart Assoc. 2012, 1, e000760. [Google Scholar] [CrossRef] [PubMed]
- Arangalage, D.; Nguyen, V.; Robert, T.; Melissopoulou, M.; Mathieu, T.; Estellat, C.; Codogno, I.; Huart, V.; Duval, X.; Cimadevilla, C.; et al. Determinants and prognostic value of galectin-3 in patients with aortic valve stenosis. Heart 2016, 102, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Baldenhofer, G.; Zhang, K.; Spethmann, S.; Laule, M.; Eilers, B.; Leonhardt, F.; Sanad, W.; Dreger, H.; Sander, M.; Grubitzsch, H.; et al. Galectin-3 predicts short- and long-term outcome in patients undergoing transcatheter aortic valve implantation (TAVI). Int. J. Cardiol. 2014, 177, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.R.; Breyley, J.G.; Schilling, J.D.; Vatterott, A.M.; Zajarias, A.; Maniar, H.S.; Damiano, R.J.; Moon, M.R.; Lawton, J.S.; Gage, B.F.; et al. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015, 101, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Liebetrau, C.; Gaede, L.; Wolter, S.; Hoffmann, J.; Troidl, C.; Dörr, O.; Kim, W.K.; Walther, T.; Hamm, C.; Nef, H.; et al. The prognostic value of galectin-3 and NT-proBNP in patients undergoing transcatheter aortic valve implantation (abstract). J. Am. Coll. Cardiol. 2014, 63, A1997. [Google Scholar] [CrossRef]
- Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carrel, T.P.; de Bonis, M.; Evangelista, A.; et al. Guidelines on the management of valvular heart disease (version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Meijers, W.C.; van der Velde, A.R.; Ruifrok, W.P.; Schroten, N.F.; Dokter, M.M.; Damman, K.; Assa, S.; Franssen, C.F.; Gansevoort, R.T.; van Gilst, W.H.; et al. Renal handling of galectin-3 in the general population, chronic heart failure, and hemodialysis. J. Am. Heart Assoc. 2014, 3, e000962. [Google Scholar] [CrossRef] [PubMed]
- Zamora, E.; Lupón, J.; de Antonio, M.; Galán, A.; Domingo, M.; Urrutia, A.; Troya, M.; Bayes-Genis, A. Renal function largely influences galectin-3 prognostic value in heart failure. Int. J. Cardiol. 2014, 177, 171–177. [Google Scholar] [CrossRef] [PubMed]
- AbouEzzeddine, O.F.; Haines, P.; Stevens, S.; Nativi-Nicolau, J.; Felker, G.M.; Borlaug, B.A.; Chen, H.H.; Tracy, R.P.; Braunwald, E.; Redfield, M.M. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure). JACC Heart Fail. 2015, 3, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, renal function, and clinical outcomes: Results from the LURIC and 4D studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, C.M.; Hwang, S.J.; Ho, J.E.; Vasan, R.S.; Levy, D.; Fox, C.S. Elevated galectin-3 precedes the development of CKD. J. Am. Soc. Nephrol. 2013, 24, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Pichard, A.D.; Satler, L.F.; Goldstein, S.A.; Syed, A.I.; Gaglia, M.A.; Weissman, G.; Maluenda, G.; Gonzalez, M.A.; Wakabayashi, K.; et al. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC Cardiovasc. Interv. 2010, 3, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dor, I.; Pichard, A.D.; Gonzalez, M.A.; Weissman, G.; Li, Y.; Goldstein, S.A.; Okubagzi, P.; Syed, A.I.; Maluenda, G.; Collins, S.D.; et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation 2010, 122, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Forcillo, J.; Beohar, N.; Doshi, D.; Parvataneni, R.; Ayele, G.M.; Kirtane, A.J.; Babaliaros, V.; Kodali, S.; Devireddy, C.; et al. Impact of preoperative chronic kidney disease in 2531 high-risk and inoperable patients undergoing transcatheter aortic valve replacement in the PARTNER trial. Ann. Thorac. Surg. 2016, 102, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.J.; Chue, C.D.; de Belder, M.A.; Moat, N.; Wendler, O.; Trivedi, U.; Ludman, P.; Townend, J.N.; UK TAVI Steering Group; National Institute for Cardiovascular Outcomes Research. Impact of renal function on survival after transcatheter aortic valve implantation (TAVI): An analysis of the UK TAVI registry. Heart 2015, 101, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Keeling, W.B.; Sarin, E.L.; Guyton, R.A.; Kilgo, P.D.; Dara, A.B.; Puskas, J.D.; Chen, E.P.; Cooper, W.A.; Vega, J.D.; et al. Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann. Thorac. Surg. 2011, 91, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Brummel, K.; Friedman, J.L.; Atri, P.; Sweis, R.N.; Russell, H.; Ricciardi, M.J.; Malaisrie, S.C.; Davidson, C.J.; Flaherty, J.D. Inhospital and post-discharge changes in renal function after transcatheter aortic valve replacement. Am. J. Cardiol. 2016, 117, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlassara, H.; Liu, F.T.; et al. Accelerated diabetic glomerulopathy in galectin-3/AGE receptor 3 knockout mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.T.; Eddy, A.A. Galectin-3 preserves renal tubules and modulates extracellular matrix remodeling in progressive fibrosis. Am. J. Physiol. Ren. Physiol. 2011, 300, F245–F253. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Oddi, G.; Menini, S.; Amadio, L.; Ricci, C.; di Pippo, C.; Sorcini, M.; Pricci, F.; Pugliese, F.; Pugliese, G. Development of age-dependent glomerular lesions in galectin-3/AGE-receptor-3 knockout mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F611–F621. [Google Scholar] [CrossRef] [PubMed]
- Fernandes Bertocchi, A.P.; Campanhole, G.; Wang, P.H.; Gonçalves, G.M.; Damião, M.J.; Cenedeze, M.A.; Beraldo, F.C.; de Paula Antunes Teixeira, V.; Dos Reis, M.A.; Mazzali, M.; et al. A role for galectin-3 in renal tissue damage triggered by ischemia and reperfusion injury. Transpl. Int. 2008, 21, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef] [PubMed]
- Frenay, A.R.; Yu, L.; van der Velde, A.R.; Vreeswijk-Baudoin, I.; López-Andrés, N.; van Goor, H.; Silljé, H.H.; Ruifrok, W.P.; de Boer, R.A. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am. J. Physiol. Ren. Physiol. 2015, 308, F500–F509. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 blockade reduces renal fibrosis in two normotensive experimental models of renal damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, M.; Essed, C.E.; di Mario, C.; Plante, S.; Mochtar, B.; de Feyter, P.J.; Suryapranata, H.; Serruys, P.W. Histological changes in the aortic valve after balloon dilatation: Evidence for a delayed healing process. Br. Heart J. 1992, 67, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Sádaba, J.R.; Martínez-Martínez, E.; Arrieta, V.; Álvarez, V.; Fernández-Celis, A.; Ibarrola, J.; Melero, A.; Rossignol, P.; Cachofeiro, V.; López-Andrés, N. Role for galectin-3 in calcific aortic valve stenosis. J. Am. Heart Assoc. 2016, 5, e004360. [Google Scholar] [CrossRef] [PubMed]
- Barone-Rochette, G.; Piérard, S.; de Meester de Ravenstein, C.; Seldrum, S.; Melchior, J.; Maes, F.; Pouleur, A.C.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.L.; et al. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J. Am. Coll. Cardiol. 2014, 64, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; D’Ambrosio, M.; Liao, T.D.; Peng, H.; Rhaleb, N.E.; Sharma, U.; André, S.; Gabius, H.J.; Carretero, O.A. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H404–H412. [Google Scholar] [CrossRef] [PubMed]
- Hundae, A.; McCullough, P.A. Cardiac and renal fibrosis in chronic cardiorenal syndromes. Nephron Clin. Pract. 2014, 127, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Perea, R.J.; Morales-Ruiz, M.; Ortiz-Perez, J.T.; Bosch, X.; Andreu, D.; Borras, R.; Acosta, J.; Penela, D.; Prat-González, S.; de Caralt, T.M.; et al. Utility of galectin-3 in predicting post-infarct remodeling after acute myocardial infarction based on extracellular volume fraction mapping. Int. J. Cardiol. 2016, 223, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Weir, R.A.; Petrie, C.J.; Murphy, C.A.; Clements, S.; Steedman, T.; Miller, A.M.; McInnes, I.B.; Squire, I.B.; Ng, L.L.; Dargie, H.J.; et al. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ. Heart Fail. 2013, 6, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, A.R.; Lexis, C.P.; Meijers, W.C.; van der Horst, I.C.; Lipsic, E.; Dokter, M.M.; van Veldhuisen, D.J.; van der Harst, P.; de Boer, R.A. Galectin-3 and sST2 in prediction of left ventricular ejection fraction after myocardial infarction. Clin. Chim. Acta 2016, 452, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, G.; Caretta, G.; de Maria, R.; Parolini, M.; Bassi, L.; Testa, S.; Pirelli, S. Galectin-3 predicts left ventricular remodelling after anterior-wall myocardial infarction treated by primary percutaneous coronary intervention. Heart 2017, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Libhaber, E.; Woodiwiss, A.J.; Raymond, A.; Gomes, M.; Maseko, M.J.; Sareli, P.; Norton, G.R. Independent associations of circulating galectin-3 concentrations with aortic pulse wave velocity and wave reflection in a community sample. Hypertension 2015, 65, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Briand, M.; Dumesnil, J.G.; Kadem, L.; Tongue, A.G.; Rieu, R.; Garcia, D.; Pibarot, P. Reduced systemic arterial compliance impacts significantly on left ventricular afterload and function in aortic stenosis: Implications for diagnosis and treatment. J. Am. Coll. Cardiol. 2005, 46, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kruszelnicka, O.; Chmiela, M.; Bobrowska, B.; Świerszcz, J.; Bhagavatula, S.; Bednarek, J.; Surdacki, A.; Nessler, J.; Hryniewiecki, T. Depressed systemic arterial compliance is associated with the severity of heart failure symptoms in moderate-to-severe aortic stenosis: A cross-sectional retrospective study. Int. J. Med. Sci. 2015, 12, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, S.; Yiu, K.H.; Clavel, M.A.; Rodés-Cabau, J.; Leong, D.; van der Kley, F.; Ajmone Marsan, N.; Bax, J.J.; Pibarot, P.; Delgado, V. Impact of valvuloarterial impedance on 2-year outcome of patients undergoing transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 2013, 26, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the valvuloarterial impedance to predict adverse outcome in asymptomatic aortic stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Nachtigal, M.; Ghaffar, A.; Mayer, E.P. Galectin-3 gene inactivation reduces atherosclerotic lesions and adventitial inflammation in apoE-deficient mice. Am. J. Pathol. 2008, 172, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Iacobini, C.; Menini, S.; Ricci, C.; Scipioni, A.; Sansoni, V.; Cordone, S.; Taurino, M.; Serino, M.; Marano, G.; Federici, M.; et al. Accelerated lipid-induced atherogenesis in galectin-3-deficient mice: Role of lipoxidation via receptor-mediated mechanisms. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.; Koenig, W.; Jaensch, A.; Mons, U.; Breitling, L.P.; Scharnagl, H.; Stojakovic, T.; Schunkert, H.; Brenner, H.; Rothenbacher, D. Prognostic utility of galectin-3 for recurrent cardiovascular events during long-term follow-up in patients with stable coronary heart disease: Results of the KAROLA study. Clin. Chem. 2016, 62, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, A.R.; Meijers, W.C.; Ho, J.E.; Brouwers, F.P.; Rienstra, M.; Bakker, S.J.; Muller Kobold, A.C.; van Veldhuisen, D.J.; van Gilst, W.H.; van der Harst, P.; et al. Serial galectin-3 and future cardiovascular disease in the general population. Heart 2016, 102, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Gruson, D.; Mancini, M.; Ahn, S.A.; Rousseau, M.F. Galectin-3 testing: Validity of a novel automated assay in heart failure patients with reduced ejection fraction. Clin. Chim. Acta 2014, 429, 189–193. [Google Scholar] [CrossRef] [PubMed]
| Age, years | 79 ± 8 |
| Gender, male | 36 (45%) |
| Body mass index, kg/m2 | 26.5 ± 4.1 |
| NYHA functional class, II/III/IV | 40/25/15 (50/31/19%) |
| eGFR, mL/min per 1.73 m2 | 58 ± 21 |
| Hypertension | 71 (89%) |
| Diabetes mellitus | 26 (33%) |
| Coronary artery disease | 36 (45%) |
| NT-proBNP, pg/mL | 2554 (1168–5244) |
| AVA, cm2 | 0.7 ± 0.2 |
| AVA index, cm2/m2 | 0.4 ± 0.1 |
| Maximal transvalvular aortic pressure gradient, mmHg | 84 ± 30 |
| Mean transvalvular aortic pressure gradient, mmHg | 52 ± 21 |
| LVMI, g/m2 | 174 ± 55 |
| EF, % | 52 ± 15 |
| Stroke volume index, mL/m2 | 34.0 ± 11.6 |
| Systolic blood pressure, mmHg | 129 ± 20 |
| Zva, mmHg/(mL/m2) | 5.9 ± 2.3 |
| Characteristic | Age | eGFR | NYHA | AVA | MPG | LVMI | EF | Zva |
|---|---|---|---|---|---|---|---|---|
| Gal-3 | 0.09 0.4 | −0.61 * <0.001 | 0.16 0.2 | 0.01 0.9 | −0.25 0.02 | 0.17 0.14 | −0.12 0.3 | −0.07 0.5 |
| NT-proBNP | 0.09 0.4 | −0.37 * 0.001 | 0.20 0.07 | −0.17 0.14 | 0.18 0.11 | 0.40 * <0.001 | −0.26 0.02 | −0.06 0.6 |
| Characteristic | Balloon Aortic Valvuloplasty (n = 25) | Other Treatment Modalities (n = 55) | p |
|---|---|---|---|
| Age, years | 84 ± 6 | 78 ± 8 | <0.001 * |
| Gender, male | 11 (44%) | 25 (45%) | 0.9 |
| Body-mass index, kg/m2 | 25.3 ± 3.7 | 27.0 ± 4.2 | 0.1 |
| NYHA functional class, II/III/IV | 8/8/9 (32/32/36%) | 32/17/6 (58/31/11%) | 0.02 |
| Hypertension | 24 (96%) | 47 (85%) | 0.2 |
| Diabetes mellitus | 12 (48%) | 14 (25%) | 0.05 |
| Coronary artery disease | 14 (56%) | 22 (40%) | 0.2 |
| NT-proBNP, pg/mL | 3635 (2349–5755) | 2294 (1004–5055) | 0.14 |
| AVA index, cm2/m2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.05 |
| Maximal transvalvular aortic pressure gradient, mmHg | 83 ± 28 | 84 ± 32 | 0.9 |
| Mean transvalvular aortic pressure gradient, mmHg | 52 ± 19 | 52 ± 22 | 0.9 |
| LVMI, g/m2 | 183 ± 37 | 170 ± 61 | 0.3 |
| EF, % | 48 ± 16 | 53 ± 15 | 0.2 |
| Stroke volume index, mL/m2 | 32.2 ± 11.9 | 34.8 ± 11.4 | 0.4 |
| Systolic blood pressure, mmHg | 128 ± 22 | 130 ± 19 | 0.7 |
| Zva, mmHg/(mL/m2) | 6.4 ± 2.7 | 5.7 ± 2.0 | 0.2 |
| Galectin-3 (ng/mL) | 19.0 (13.1–25.2) | 14.5 (11.5–19.5) | 0.06 |
| Predictor Variable | β ± SEM | Hazard Ratio (HR) | |
|---|---|---|---|
| Mean HR (95% CI) | p | ||
| Gal-3, unadjusted | |||
| Gal-3 (Gal-3 >17.8 vs. <17.8 ng/mL) | 0.71 ± 0.43 | 2.03 (0.88–4.69) | 0.09 |
| Gal-3 (per 1-SD increment) | 0.40 ± 0.20 | 1.49 (1.00–2.21) | 0.05 |
| Gal-3, adjusted for eGFR | |||
| Gal-3 (Gal-3 >17.8 vs. <17.8 ng/mL) | 0.53 ± 0.52 | 1.70 (0.61–4.73) | 0.31 |
| Gal-3 (per 1-SD increment) | 0.38 ± 0.29 | 1.46 (0.83–2.57) | 0.19 |
| Predictor Variable | β ± SEM | Hazard Ratio (HR) | |
|---|---|---|---|
| Mean HR (95% CI) | p | ||
| Gal-3, unadjusted | |||
| Gal-3 (Gal-3 >17.8 vs. <17.8 ng/mL) | 0.84 ± 0.56 | 2.32 (0.78–6.96) | 0.13 |
| Gal-3, adjusted for eGFR | |||
| Gal-3 (Gal-3 >17.8 vs. <17.8 ng/mL) | 1.19 ± 0.69 | 3.30 (0.86–12.7) | 0.08 |
| Gal-3, adjusted for eGFR and Zva | |||
| Gal-3 (Gal-3 >17.8 vs. <17.8 ng/mL) | 2.00 ± 0.81 | 7.41 (1.52–36.1) | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bobrowska, B.; Wieczorek-Surdacka, E.; Kruszelnicka, O.; Chyrchel, B.; Surdacki, A.; Dudek, D. Clinical Correlates and Prognostic Value of Plasma Galectin-3 Levels in Degenerative Aortic Stenosis: A Single-Center Prospective Study of Patients Referred for Invasive Treatment. Int. J. Mol. Sci. 2017, 18, 947. https://doi.org/10.3390/ijms18050947
Bobrowska B, Wieczorek-Surdacka E, Kruszelnicka O, Chyrchel B, Surdacki A, Dudek D. Clinical Correlates and Prognostic Value of Plasma Galectin-3 Levels in Degenerative Aortic Stenosis: A Single-Center Prospective Study of Patients Referred for Invasive Treatment. International Journal of Molecular Sciences. 2017; 18(5):947. https://doi.org/10.3390/ijms18050947
Chicago/Turabian StyleBobrowska, Beata, Ewa Wieczorek-Surdacka, Olga Kruszelnicka, Bernadeta Chyrchel, Andrzej Surdacki, and Dariusz Dudek. 2017. "Clinical Correlates and Prognostic Value of Plasma Galectin-3 Levels in Degenerative Aortic Stenosis: A Single-Center Prospective Study of Patients Referred for Invasive Treatment" International Journal of Molecular Sciences 18, no. 5: 947. https://doi.org/10.3390/ijms18050947
APA StyleBobrowska, B., Wieczorek-Surdacka, E., Kruszelnicka, O., Chyrchel, B., Surdacki, A., & Dudek, D. (2017). Clinical Correlates and Prognostic Value of Plasma Galectin-3 Levels in Degenerative Aortic Stenosis: A Single-Center Prospective Study of Patients Referred for Invasive Treatment. International Journal of Molecular Sciences, 18(5), 947. https://doi.org/10.3390/ijms18050947





