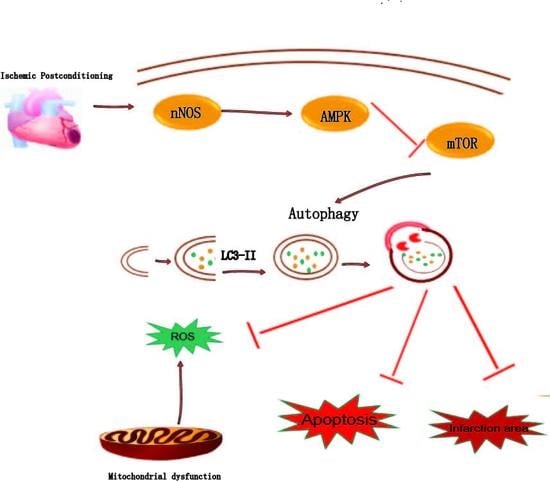
Myocardial Ischemic Postconditioning Promotes Autophagy against Ischemia Reperfusion Injury via the Activation of the nNOS/AMPK/mTOR Pathway
Abstract
:1. Introduction
2. Results
2.1. IPostC Restored nNOS Activity in Myocadium and H9c2 Cells
2.2. IPostC Promoted Autophagy via nNOS in I/R Injured Myocardium
2.3. IPostC Enhanced Autophagic Activity via nNOS in H9c2 Cells In Vitro
2.4. Activation of the nNOS/AMPK/mTOR Signaling Pathway Was Involved in the Regulation of IPostC on Autophagy
2.5. Autophagy Inhibitor Abolished the Protective Effect of IPostC in the Myocardium
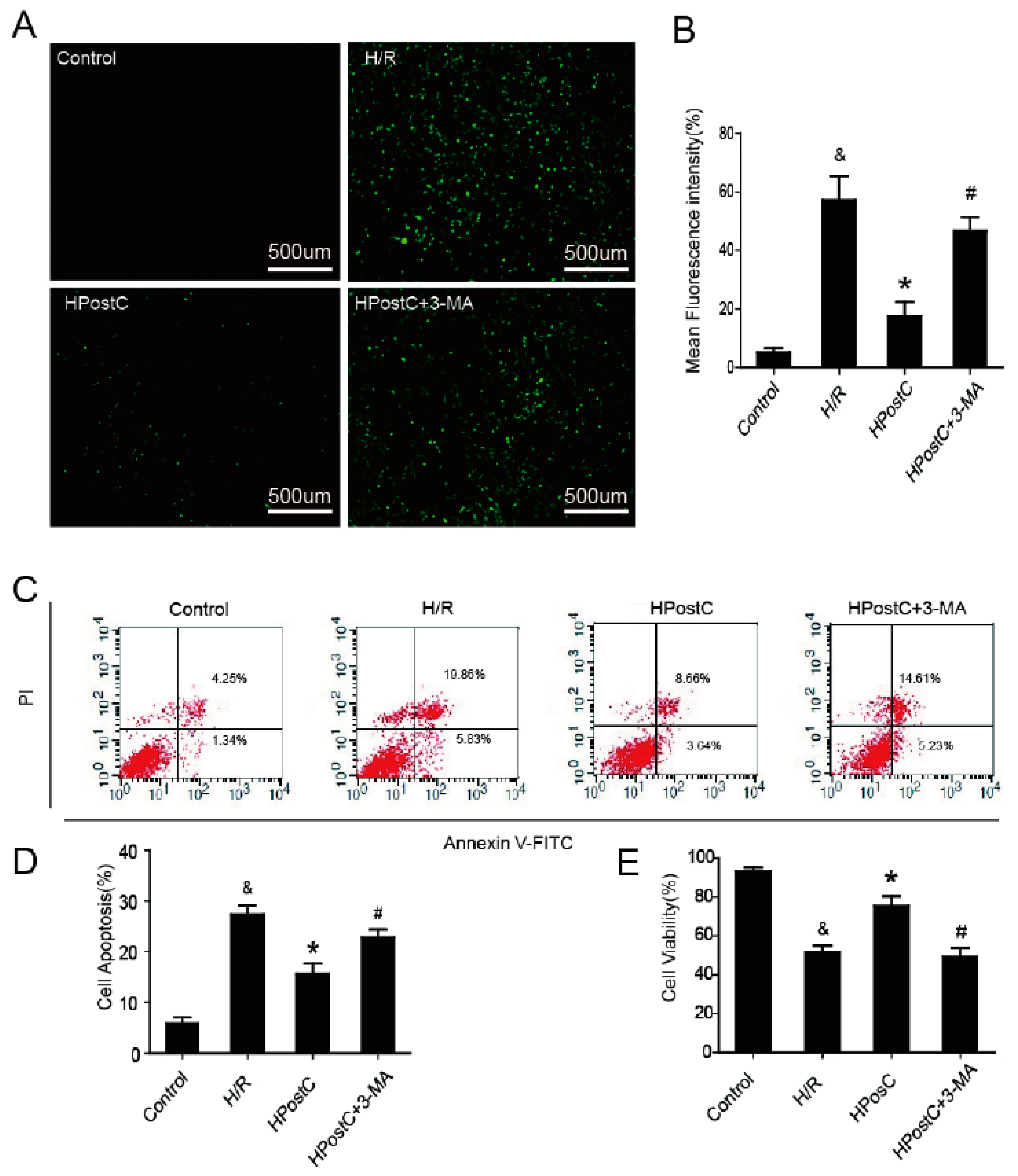
2.6. IPostC Protected H9c2 Cells by Promoting Autophagy In Vitro
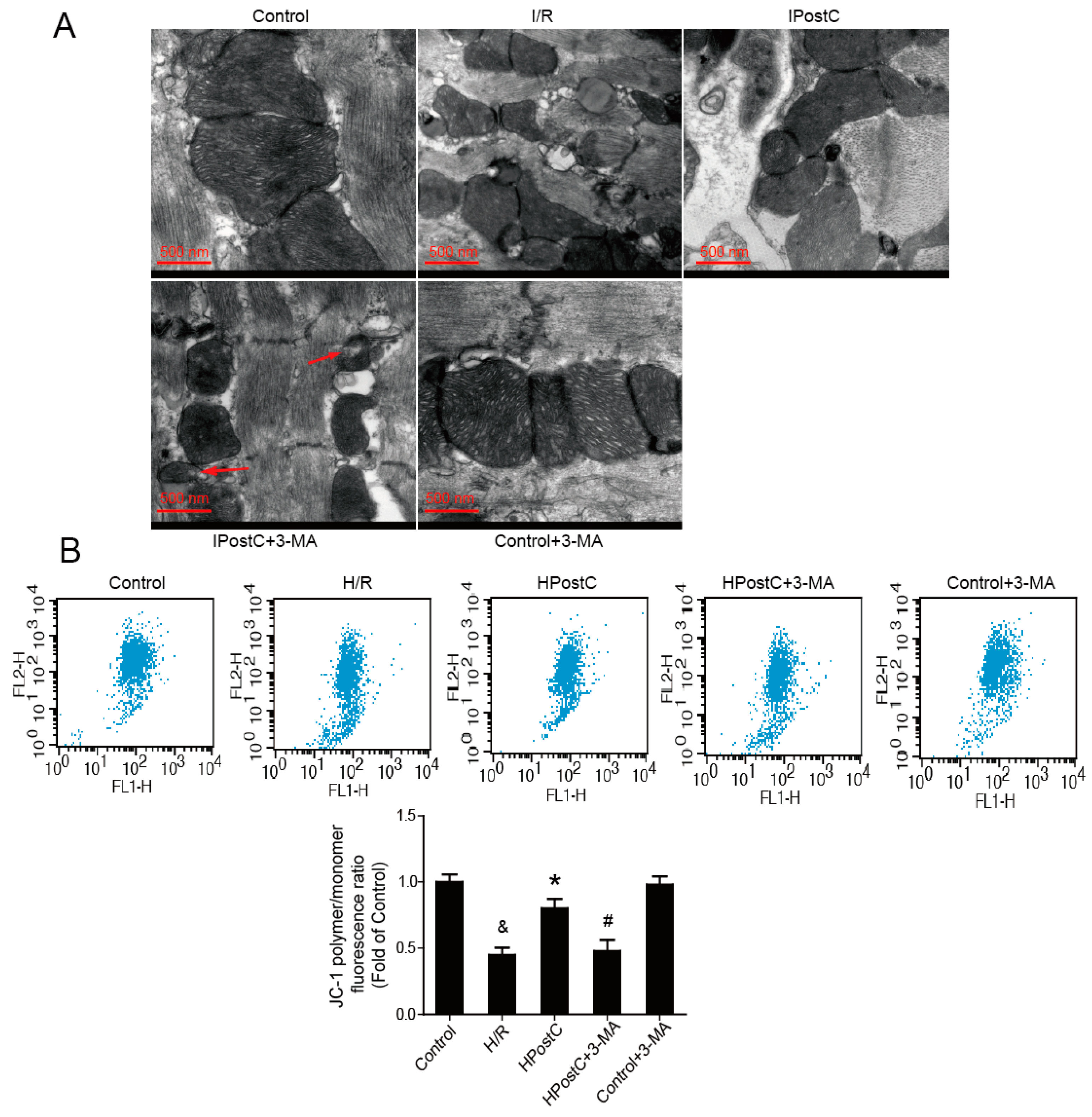
2.7. IPostC Improved Mitochondrial Function by Enhancing Autophagic Activity
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Reagents and Antibodies
4.3. I/R Modeling
4.4. Culture and Experimental Protocols for H9C2 Cells
4.5. Infarct Size Measurement
4.6. Hematoxylin and Eosin Staining (HE)
4.7. Intracellular ROS Detection
4.8. Evaluation of Cell Death
4.9. Western Blot
4.10. Transmission Electron Microscopy
4.11. GFP-LC3 Adenovirus Transfection Assay
4.12. Mitochondrial Membrane Potenital Detection
4.13. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| I/R | ischemia/reperfusion |
| nNOS | neuronal nitric oxide synthase |
| IPostC | ischemic postconditioning |
| H/R | hypoxia/reoxygenation |
| HPostC | hypoxic postconditioning |
| AMPK | adenosine monophosphate-activated protein kinase |
| mTOR | mammalian target rapamycin |
| ROS | reactive oxygen species |
| IPre | ischemic preconditioning |
| SR | sarcoplasmic reticulum |
| NO | nitric oxide |
| HE | hematoxylin and eosin Staining |
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update a report from the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, X.; Zhang, Y.; Pang, L.; Ma, X.; Song, M.; Kou, J.; Yu, B. Cardioprotection by combination of three compounds from ShengMai preparations in mice with myocardial ischemia/reperfusion injury through AMPK activation-mediated mitochondrial fission. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.B.; Yuan, T.Y.; Wu, Y.J.; Yan, Y.; Li, L.; Xu, X.N.; Gong, L.L.; Qin, H.L.; Fang, L.H.; et al. Coptisine protects rat heart against myocardial ischemia/reperfusion injury by suppressing myocardial apoptosis and inflammation. Atherosclerosis 2013, 231, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 24–36. [Google Scholar] [CrossRef]
- Shahzad, T.; Kasseckert, S.A.; Iraqi, W.; Johnson, V.; Schulz, R.; Schlüter, K.D.; Dörr, O.; Parahuleva, M.; Hamm, C.; Ladilov, Y.; et al. Mechanisms involved in postconditioning protection of cardiomyocytes against acute reperfusion injury. Mol. Cell. Cardiol. 2013, 58, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Babiker, F.A.; Al-Jarallah, A.; Joseph, S. Understanding pacing postconditioning-mediated cardiac protection: A role of oxidative stress and a synergistic effect of adenosine. J. Physiol. Biochem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.; Engelbrecht, A.M.; Lockshin, R.A.; Klionsky, D.J.; Zakeri, Z. The variability of autophagy and cell death susceptibility: Unanswered questions. Autophagy 2013, 9, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, Y.; Chen, Y.; Cao, F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys. Acta 2015, 1852, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Tannous, P.; Zhu, H.; Nemchenko, A.; Berry, J.M.; Johnstone, J.L.; Shelton, J.M.; Miller, F.J.; Rothermel, B.A.; Hill, J.A. Introcellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation 2008, 117, 3070–3078. [Google Scholar] [CrossRef] [PubMed]
- Quinsay, M.N.; Thomas, R.L.; Lee, Y.; Gustafsson, Å.B. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophag 2010, 6, 855–862. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Diwan, A. Autophagy is impaired in cardiac ischemia-reperfusion injury. Autophagy 2012, 8, 1394–1396. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, H.; Han, L.; Zhang, L.; Yang, X. Activation of autophagy in ischemic postconditioning contributes to cardioprotective effects against ischemia/reperfusion injury in rat hearts. J. Cardiovasc. Pharmacol. 2013, 61, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yao, Y.T.; Fang, N.X.; Zhou, C.H.; Gong, J.S.; Li, L.H. Restoration of autophagic flux in myocardial tissues is required for cardioprotection of sevoflurane postconditioning in rats. Acta Pharmacol. Sin. 2014, 35, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Dawson, D.; Lygate, C.A.; Zhang, M.H.; Hulbert, K.; Neubauer, S.; Casadei, B. nNOS gene deletion exacerbates pathological left ventricular remodeling and functional deterioration after myocardial infarction. Circulation 2005, 112, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Dedkova, E.N.; Blatter, L.A. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J. Physiol. 2009, 587, 851–872. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Skaf, M.W.; Harrison, R.W.; Lee, K.; Minhas, K.M.; Kumar, A.; Fradley, M.; Shoukas, A.A.; Berkowitz, D.E.; Hare, J.M. Nitric oxide regulation of myocardial contractility and calcium cycling: Independent impact of neuronal and endothelial nitric oxide synthases. Circ. Res. 2003, 92, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhu, H.; Wu, X.; Zhou, L.; Song, Y.; Zhu, S.; Hao, M.; Liu, C.; Fan, Y.; et al. Ischemic postconditioning protects the heart against ischemia—Reperfusion injury via neuronal nitric oxide synthase in the sarcoplasmic reticulum and mitochondria. Cell Death Dis. 2016, 7, e2222. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Korolchuk, V.I.; Renna, M.; Imarisio, S.; Fleming, A.; Williams, A.; Garcia-Arencibia, M.; Rose, C.; Luo, S.; Underwood, B.R.; et al. Complex Inhibitory Effects of Nitric Oxide on Autophagy. Mol. Cell. 2011, 43, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, J.M.; Mo, X.Y. Ischemic postconditioning regulates cardiomyocyte autophagic activity following ischemia/reperfusion injury. Mol. Med. Rep. 2015, 12, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Strasen, J.; Ritter, O. Role of nNOS in Cardiac Ischemia-Reperfusion Injury. Trends Cardiovasc. Med. 2011, 21, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Y.; Jia, L.; Wu, H.; Fan, C.; Sun, Y.; Ye, C.; Liao, M.; Zhou, J. Binding of the pathogen receptor HSP90AA1 to avibirnavirus VP2 induces autophagy by inactivating the AKT-MTOR pathway. Autophagy 2015, 11, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Bohensky, J.; Leshinsky, S.; Srinivas, V.; Shapiro, I.M. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activaton and mTOR Suppression. Pediatr. Nephrol. 2010, 25, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of Myocardial Injury by Ischemic Postconditioning During Reperfusion: Comparison with Ischemic Preconditioning. Am. J. Physiol. 2003, 285, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Kanai, E.; Yuan, H.; Huang, C.; Sayen, M.R.; Perry-Garza, C.N.; Kim, L.; Gottlieb, R.A. A method to measure cardiac autophagic flux in vivo. Autophagy 2008, 4, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.M.; Bockus, A.; Wozniak, A.L.; Jones, K.; Weinman, S.; Yin, X.M.; Ding, W.X. Dissecting the dynamic turnover of GFP-LC3 in the autolysosome. Autophagy 2011, 7, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Ichimura, Y. Physiological significance of selective degradation of p62 by autophagy. FEBS Lett. 2010, 584, 1374–1378. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Agnello, M.; Agostinis, P.; Aguirre-ghiso, J.A.; Ahn, H.J.; Ait-mohamed, O.; Brown, E.J.; Brumell, J.H.; Brunetti-Pierri, N.; Brunk, U.T.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 2012, 8, 445–544. [Google Scholar] [Green Version]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Egan, D.F.; Kim, J.; Shaw, R.J.; Guan, K. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 1, 645–646. [Google Scholar] [CrossRef]
- Oakhill, J.S.; Scott, J.W.; Kemp, B.E. AMPK functions as an adenylate charge-regulated protein kinase. Trends Endocrinol. Metab. 2012, 23, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Cleeter, M.W.J.; Cooper, J.M.; Darley-Usmar, V.M.; Moncada, S.; Schapira, H.V. Reversible inhibition of cytochrome C oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide: Implications for neurodegenerative diseases. FEBS Lett. 1994, 345, 50–54. [Google Scholar] [CrossRef]
- Gross, W.L.; Bak, M.I.; Ingwall, J.S.; Arstall, M.a.; Smith, T.W.; Balligand, J.L.; Kelly, R.A. Nitric oxide inhibits creatine kinase and regulates rat heart contractile reserve. Proc. Natl. Acad. Sci. USA 1996, 93, 5604–5609. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired Autophagosome Clearance Contributes to Cardiomyocyte Death in Ischemia/Reperfusion Injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Xu, J.; Kim, J.S.; Dunn, W.A., Jr.; Leeuwenburgh, C. Upregulated autophagy protects cardiomyocytes from oxidative stress-induced toxicity. Autophagy 2013, 9, 328–344. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, M.; Zhu, S.; Hu, L.; Zhu, H.; Wu, X.; Li, Q. Myocardial Ischemic Postconditioning Promotes Autophagy against Ischemia Reperfusion Injury via the Activation of the nNOS/AMPK/mTOR Pathway. Int. J. Mol. Sci. 2017, 18, 614. https://doi.org/10.3390/ijms18030614
Hao M, Zhu S, Hu L, Zhu H, Wu X, Li Q. Myocardial Ischemic Postconditioning Promotes Autophagy against Ischemia Reperfusion Injury via the Activation of the nNOS/AMPK/mTOR Pathway. International Journal of Molecular Sciences. 2017; 18(3):614. https://doi.org/10.3390/ijms18030614
Chicago/Turabian StyleHao, Maojuan, Suhua Zhu, Liang Hu, Hongyi Zhu, Xiaowei Wu, and Qingping Li. 2017. "Myocardial Ischemic Postconditioning Promotes Autophagy against Ischemia Reperfusion Injury via the Activation of the nNOS/AMPK/mTOR Pathway" International Journal of Molecular Sciences 18, no. 3: 614. https://doi.org/10.3390/ijms18030614