In Vivo Assessment of Clobetasol Propionate-Loaded Lecithin-Chitosan Nanoparticles for Skin Delivery
Abstract
:1. Introduction
2. Results
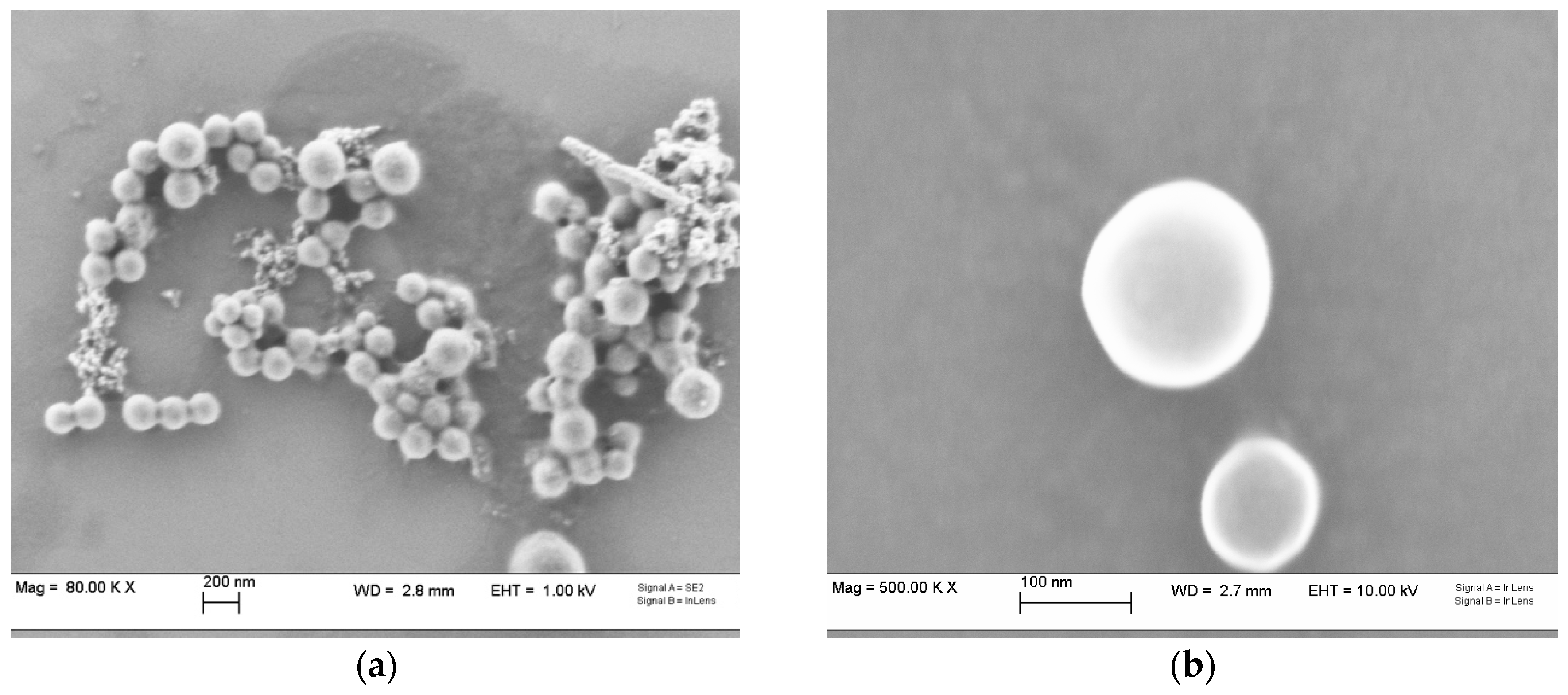
2.1. Nanoparticle Characterization
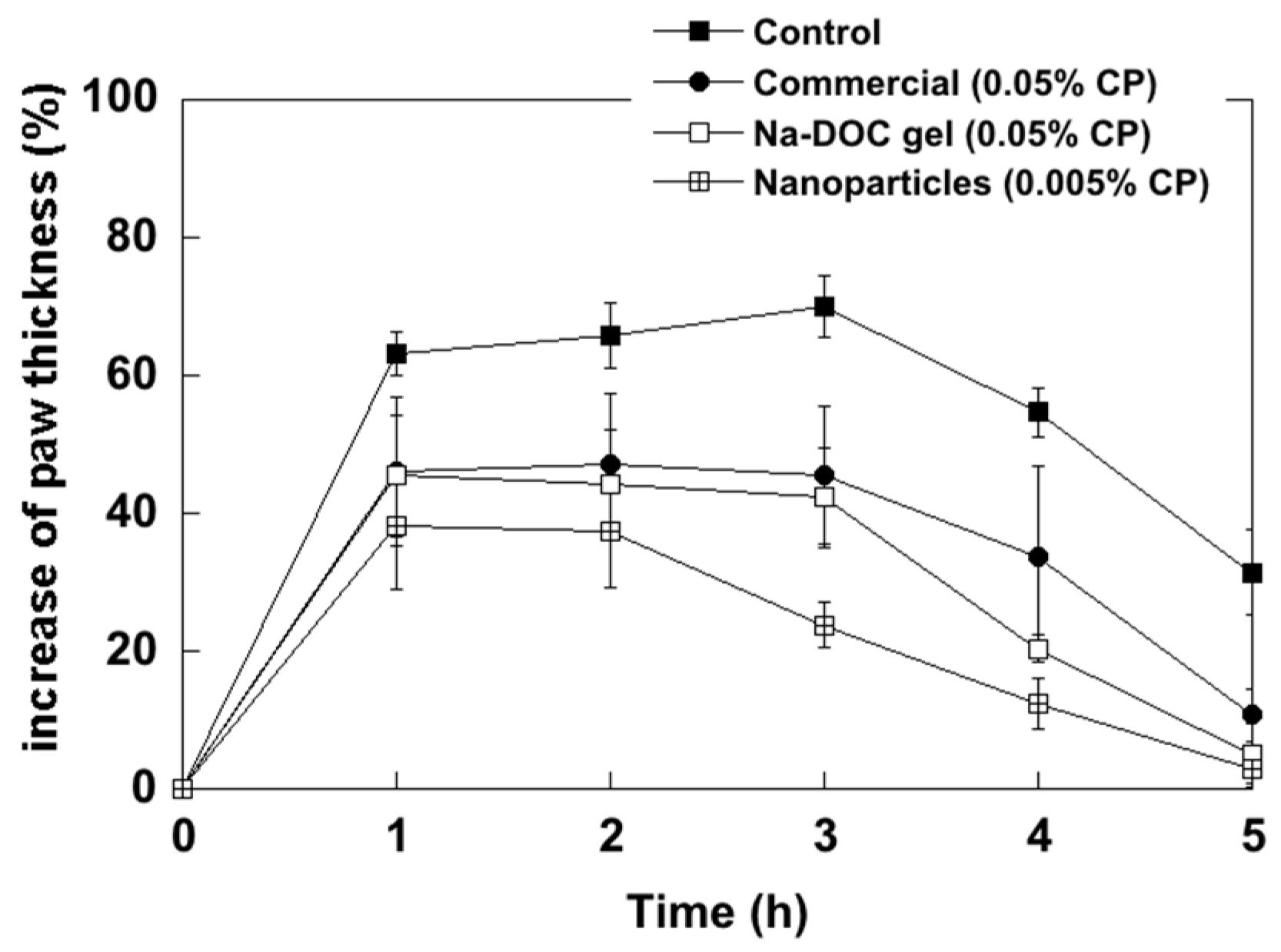
2.2. Anti-Inflammatory Activity Studies
- A 10-fold higher CP accumulation in the epidermis starting from the Na-DOC gel compared to NP and commercial cream;
- A comparable CP level in the dermis for the three formulations (see the very high data variability).
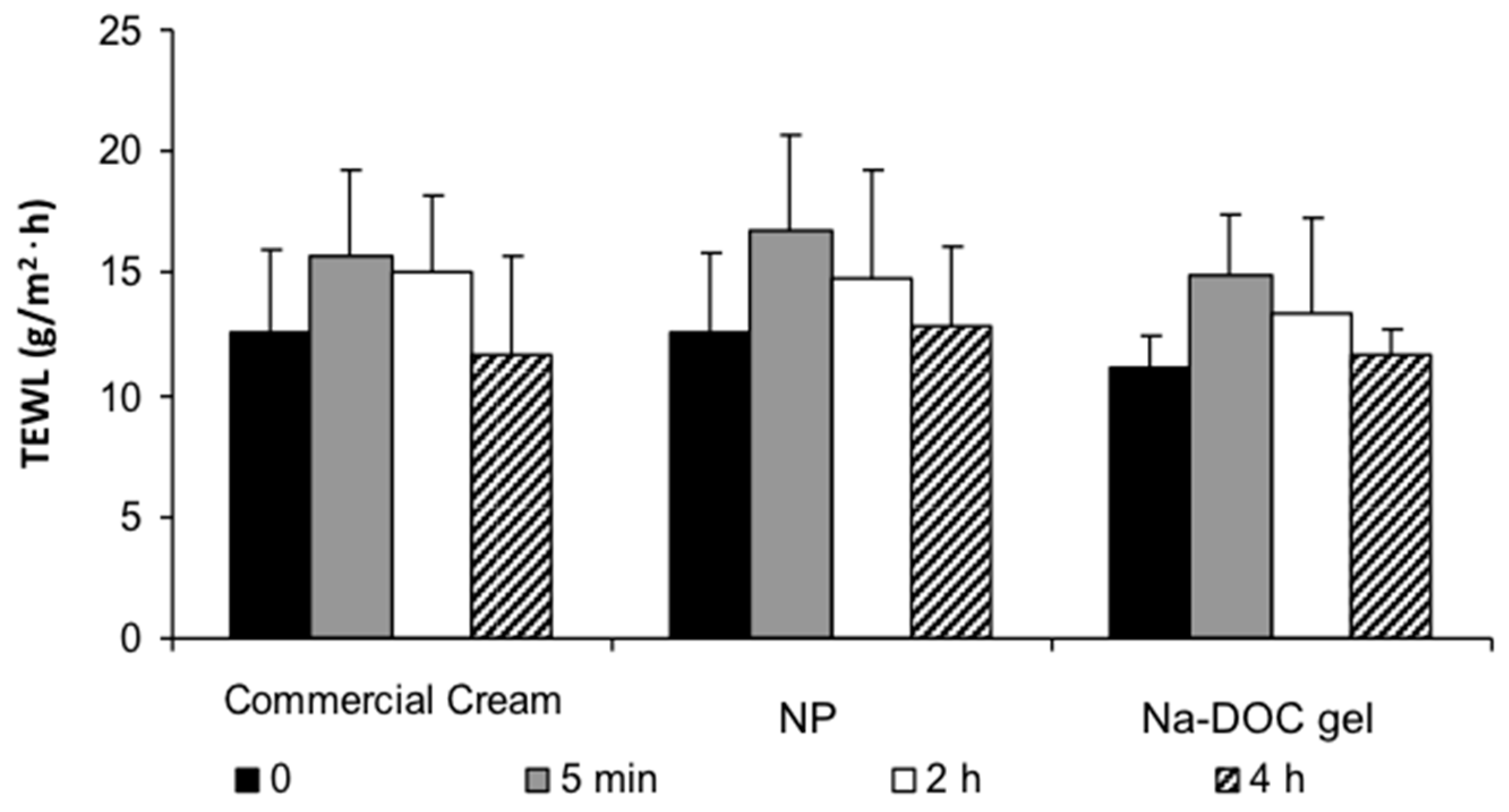
2.3. Evaluation of Skin Barrier Function and Damage
3. Discussion
- By forming a reservoir of the drug;
- Through a progressive enzyme-controlled delivery of the drug from the nanometric delivery system.
4. Materials and Methods
4.1. Materials
4.2. Nanoparticle Preparation and Characterization
4.3. Preparation of Na-DOC Gel
4.4. Preparation of NP Loaded Chitosan Gel
4.5. In Vivo Studies
4.5.1. Anti-Inflammatory Activity Studies
4.5.2. Transepidermal Water Loss (TEWL)
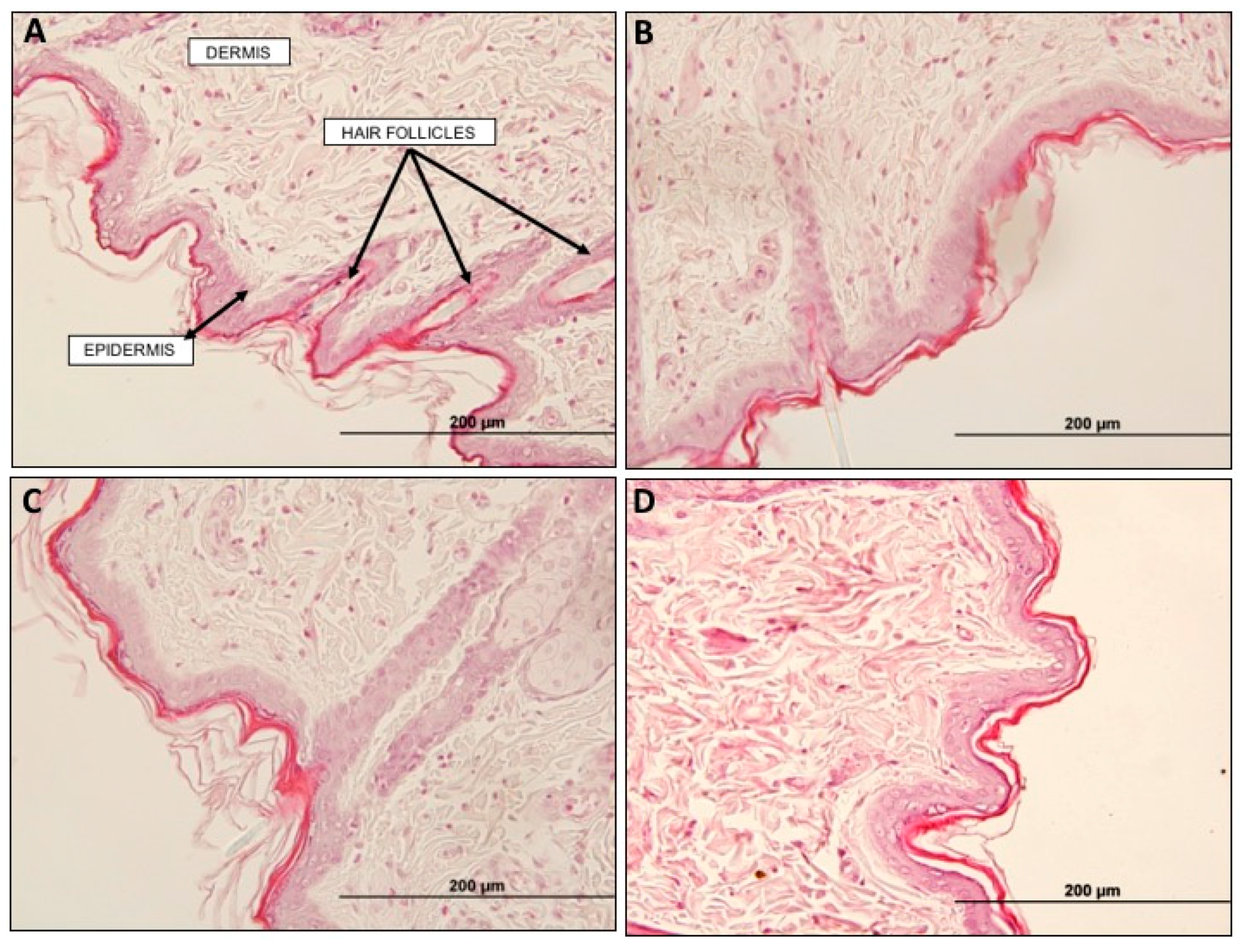
4.5.3. Histological Analysis
2.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sonvico, F.; Dubernet, C.; Colombo, P.; Couvreur, P. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Cur. Pharm. Des. 2005, 11, 2091–2105. [Google Scholar] [CrossRef]
- Yamamoto, E.; Kuroda, K. Colloidal Mesoporous Silica Nanoparticles. BCSJ 2016, 89, 501–539. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ϵ-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Kawakami, K.; Ebara, M.; Kotsuchibashi, Y.; Ji, Q.; Hill, J.P. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J. Chem. 2014, 38, 5149–5163. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed]
- Wiedersberg, S.; Leopold, C.S.; Guy, R.H. Bioavailability and bioequivalence of topical glucocorticoids. Eur. J. Pharm. Biopharm. 2008, 68, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Brazzini, B.; Pimpinelli, N. New and established topical corticosteroids in dermatology. Am. J. Clin. Dermatol. 2002, 3, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.W.; Hunter, J.A. The use and abuse of 0.05 per cent clobetasol propionate in dermatology. Dermatol. Clin. 1988, 6, 643–647. [Google Scholar] [PubMed]
- Tosti, A.; Iorizzo, M.; Botta, G.L.; Milani, M. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: A randomized, double-blind placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Bhutani, T.; Koo, J.; Maibach, H.I. Efficacy of clobetasol spray: Factors beyond patient compliance. J. Dermatol. Treat. 2012, 23, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Fang, C.; Sung, K.C.; CHEN, H. Effect of low frequency ultrasound on the in vitro percutaneous absorption of clobetasol 17-propionate. Int. J. Pharm. 1999, 191, 33–42. [Google Scholar] [CrossRef]
- Patel, H.K.; Barot, B.S.; Parejiya, P.B.; Shelat, P.K.; Shukla, A. Topical delivery of clobetasol propionate loaded microemulsion based gel for effective treatment of vitiligo: ex vivo permeation and skin irritation studies. Colloids Surf. B Biointerfaces 2013, 102, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Panonnummal, R.; Jayakumar, R.; Sabitha, M. Comparative anti-psoriatic efficacy studies of clobetasol loaded chitin nanogel and marketed cream. Eur. J. Pharm. Sci. 2016, 96, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.C.; Rezer, J.F.P.; Coradini, K.; Leal, D.B.R.; Beck, R.C.R. Improved efficacy in the treatment of contact dermatitis in rats by a dermatological nanomedicine containing clobetasol propionate. Eur. J. Pharm. Biopharm. 2011, 79, 241–249. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, D.F.; Fontana, M.C.; Pohlmann, A.R.; Guterres, S.S.; Carlos, R.; Beck, R. Nanoencapsulation of clobetasol propionate decreases its penetration to skin layers without changing its relative skin distribution. J. Nanosci. Nanotechnol. 2015, 15, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Mathes, C.; Melero, A.; Conrad, P.; Vogt, T.; Rigo, L.; Selzer, D.; Prado, W.A.; de Rossi, C.; Garrigues, T.M.; Hansen, S.; et al. Nanocarriers for optimizing the balance between interfollicular permeation and follicular uptake of topically applied clobetasol to minimize adverse effects. J. Control. Release 2016, 223, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nagaich, U.; Gulati, N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv. Transl. Res. 2016, 6, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.D.; Andrade, L.M.; de Sá, F.A.P.; Marreto, R.N.; Lima, E.M.; Gratieri, T.; Taveira, S.F. Clobetasol-loaded nanostructured lipid carriers for epidermal targeting. J. Pharm. Pharmacol. 2016, 68, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Buttini, F.; Rossi, A.; Bettini, R.; Colombo, P.; Ponchel, G.; Sonvico, F.; Colombo, G. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015, 491, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Senyiğit, T.; Sonvico, F.; Barbieri, S.; Ozer, O.; Santi, P.; Colombo, P. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J. Control. Release 2010, 142, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Clementino, A.; Batger, M.; Garrastazu, G.; Pozzoli, M.; del Favero, E.; Rondelli, V.; Gutfilen, B.; Barboza, T.; Sukkar, M.B.; Souza, S.A.L.; et al. The nasal delivery of nanoencapsulated statins–an approach for brain delivery. Int. J. Nanomed. 2016, 11, 6575–6590. [Google Scholar] [CrossRef] [PubMed]
- Senyigit, T.; Padula, C.; Ozer, O.; Santi, P. Different approaches for improving skin accumulation of topical corticosteroids. Int. J. Pharm. 2009, 380, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Raphael, A.P.; Garrastazu, G.; Sonvico, F.; Prow, T.W. Formulation design for topical drug and nanoparticle treatment of skin disease. Ther. Deliv. 2015, 6, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Leme, J.G.; Hamamura, L.; Leite, M.P. Pharmacological analysis of the acute inflammatory process induced in the rat’s paw by local injection of carrageenin and by heating. Br. J. Pharm. 1973, 48, 88–96. [Google Scholar] [CrossRef]
- Sonvico, F.; di Bari, M.T.; Bove, L.; Deriu, A.; Cavatorta, F.; Albanese, G. Mean square hydrogen fluctuations in chitosan/lecithin nanoparticles from elastic neutron scattering experiments. Phys. B Condens. Matter 2006, 385–386, 725–727. [Google Scholar] [CrossRef]
- Gerelli, Y.; Di Bari, M.T.; Deriu, A.; Cantù, L.; Colombo, P.; Como, C.; Motta, S.; Sonvico, F.; May, R. Structure and organization of phospholipid/polysaccharide nanoparticles. J. Phys. Condens. Matter 2008, 20, 104211. [Google Scholar] [CrossRef]
- Gerelli, Y.; Bari, M.; Barbieri, S.; Sonvico, F.; Colombo, P.; Natali, F.; Deriu, A. Flexibility and drug release features of lipid/saccharide nanoparticles. Soft Matter 2010, 6, 685–691. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Boddu, S.H.S.; Alsaab, H.; Umar, S.; Bonam, S.P.; Gupta, H.; Ahmed, S. Anti-inflammatory effects of a novel ricinoleic acid poloxamer gel system for transdermal delivery. Int. J. Pharm. 2015, 479, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Sarigüllü Ozgüney, I.; Yeşim Karasulu, H.; Kantarci, G.; Sözer, S.; Güneri, T.; Ertan, G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech 2006, 7, 88. [Google Scholar] [PubMed]
- Arévalo, M.I.; Escribano, E.; Calpena, A.; Domenech, J.; Queralt, J. Rapid skin anesthesia using a new topical amethocaine formulation: a preclinical study. Anesth. Analg. 2004, 98, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Maibach, H. The correlation between transepidermal water loss and percutaneous absorption: An overview. J. Control. Release 2005, 103, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Heylings, J.R.; Clowes, H.M.; Hughes, L. Comparison of tissue sources for the skin integrity function test (SIFT). Toxicol. In Vitro 2001, 15, 597–600. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Leu, Y.-L.; Wang, Y.-Y.; Tsai, Y.-H. In vitro topical application and in vivo pharmacodynamic evaluation of nonivamide hydrogels using Wistar rat as an animal model. Eur. J. Pharm. Sci. 2002, 15, 417–423. [Google Scholar] [CrossRef]
- Sheu, H.M.; Lee, J.Y.; Chai, C.Y.; Kuo, K.W. Depletion of stratum corneum intercellular lipid lamellae and barrier function abnormalities after long-term topical corticosteroids. Br. J. Dermatol. 1997, 136, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Stracke, F.; Hansen, S.; Schaefer, U.F. Nanoparticles and their interactions with the dermal barrier. Dermatoendocrinology 2009, 1, 197–206. [Google Scholar] [CrossRef]
- Swingle, K.F. Evaluation for Antiinflammatory Activity; Elsevier: Amsterdam, The Netherlands, 1974; pp. 33–122. [Google Scholar]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiss, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R.; et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-L.; Aljuffali, I.A.; Li, Y.-C.; Fang, J.-Y. Delivery and targeting of nanoparticles into hair follicles. Ther. Deliv. 2014, 5, 991–1006. [Google Scholar] [CrossRef] [PubMed]
- Gröschel-Stewart, U.; Bardini, M.; Robson, T.; Burnstock, G. Localisation of P2X5 and P2X7 receptors by immunohistochemistry in rat stratified squamous epithelia. Cell Tissue Res. 1999, 296, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, S.; Sonvico, F.; Como, C.; Zani, F.; Buttini, F.; Bettini, R.; Rossi, A.; Colombo, P. Lecithin/chitosan controlled release nanopreparations of tamoxifen citrate: loading, enzyme-trigger release and cell uptake. J. Control. Release 2013, 167, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.M.; Harder, J. Antimicrobial skin peptides and proteins. Cell. Mol. Life Sci. 2006, 63, 469–486. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Park, G.-T.; Cho, I.H.; Sim, S.-M.; Yang, J.-M.; Lee, D.-Y. An antimicrobial protein, lactoferrin exists in the sweat: proteomic analysis of sweat. Exp. Dermatol. 2011, 20, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Sonvico, F.; Cagnani, A.; Rossi, A.; Motta, S.; di Bari, M.T.; Cavatorta, F.; Alonso, M.J.; Deriu, A.; Colombo, P. Formation of self-organized nanoparticles by lecithin/chitosan ionic interaction. Int. J. Pharm. 2006, 324, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C.; Nowack, E.; Bernkop-Schnürch, A. Deoxycholate-hydrogels: Novel drug carrier systems for topical use. Int. J. Pharm. 1999, 185, 103–111. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Hamidian, M. Anti-inflammatory and analgesic activity of the topical preparation of Glaucium grandiflorum. Fitoterapia 2004, 75, 123–129. [Google Scholar] [CrossRef] [PubMed]
| Formulation | CP Loading (% w/w) | In Vivo Efficacy | In Vitro CP Skin Accumulation | ||
|---|---|---|---|---|---|
| AUC0–5 (%·h) 1 | Edema Inhibition (%) 2 | Epidermis (µg/mg) | Dermis (µg/mg) | ||
| Dermovate | 0.05 | 178 ± 44 | 34 ± 16 | 0.03 ± 0.03 | 0.0042 ± 0.0027 |
| Na-DOC Gel | 0.05 | 151 ± 23 | 44 ± 9 | 0.67 ± 0.19 | 0.0115 ± 0.0043 |
| NP | 0.005 | 113 ± 16 | 58 ± 6 | 0.06 ± 0.03 | 0.0053 ± 0.0012 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şenyiğit, T.; Sonvico, F.; Rossi, A.; Tekmen, I.; Santi, P.; Colombo, P.; Nicoli, S.; Özer, Ö. In Vivo Assessment of Clobetasol Propionate-Loaded Lecithin-Chitosan Nanoparticles for Skin Delivery. Int. J. Mol. Sci. 2017, 18, 32. https://doi.org/10.3390/ijms18010032
Şenyiğit T, Sonvico F, Rossi A, Tekmen I, Santi P, Colombo P, Nicoli S, Özer Ö. In Vivo Assessment of Clobetasol Propionate-Loaded Lecithin-Chitosan Nanoparticles for Skin Delivery. International Journal of Molecular Sciences. 2017; 18(1):32. https://doi.org/10.3390/ijms18010032
Chicago/Turabian StyleŞenyiğit, Taner, Fabio Sonvico, Alessandra Rossi, Işıl Tekmen, Patrizia Santi, Paolo Colombo, Sara Nicoli, and Özgen Özer. 2017. "In Vivo Assessment of Clobetasol Propionate-Loaded Lecithin-Chitosan Nanoparticles for Skin Delivery" International Journal of Molecular Sciences 18, no. 1: 32. https://doi.org/10.3390/ijms18010032






