Involvement of Host Non-Coding RNAs in the Pathogenesis of the Influenza Virus
Abstract
:1. Introduction
2. Biology of ncRNAs
2.1. miRNAs
2.2. lncRNAs
2.3. vtRNAs
3. Critical Roles of miRNAs in Influenza A Virus (IAV) Infection
3.1. Functional Involvement of miRNAs in Host–Virus Interaction
3.2. miRNAs Play Important Roles in Antiviral Response to IAV Infection
3.3. Influenza Virus Infection Induces Differential Expression of Distinct miRNAs
3.4. miRNAs Regulate Interaction between Host and Influenza Virus
3.5. miRNAs Inhibit IAV by Directly Suppressing Viral Gene Expression
3.6. Diagnostic and Therapeutic Applications of miRNAs
4. Involvement of lncRNAs in Anti-IAV Innate Immunity
5. vtRNAs Are Involved in IAV Pathogenesis by Inhibiting PKR Activation
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ncRNA | non-coding RNA |
| lncRNA | long non-coding RNA |
| IAV | influenza A virus |
| PAMP | pathogen-associated molecular pattern |
| PRR | pattern recognition receptor |
| TLR | toll-like receptor |
| RIG-I | retinoic acid-inducible gene 1 |
| MDA5 | melanoma differentiation-associated gene 5 |
| IFN | interferon |
| ISG | IFN-stimulated gene |
| vtRNA | vault RNA |
| JAK-STAT | Janus kinase-signal transducer and activator of transcription |
| TNF | tumor necrosis factor |
| NF-κB | nuclear factor κ-light-chain-enhancer of activated B cells |
| IRF | interferon regulatory factor |
| IFIT | interferon-induced protein with tetratricopeptide repeats |
| OASL | 2′-5′-oligoadenylate synthetase-like protein |
| MxA | Myxovirus resistance protein 1 |
| IFITM | interferon-induced transmembrane protein |
| TGF | transforming growth factor |
| NRAV | negative regulator of anti-viral |
| NEAT1 | nuclear enriched abundant transcript 1 |
| VIN | virus inducible lincRNA |
| SFPQ/PSF | splicing factor proline/glutamine-rich |
References
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef] [PubMed]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, M.; Barciszewska, M.Z.; Zywicki, M.; Barciszewski, J. Noncoding RNA transcripts. J. Appl. Genet. 2003, 44, 1–19. [Google Scholar] [PubMed]
- Wilusz, J.E.; Sunwoo, H.; Spector, D.L. Long noncoding RNAs: Functional surprises from the RNA world. Genes Dev. 2009, 23, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Mattick, J.S. RNA regulation: A new genetics? Nat. Rev. Genet. 2004, 5, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mattick, J.S. The eukaryotic genome as an RNA machine. Science 2008, 319, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.; de Jong, M.D.; Guan, Y. Avian influenza virus (H5N1): A threat to human health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Webster, R.G. The influenza virus enigma. Cell 2009, 136, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Stohr, K. Avian influenza and pandemics—Research needs and opportunities. N. Engl. J. Med. 2005, 352, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Mehle, A. Unusual influenza A viruses in bats. Viruses 2014, 6, 3438–3449. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Planz, O.; Pleschka, S.; Wolff, T. Influenza-virus-induced signaling cascades: Targets for antiviral therapy? Trends Mol. Med. 2003, 9, 46–52. [Google Scholar] [CrossRef]
- Ouyang, J.; Hu, J.; Chen, J.L. lncRNAs regulate the innate immune response to viral infection. Wiley Interdiscip. Rev. RNA 2016, 7, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chi, X.; Wei, H.; Chen, Y.; Chen, Z.; Huang, S.; Chen, J.L. Influenza A virus-induced degradation of eukaryotic translation initiation factor 4B contributes to viral replication by suppressing IFITM3 protein expression. J. Virol. 2014, 88, 8375–8385. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, I.; Ye, F.; McNally, B.; Willette, M.; Flano, E. Toll-like receptor expression and induction of type I and type III interferons in primary airway epithelial cells. J. Virol. 2013, 87, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Josset, L.; Tchitchek, N.; Gralinski, L.E.; Ferris, M.T.; Eisfeld, A.J.; Green, R.R.; Thomas, M.J.; Tisoncik-Go, J.; Schroth, G.P.; Kawaoka, Y.; et al. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014, 11, 875–890. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Davis-Dusenbery, B.N.; Hata, A. Mechanisms of control of microRNA biogenesis. J. Biochem. 2010, 148, 381–392. [Google Scholar] [PubMed]
- Miyoshi, K.; Uejima, H.; Nagami-Okada, T.; Siomi, H.; Siomi, M.C. In vitro RNA cleavage assay for Argonaute-family proteins. Methods Mol. Biol. 2008, 442, 29–43. [Google Scholar] [PubMed]
- Li, L.; Liu, Y. Diverse small non-coding RNAs in RNA interference pathways. Methods Mol. Biol. 2011, 764, 169–182. [Google Scholar] [PubMed]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: An expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Okamura, K.; Hagen, J.W.; Duan, H.; Tyler, D.M.; Lai, E.C. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007, 130, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, H.; Gao, S.; Jiang, W.; Huang, W. Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J. Virol. 2010, 84, 8849–8860. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.; Chang, H.Y. Long noncoding RNA in genome regulation: Prospects and mechanisms. RNA Biol. 2010, 7, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.G.; Yang, M.F.; Ren, Y.Q.; Wu, C.H.; Wang, L.Q. Exosomes mediated transfer of lncRNA UCA1 results in increased tamoxifen resistance in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4362–4368. [Google Scholar] [PubMed]
- Kedersha, N.L.; Rome, L.H. Isolation and characterization of a novel ribonucleoprotein particle: Large structures contain a single species of small RNA. J. Cell Biol. 1986, 103, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.L.; Heuser, J.E.; Chugani, D.C.; Rome, L.H. Vaults. III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J. Cell Biol. 1991, 112, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.B.; Siva, A.C.; Kickhoefer, V.A.; Rome, L.H.; Stewart, P.L. RNA location and modeling of a WD40 repeat domain within the vault. RNA 2000, 6, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Nandy, C.; Mrazek, J.; Stoiber, H.; Grasser, F.A.; Huttenhofer, A.; Polacek, N. Epstein-barr virus-induced expression of a novel human vault RNA. J. Mol. Biol. 2009, 388, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.; Kvist, A.; Vallon-Christersson, J.; Medstrand, P.; Borg, A.; Rovira, C. The non-coding RNA of the multidrug resistance-linked vault particle encodes multiple regulatory small RNAs. Nat.Cell Biol. 2009, 11, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Kunkeaw, N.; Jeon, S.H.; Lee, K.; Johnson, B.H.; Tanasanvimon, S.; Javle, M.; Pairojkul, C.; Chamgramol, Y.; Wongfieng, W.; Gong, B.; et al. Cell death/proliferation roles for nc886, a non-coding RNA, in the protein kinase R pathway in cholangiocarcinoma. Oncogene 2013, 32, 3722–3731. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Y.; Zhang, Z.; Ouyang, J.; Wang, Y.; Yan, R.; Huang, S.; Gao, G.F.; Guo, G.; Chen, J.L. Robust expression of vault RNAs induced by influenza A virus plays a critical role in suppression of PKR-mediated innate immunity. Nucleic Acids Res. 2015, 43, 10321–10337. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kunkeaw, N.; Jeon, S.H.; Lee, I.; Johnson, B.H.; Kang, G.Y.; Bang, J.Y.; Park, H.S.; Leelayuwat, C.; Lee, Y.S. Precursor miR-886, a novel noncoding RNA repressed in cancer, associates with PKR and modulates its activity. RNA 2011, 17, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Mrazek, J.; Kreutmayer, S.B.; Grasser, F.A.; Polacek, N.; Huttenhofer, A. Subtractive hybridization identifies novel differentially expressed ncRNA species in EBV-infected human B cells. Nucleic Acids Res. 2007, 35, e73. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Schaffert, S.; Fragoso, R.; Loh, C. Regulation of immune responses and tolerance: The microRNA perspective. Immunol. Rev. 2013, 253, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Q.; Papp, G.; Szodoray, P.; Zeher, M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun. Rev. 2016, 15, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Danger, R.; Braza, F.; Giral, M.; Soulillou, J.P.; Brouard, S. MicroRNAs, major players in B cells homeostasis and function. Front. Immunol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Baltimore, D.; Boldin, M.P.; O’Connell, R.M.; Rao, D.S.; Taganov, K.D. MicroRNAs: New regulators of immune cell development and function. Nat. Immunol. 2008, 9, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Umbach, J.L.; Cullen, B.R. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 2009, 23, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, F.; Argyris, E.; Chen, K.; Liang, Z.; Tian, H.; Huang, W.; Squires, K.; Verlinghieri, G.; Zhang, H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 2007, 13, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, I.M.; Cheng, G.; Wieland, S.; Volinia, S.; Croce, C.M.; Chisari, F.V.; David, M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature 2007, 449, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Lecellier, C.H.; Dunoyer, P.; Arar, K.; Lehmann-Che, J.; Eyquem, S.; Himber, C.; Saib, A.; Voinnet, O. A cellular microRNA mediates antiviral defense in human cells. Science 2005, 308, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Jing, Q.; Georgel, P.; New, L.; Chen, J.; Mols, J.; Kang, Y.J.; Jiang, Z.; Du, X.; Cook, R.; et al. Hypersusceptibility to vesicular stomatitis virus infection in Dicer1-deficient mice is due to impaired miR24 and miR93 expression. Immunity 2007, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.L.; Rice, A.P. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009, 5, e1000263. [Google Scholar] [CrossRef] [PubMed]
- Bellon, M.; Lepelletier, Y.; Hermine, O.; Nicot, C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood 2009, 113, 4914–4917. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.L.; Yi, M.; Lancaster, A.M.; Lemon, S.M.; Sarnow, P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 2005, 309, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Henke, J.I.; Goergen, D.; Zheng, J.; Song, Y.; Schuttler, C.G.; Fehr, C.; Junemann, C.; Niepmann, M. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 2008, 27, 3300–3310. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Z.; Weber, F.; Croce, C.; Liu, C.G.; Liao, X.; Pellett, P.E. Human cytomegalovirus infection alters the expression of cellular microRNA species that affect its replication. J. Virol. 2008, 82, 9065–9074. [Google Scholar] [CrossRef] [PubMed]
- Skalsky, R.L.; Cullen, B.R. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 2010, 64, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Bellutti, F.; Kauer, M.; Kneidinger, D.; Lion, T.; Klein, R. Identification of RISC-associated adenoviral microRNAs, a subset of their direct targets, and global changes in the targetome upon lytic adenovirus 5 infection. J. Virol. 2015, 89, 1608–1627. [Google Scholar] [CrossRef] [PubMed]
- Makkoch, J.; Poomipak, W.; Saengchoowong, S.; Khongnomnan, K.; Praianantathavorn, K.; Jinato, T.; Poovorawan, Y.; Payungporn, S. Human microRNAs profiling in response to influenza A viruses (subtypes pH1N1, H3N2, and H5N1). Exp. Biol. Med. 2016, 241, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Q.; Guo, Y.; Liu, S.; Song, R.; Gao, X.; Dai, L.; Li, B.; Zhang, D.; Cheng, J. Microarray analysis of microRNA expression in peripheral blood mononuclear cells of critically ill patients with influenza A (H1N1). BMC Infect. Dis. 2013, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Gao, Y.; Jin, Y.; Cong, W.; Pan, X.; Cui, X. MicroRNA expression profiles and networks in mouse lung infected with H1N1 influenza virus. Mol. Genet. Genom. 2015, 290, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Tambyah, P.A.; Sepramaniam, S.; Mohamed Ali, J.; Chai, S.C.; Swaminathan, P.; Armugam, A.; Jeyaseelan, K. microRNAs in circulation are altered in response to influenza A virus infection in humans. PLoS ONE 2013, 8, e76811. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, M.R.; Lizano, E.; Houben, A.J.; Bezdan, D.; Banez-Coronel, M.; Kudla, G.; Mateu-Huertas, E.; Kagerbauer, B.; Gonzalez, J.; Chen, K.C.; et al. Evidence for the biogenesis of more than 1000 novel human microRNAs. Genome Biol. 2014, 15, R57. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Othumpangat, S.; Noti, J.D.; Beezhold, D.H. Lung epithelial cells resist influenza A infection by inducing the expression of cytochrome c oxidase VIc which is modulated by miRNA 4276. Virology 2014, 468–470, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Pichulik, T.; Khatamzas, E.; Liu, X.; Brain, O.; Delmiro Garcia, M.; Leslie, A.; Danis, B.; Mayer, A.; Baban, D.; Ragoussis, J.; et al. Pattern recognition receptor mediated downregulation of microRNA-650 fine-tunes MxA expression in dendritic cells infected with influenza A virus. Eur. J. Immunol. 2016, 46, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, C.M.; Podyminogin, R.L.; Navarro, G.; Zhao, G.W.; Askovich, P.S.; Weiss, M.J.; Aderem, A. miR-451 regulates dendritic cell cytokine responses to influenza infection. J. Immunol. 2012, 189, 5965–5975. [Google Scholar] [CrossRef] [PubMed]
- Othumpangat, S.; Noti, J.D.; Blachere, F.M.; Beezhold, D.H. Expression of non-structural-1A binding protein in lung epithelial cells is modulated by miRNA-548an on exposure to influenza A virus. Virology 2013, 447, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Buggele, W.A.; Johnson, K.E.; Horvath, C.M. Influenza A virus infection of human respiratory cells induces primary microRNA expression. J. Biol. Chem. 2012, 287, 31027–31040. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Sun, X.; Guan, Z.; Zhang, M.; Duan, M. Modulation of influenza A virus replication by microRNA-9 through targeting MCPIP1. J. Med. Virol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Loveday, E.K.; Diederich, S.; Pasick, J.; Jean, F. Human microRNA-24 modulates highly pathogenic avian-origin H5N1 influenza A virus infection in A549 cells by targeting secretory pathway furin. J. Gen. Virol. 2015, 96, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Shi, N.; Song, Y.; Zhang, X.; Zhang, M.; Duan, M. Induction of the cellular microRNA-29c by influenza virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors BCL2L2. Biochem. Biophys. Res. Commun. 2012, 425, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Hao, Q.; Liu, L.; Li, Y.; Wu, J.; Huo, X.; Zhu, Y. Epigenetic changes mediated by microRNA miR29 activate cyclooxygenase 2 and lambda-1 interferon production during viral infection. J. Virol. 2012, 86, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, C.; Sun, X.; Li, Z.; Zhang, M.; Guan, Z.; Duan, M. Induction of the cellular miR-29c by influenza virus inhibits the innate immune response through protection of A20 mRNA. Biochem. Biophys. Res. Commun. 2014, 450, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Lai, W.; Qin, Y.; Zhang, T.; Wang, S.; Ye, X. MicroRNA-33a disturbs influenza A virus replication by targeting ARCN1 and inhibiting viral ribonucleoprotein activity. J. Gen. Virol. 2016, 97, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Zhou, H.; Zhao, Z.; Zou, Z.; Liu, X.; Lin, X.; Zhang, X.; Deng, X.; Wang, R.; et al. Identification of cellular microRNA-136 as a dual regulator of RIG-I-mediated innate immunity that antagonizes H5N1 IAV replication in A549 cells. Sci. Rep. 2015, 5, 14991. [Google Scholar] [CrossRef] [PubMed]
- Lam, W.Y.; Yeung, A.C.; Ngai, K.L.; Li, M.S.; To, K.F.; Tsui, S.K.; Chan, P.K. Effect of avian influenza A H5N1 infection on the expression of microRNA-141 in human respiratory epithelial cells. BMC Microbiol. 2013, 13, 104. [Google Scholar] [CrossRef] [PubMed]
- Terrier, O.; Textoris, J.; Carron, C.; Marcel, V.; Bourdon, J.C.; Rosa-Calatrava, M. Host microRNA molecular signatures associated with human H1N1 and H3N2 influenza A viruses reveal an unanticipated antiviral activity for miR-146a. J. Gen. Virol. 2013, 94, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Chen, X.; Zhang, M.; Zhao, F.; Wan, Y.; Wang, L.; Xu, G.; Zhou, L.; Yue, X.; Zhu, Y.; et al. Mir-302c mediates influenza A virus-induced IFNβ expression by targeting NF-κB inducing kinase. FEBS Lett. 2015, 589, 4112–4118. [Google Scholar] [CrossRef] [PubMed]
- Ingle, H.; Kumar, S.; Raut, A.A.; Mishra, A.; Kulkarni, D.D.; Kameyama, T.; Takaoka, A.; Akira, S.; Kumar, H. The microRNA miR-485 targets host and influenza virus transcripts to regulate antiviral immunity and restrict viral replication. Sci. Signal. 2015, 8, ra126. [Google Scholar] [CrossRef] [PubMed]
- Khongnomnan, K.; Makkoch, J.; Poomipak, W.; Poovorawan, Y.; Payungporn, S. Human miR-3145 inhibits influenza A viruses replication by targeting and silencing viral PB1 gene. Exp. Biol. Med. 2015, 240, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Yang, J.; Fan, X.L.; Zhao, H.B.; Hu, W.; Li, Z.P.; Yu, G.C.; Ding, X.R.; Wang, J.Z.; Bo, X.C.; et al. Cellular microRNA let-7c inhibits M1 protein expression of the H1N1 influenza A virus in infected human lung epithelial cells. J. Cell. Mol. Med. 2012, 16, 2539–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhu, X.; Chen, Y.; Wei, H.; Chen, Q.; Chi, X.; Qi, B.; Zhang, L.; Zhao, Y.; Gao, G.F.; et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe 2014, 16, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Imamura, K.; Imamachi, N.; Akizuki, G.; Kumakura, M.; Kawaguchi, A.; Nagata, K.; Kato, A.; Kawaguchi, Y.; Sato, H.; Yoneda, M.; et al. Long noncoding RNA NEAT1-dependent SFPQ relocation from promoter region to paraspeckle mediates IL8 expression upon immune stimuli. Mol. Cell 2014, 53, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, P.; Zhao, Y.; Zhang, S.; Lu, J.; Xie, W.; Jiang, Y.; Lei, F.; Xu, N.; Zhang, Y. NEAT1 modulates herpes simplex virus-1 replication by regulating viral gene transcription. Cell. Mol. Life Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Winterling, C.; Koch, M.; Koeppel, M.; Garcia-Alcalde, F.; Karlas, A.; Meyer, T.F. Evidence for a crucial role of a host non-coding RNA in influenza A virus replication. RNA Biol. 2014, 11, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Landeras-Bueno, S.; Ortin, J. Regulation of influenza virus infection by long non-coding RNAs. Virus Res. 2016, 212, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Loveday, E.K.; Svinti, V.; Diederich, S.; Pasick, J.; Jean, F. Temporal- and strain-specific host microRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection. J. Virol. 2012, 86, 6109–6122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chan, E.Y.; Li, J.; Ni, C.; Peng, X.; Rosenzweig, E.; Tumpey, T.M.; Katze, M.G. MicroRNA expression and virulence in pandemic influenza virus-infected mice. J. Virol. 2010, 84, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brahmakshatriya, V.; Zhu, H.; Lupiani, B.; Reddy, S.M.; Yoon, B.J.; Gunaratne, P.H.; Kim, J.H.; Chen, R.; Wang, J.; et al. Identification of differentially expressed miRNAs in chicken lung and trachea with avian influenza virus infection by a deep sequencing approach. BMC Genom. 2009, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Belisle, S.; Baskin, C.R.; Tumpey, T.M.; Katze, M.G. Differential microRNA expression and virulence of avian, 1918 reassortant, and reconstructed 1918 influenza A viruses. Virology 2011, 421, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Barr, T.; Rais, M.; Engelmann, F.; Messaoudi, I. MicroRNAs regulate host immune response and pathogenesis during influenza infection in rhesus macaques. Viral Immunol. 2016, 29, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qi, Y.; Ge, A.; Zhu, Y.; Xu, K.; Ji, H.; Shi, Z.; Cui, L.; Zhou, M. Comprehensive characterization of serum microRNA profile in response to the emerging avian influenza A (H7N9) virus infection in humans. Viruses 2014, 6, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; He, X.; Zheng, Z.; Zhang, Z.; Wei, C.; Guan, K.; Hou, L.; Zhang, B.; Zhu, L.; Cao, Y.; et al. Downregulation of microRNA miR-526a by enterovirus inhibits RIG-I-dependent innate immune response. J. Virol. 2014, 88, 11356–11368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brahmakshatriya, V.; Lupiani, B.; Reddy, S.M.; Soibam, B.; Benham, A.L.; Gunaratne, P.; Liu, H.C.; Trakooljul, N.; Ing, N.; et al. Integrated analysis of microRNA expression and mRNA transcriptome in lungs of avian influenza virus infected broilers. BMC Genom. 2012, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.H.; Jang, J.Y.; Jeon, Y.K.; Kim, W.Y.; Kim, T.M.; Heo, D.S.; Kim, C.W. MicroRNA-146a downregulates NFκB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 2011, 17, 4761–4771. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhou, R.; Liu, J.; Gong, A.Y.; Eischeid, A.N.; Dittman, J.W.; Chen, X.M. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J. Immunol. 2009, 183, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sahu, S.K.; Kumar, R.; Subuddhi, A.; Maji, R.K.; Jana, K.; Gupta, P.; Raffetseder, J.; Lerm, M.; Ghosh, Z.; et al. MicroRNA let-7 modulates the immune response to Mycobacterium tuberculosis infection via control of A20, an inhibitor of the NF-κB pathway. Cell Host Microbe 2015, 17, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Choi, H.; Jiang, X.; Yin, L.; Seet, J.E.; Patzel, V.; Engelward, B.P.; Chow, V.T. Micro-RNAs in regenerating lungs: An integrative systems biology analysis of murine influenza pneumonia. BMC Genom. 2014, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Lonze, B.E.; Riccio, A.; Cohen, S.; Ginty, D.D. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 2002, 34, 371–385. [Google Scholar] [CrossRef]
- Kash, J.C.; Tumpey, T.M.; Proll, S.C.; Carter, V.; Perwitasari, O.; Thomas, M.J.; Basler, C.F.; Palese, P.; Taubenberger, J.K.; Garcia-Sastre, A.; et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 2006, 443, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Kobasa, D.; Jones, S.M.; Shinya, K.; Kash, J.C.; Copps, J.; Ebihara, H.; Hatta, Y.; Kim, J.H.; Halfmann, P.; Hatta, M.; et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 2007, 445, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Balkhi, M.Y.; Iwenofu, O.H.; Bakkar, N.; Ladner, K.J.; Chandler, D.S.; Houghton, P.J.; London, C.A.; Kraybill, W.; Perrotti, D.; Croce, C.M.; et al. miR-29 acts as a decoy in sarcomas to protect the tumor suppressor A20 mRNA from degradation by HuR. Sci. Signal. 2013, 6, ra63. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Feng, G.; Chen, H.; Wang, L.; Wang, Y. Identification of host encoded microRNAs interacting with novel swine-origin influenza A (H1N1) virus and swine influenza virus. Bioinformation 2009, 4, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Morán, J.; Ramírez-Martínez, G.; Jiménez-Alvarez, L.; Cruz, A.; Pérez-Patrigeon, S.; Hidalgo, A.; Orozco, L.; Martínez, A.; Padilla-Noriega, L.; Avila-Moreno, F.; et al. Circulating levels of miR-150 are associated with poorer outcomes of A/H1N1 infection. Exp. Mol. Pathol. 2015, 99, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Brogaard, L.; Heegaard, P.M.; Larsen, L.E.; Mortensen, S.; Schlegel, M.; Durrwald, R.; Skovgaard, K. Late regulation of immune genes and microRNAs in circulating leukocytes in a pig model of influenza A (H1N2) infection. Sci. Rep. 2016, 6, 21812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, F.; He, J.; Loo, J.F.; Yao, J.; Shi, L.; Liu, C.; Zhao, C.; Xie, W.; Shao, Y.; Kong, S.K.; et al. Identification of microRNAs in throat swab as the biomarkers for diagnosis of influenza. Int. J. Med. Sci. 2016, 13, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Sun, W.; Shi, Y.; Xing, Z.; Su, X. Altered viral replication and cell responses by inserting microRNA recognition element into PB1 in pandemic influenza A virus (H1N1) 2009. Mediat. Inflamm. 2015, 2015, 976575. [Google Scholar] [CrossRef] [PubMed]
- Dormitzer, P.R.; Tsai, T.F.; Del Giudice, G. New technologies for influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Barriocanal, M.; Carnero, E.; Segura, V.; Fortes, P. Long non-coding RNA BST2/BISPR is induced by IFN and regulates the expression of the antiviral factor tetherin. Front. Immunol. 2015, 5, 655. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Barriocanal, M.; Prior, C.; Pablo Unfried, J.; Segura, V.; Guruceaga, E.; Enguita, M.; Smerdou, C.; Gastaminza, P.; Fortes, P. Long noncoding RNA EGOT negatively affects the antiviral response and favors HCV replication. EMBO Rep. 2016, 17, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Carmichael, G.G. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: Functional role of a nuclear noncoding RNA. Mol. Cell 2009, 35, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Clemson, C.M.; Hutchinson, J.N.; Sara, S.A.; Ensminger, A.W.; Fox, A.H.; Chess, A.; Lawrence, J.B. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol. Cell 2009, 33, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.S.; Zhang, B.; Spector, D.L. Biogenesis and function of nuclear bodies. Trends Genet. 2011, 27, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Benard, M.; Fox, A.H.; et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies. Mol. Biol. Cell 2014, 25, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Landeras-Bueno, S.; Jorba, N.; Perez-Cidoncha, M.; Ortin, J. The splicing factor proline-glutamine rich (SFPQ/PSF) is involved in influenza virus transcription. PLoS Pathog. 2011, 7, e1002397. [Google Scholar] [CrossRef] [PubMed]
- Kambara, H.; Gunawardane, L.; Zebrowski, E.; Kostadinova, L.; Jobava, R.; Krokowski, D.; Hatzoglou, M.; Anthony, D.D.; Valadkhan, S. Regulation of interferon-stimulated gene BST2 by a lncRNA transcribed from a shared bidirectional promoter. Front. Immunol. 2014, 5, 676. [Google Scholar] [CrossRef] [PubMed]
- Carnero, E.; Barriocanal, M.; Segura, V.; Guruceaga, E.; Prior, C.; Borner, K.; Grimm, D.; Fortes, P. Type I interferon regulates the expression of long non-coding RNAs. Front. Immunol. 2014, 5, 548. [Google Scholar] [CrossRef] [PubMed]
- Kambara, H.; Niazi, F.; Kostadinova, L.; Moonka, D.K.; Siegel, C.T.; Post, A.B.; Carnero, E.; Barriocanal, M.; Fortes, P.; Anthony, D.D.; et al. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014, 42, 10668–10680. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Park, J.L.; Lee, K.; Richardson, L.E.; Johnson, B.H.; Lee, H.S.; Lee, J.S.; Kim, S.B.; Kwon, O.H.; Song, K.S.; et al. nc886, a non-coding RNA of anti-proliferative role, is suppressed by CpG DNA methylation in human gastric cancer. Oncotarget 2014, 5, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
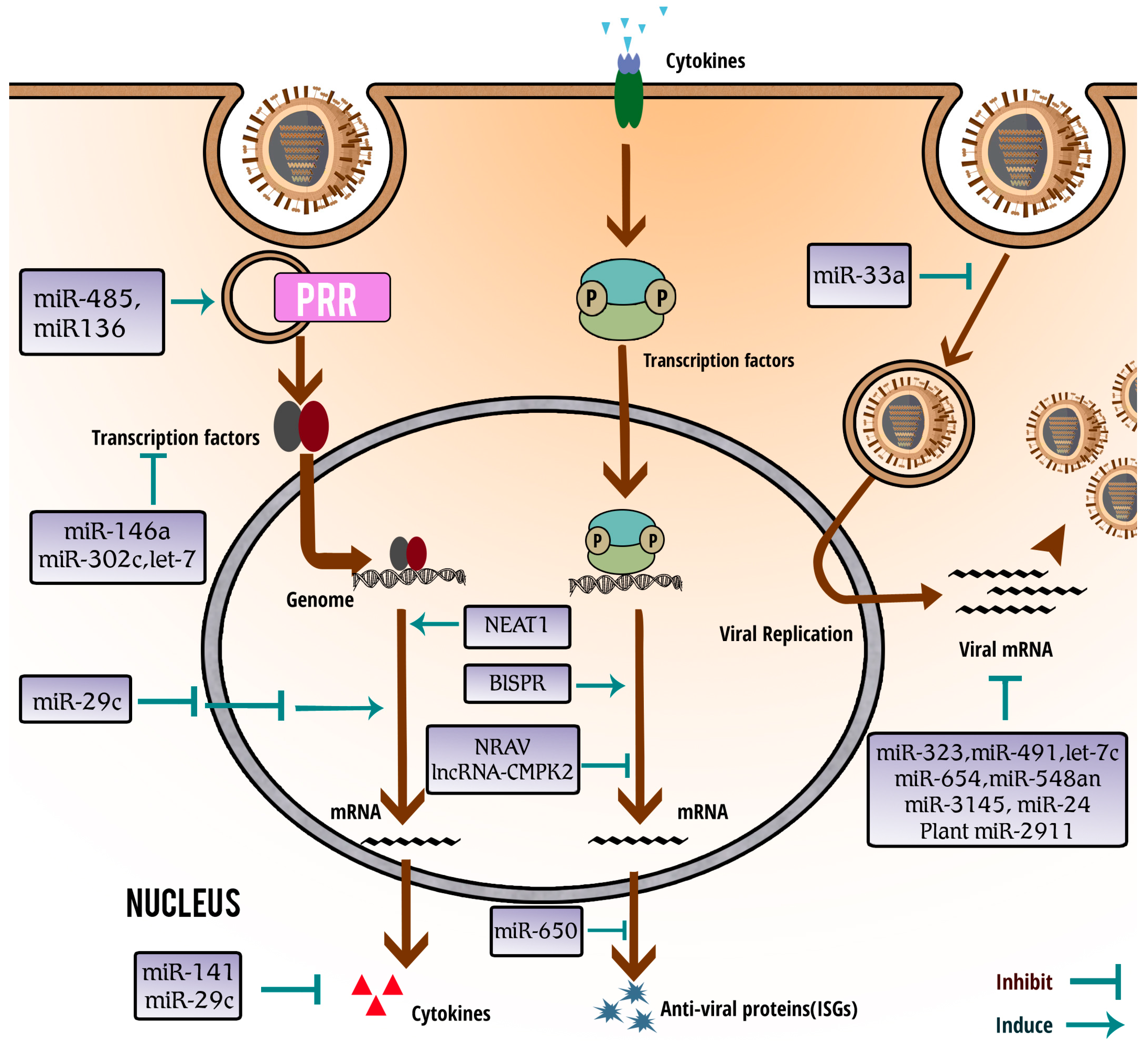
| ncRNAs | Stimuli | Differential Expression | Functions/Mechanisms | References |
|---|---|---|---|---|
| microRNA (miR)-7, miR-132, miR-187, miR-200c, and miR-1275 | H1N1 | Up | cause down-regulation of antiviral proteins such as IL-1R-associated kinase 1 (IRAK1)and mitogen-activated protein kinase 3 (MAPK3) | [64] |
| miR-9 | H1N1 and H3N2 | Up | promotes IAV replication through suppression of monocyte chemoattractant protein 1-induced protein 1 (MCPIP1) | [65] |
| miR-24 | H5N1 | Down | governs furin-mediated proteolytic activation of hemagglutinin precursor (HA0) glycoproteins and production of infectious virions | [66] |
| miR-29 | H3N2 | Up | suppresses DNA methyltransferase (DNMT)3a/3b activity and induces expression of cyclooxygenase-2 (COX2) and IFN-λ1 | [67,68] |
| miR-29c | H3N2 and H1N1 | Up | induces virus-mediated apoptosis through repression of antiapoptotic factors B-cell lymphoma 2 like 2 (BCL2L2), and inhibits the innate immune response through protection of deubiquitinating enzyme A20 mRNA | [67,69] |
| miR-33a | H1N1, H9N2 and H3N2 | Up | disturbs IAV replication by targeting archain 1 (ARCN1) and inhibiting viral ribonucleoprotein activity | [70] |
| miR-136 | H5N1 | Up | acts as an immune agonist of RIG-I, causing interleukin-6 (IL-6) and IFN-β accumulation | [71] |
| miR-141 | H5N1 | Up | suppresses the expression of transforming growth factor β2 (TGF-β2) mRNA | [72] |
| miR-146a | H1N1and H3N2 | Up | suppressesIRAK1, MAPK3, tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) expression and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, and decreases virus replication | [64,73] |
| miR-302c | H3N2 | Down | prevents the translocation of NF-κB from the cytosol to the nucleus, leading to the suppression of IFN-β expression | [74] |
| miR-323, miR-491, miR-654 | H1N1 | NA | bind with the polymerase basic 1 (PB1) gene, and downregulates PB1 expression through mRNA degradation | [26] |
| miR-451 | H1N1 | Up | negatively regulates the levels of YWHAZ protein which controls the activity of two negative regulators forkhead box O3 (FOXO3) and zinc finger protein 36 (ZFP36) of cytokine production | [62] |
| miR-485 | H5N1 | Up | exhibits bispecificity, targeting RIG-I with a low abundance of H5N1 virus and targeting PB1 with increased amounts of the H5N1 virus | [75] |
| miR-548an | H1N1 | Down | triggers the overexpression of an anti-apoptotic protein non-structural-1A binding protein (NS1ABP) | [63] |
| miR-650 | H1N1 | Down | directly targets the antiviral ISG myxovirus resistance protein 1 (MxA) and fine-tunes its expression | [61] |
| miR-3145 | pH1N1, H5N1 and H3N2 | NA | inhibits IAV replication by targeting and silencing viral PB1 gene | [76] |
| miR-4276 | H1N1, H3N2 | Down | downregulates the expression of apoptotic protein cytochrome c oxidase VIc (COX6C) | [60] |
| let-7c | H1N1 | Up | inhibits matrix protein 1 (M1) expression | [77] |
| plant miR-2911 | NA | NA | suppresses H1N1,H5N1 and H7N9 replication, and inhibits H1N1-encoded PB2 and non-structural protein 1 (NS1) expression | [78] |
| NRAV | H1N1 | Down | negatively modulates antiviral responses through suppressing of ISGs‘ transcription, including interferon-induced protein with tetratricopeptide repeats 2 (IFIT2), IFIT3, 2'-5'-oligoadenylate synthetase-like protein (OASL), MxA and interferon-induced transmembrane protein 3 (IFITM3) | [79] |
| NEAT1 | H1NI | Up | regulates the expression of IL-8 through sequestring splicing factor proline-glutamine rich (SFPQ/PSF) in paraspeckles | [80,81] |
| virus inducible lincRNA (VIN) | H1N1, H3N2 and H7N7 | Up | is induced by IAV to benefit viral replication and viral gene expression | [82,83] |
| vtRNAs | H1N1 | Up | promote viral replication through repressing the activation of PKR and the subsequent antiviral interferon response | [35] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Ouyang, J.; Wei, J.; Maarouf, M.; Chen, J.-L. Involvement of Host Non-Coding RNAs in the Pathogenesis of the Influenza Virus. Int. J. Mol. Sci. 2017, 18, 39. https://doi.org/10.3390/ijms18010039
Ma Y, Ouyang J, Wei J, Maarouf M, Chen J-L. Involvement of Host Non-Coding RNAs in the Pathogenesis of the Influenza Virus. International Journal of Molecular Sciences. 2017; 18(1):39. https://doi.org/10.3390/ijms18010039
Chicago/Turabian StyleMa, Yanmei, Jing Ouyang, Jingyun Wei, Mohamed Maarouf, and Ji-Long Chen. 2017. "Involvement of Host Non-Coding RNAs in the Pathogenesis of the Influenza Virus" International Journal of Molecular Sciences 18, no. 1: 39. https://doi.org/10.3390/ijms18010039






