Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy
Abstract
:1. Introduction
2. Results
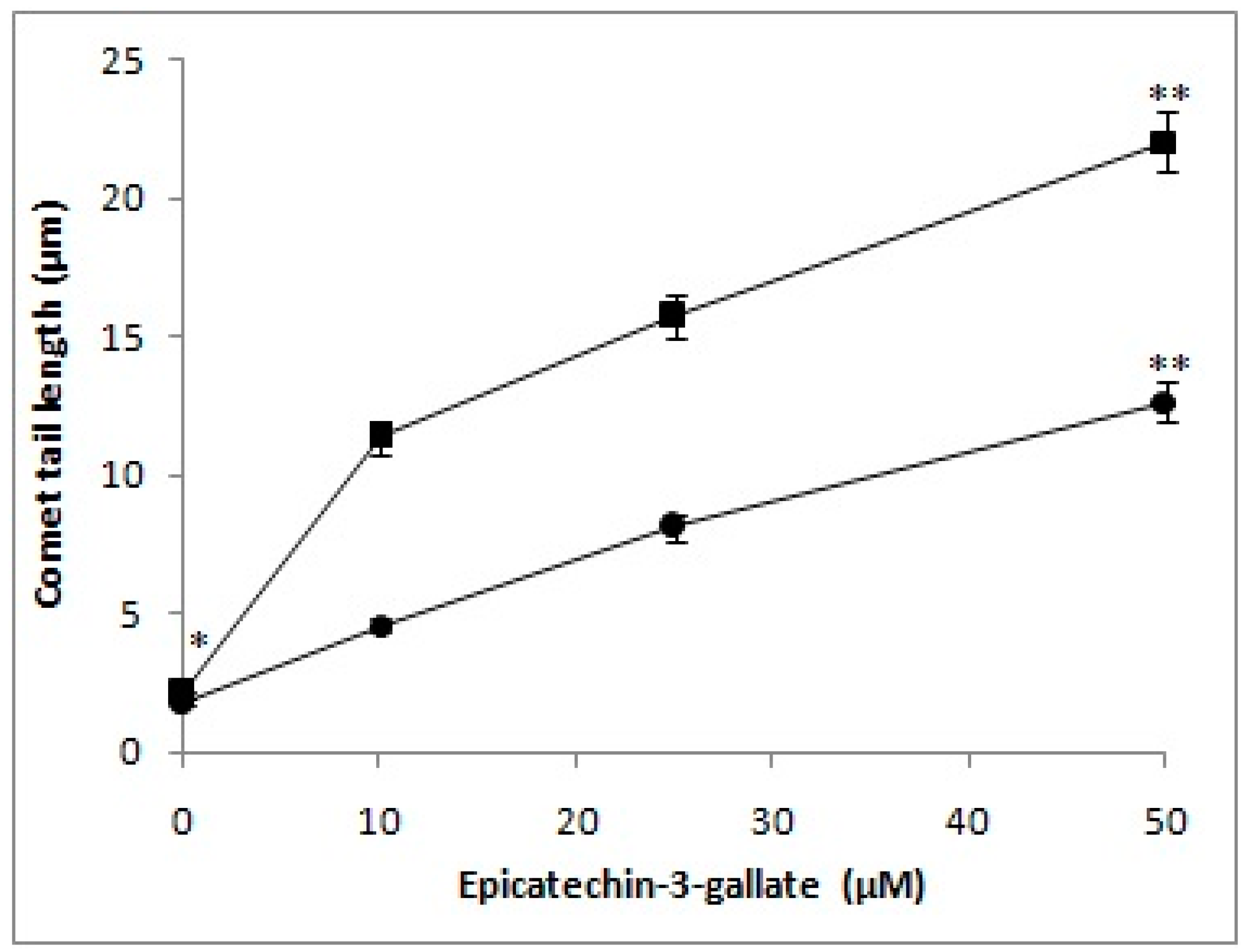
2.1. Cellular DNA Breakage by Epicatechin-3-Gallate in Intact Cells and Permeabilized Cells as Measured by Comet Assay
2.2. Effect of Reactive Oxygen Scavengers on the Epicatechin-3-Gallate Induced Cellular DNA Breakage in Permeabilized Cells
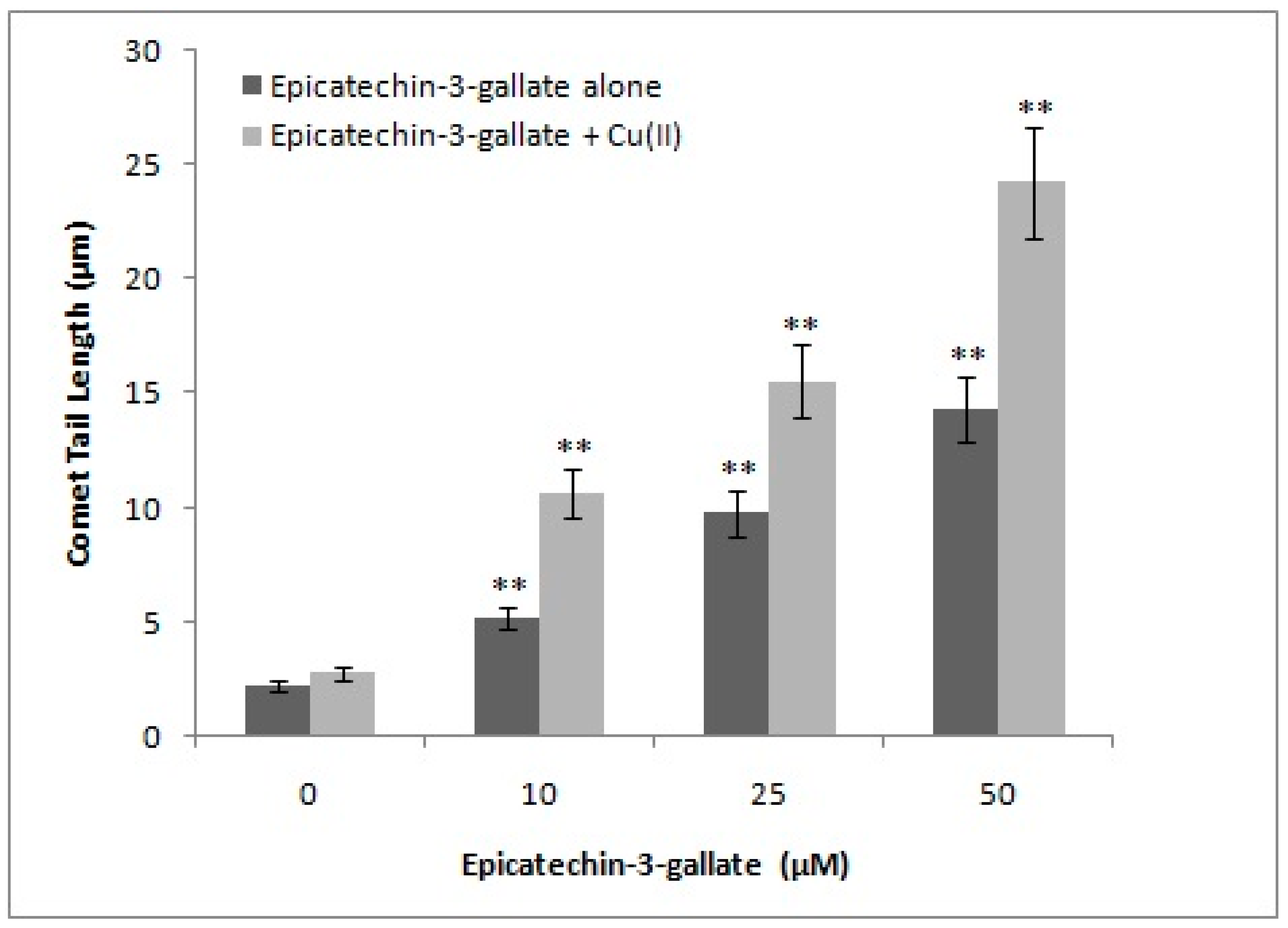
2.3. Effect of Metal-Specific Chelators on the Epicatechin-3-Gallate Induced Cellular DNA Breakage in Intact Cells and Permeabilized Cells
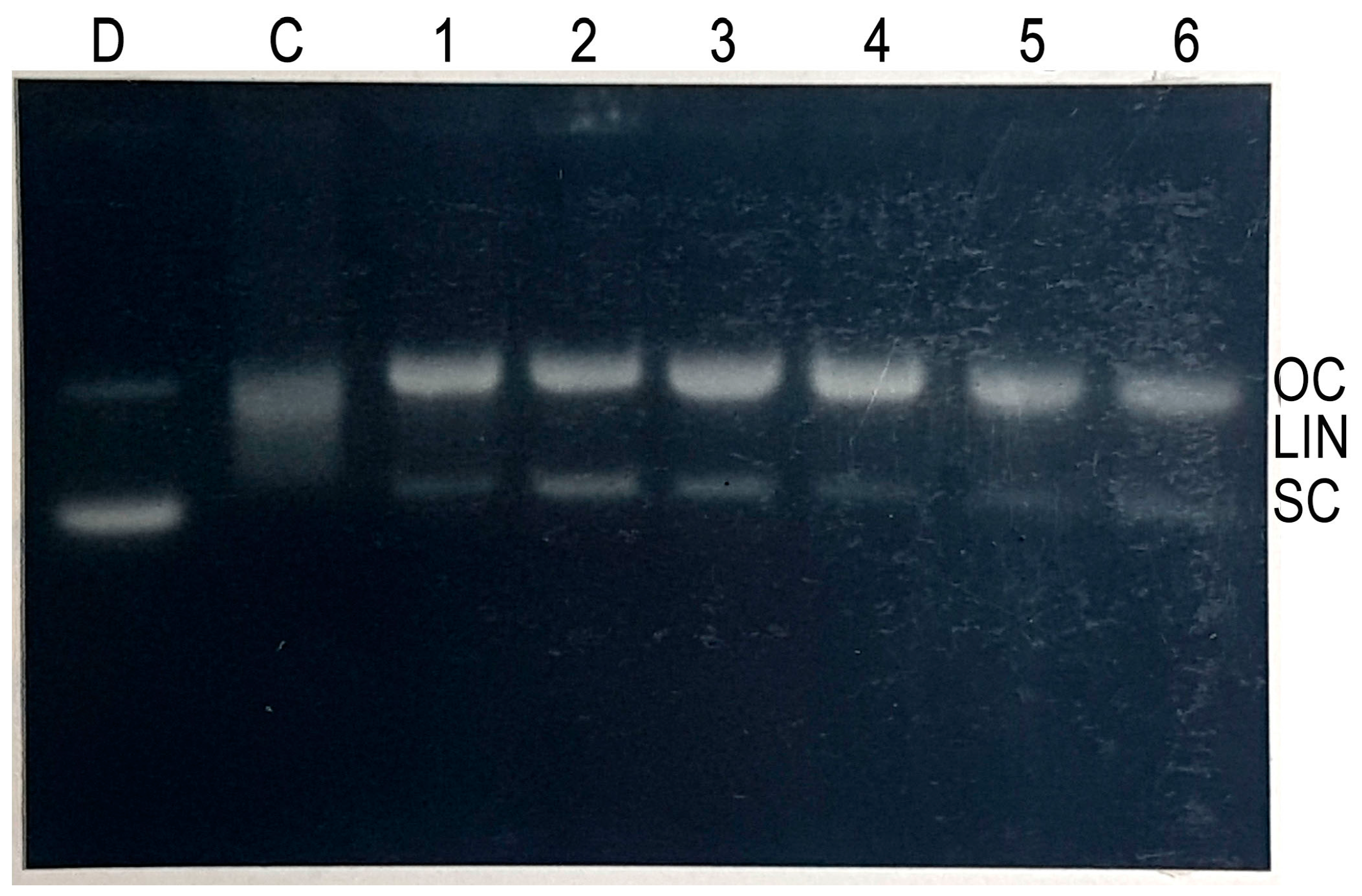
2.4. Cleavage of Plasmid pBR322 DNA by Epicatechin-3-Gallate
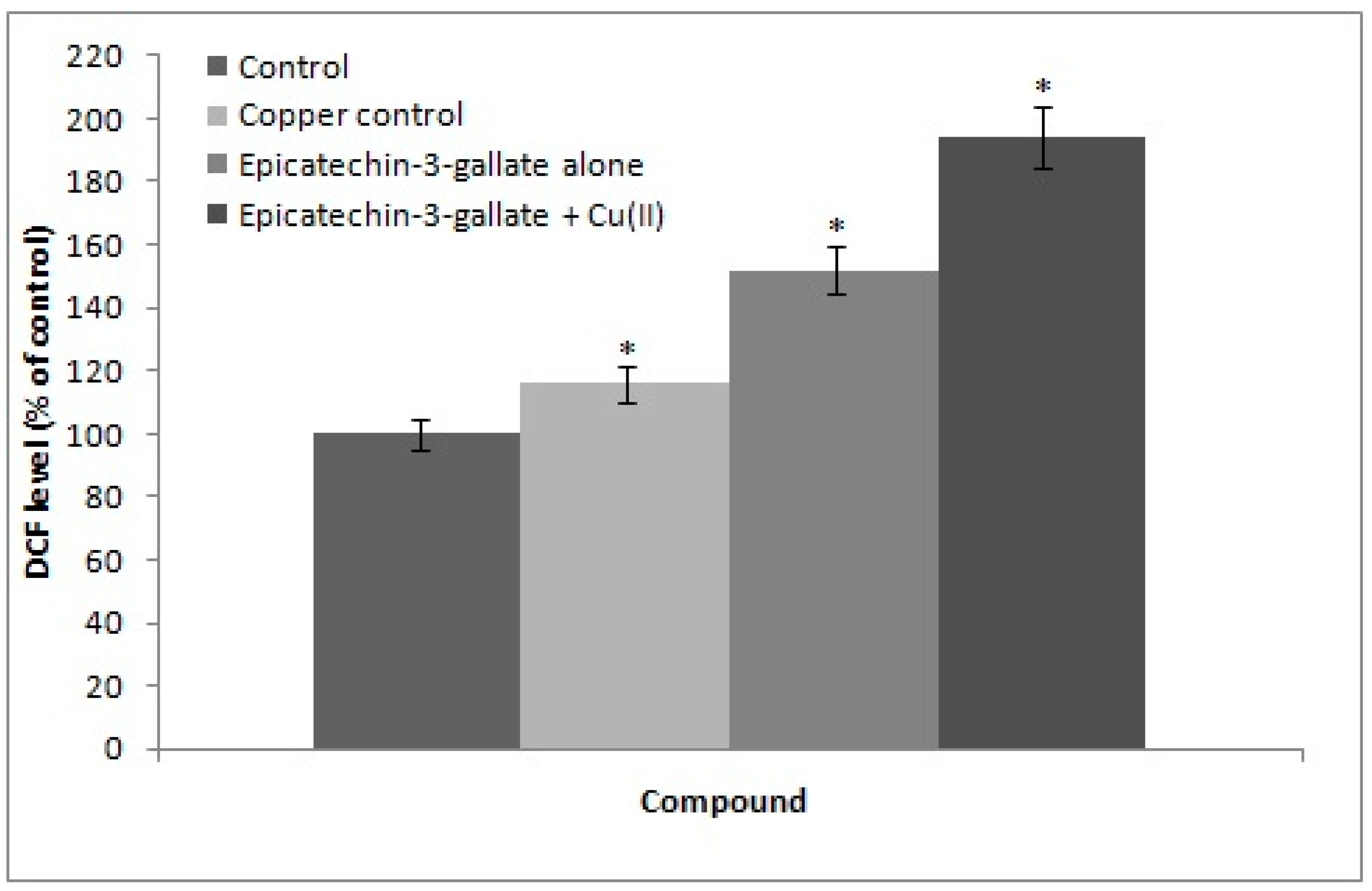
2.5. Cu(II) Mediated Formation of ROS: DCFH-DA Assay
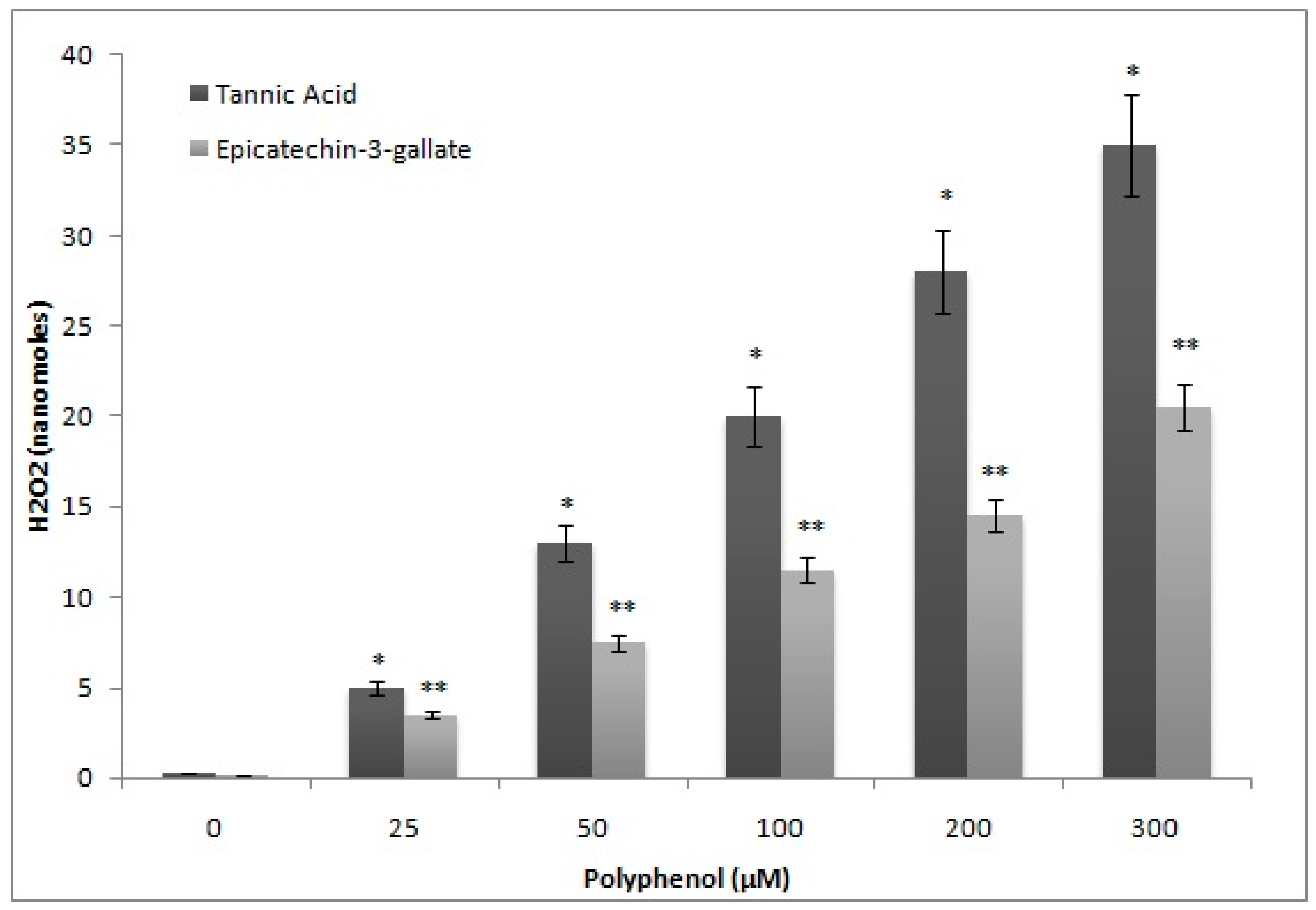
2.6. H2O2 Generation by Epicatechin-3-Gallate in the Incubation Medium
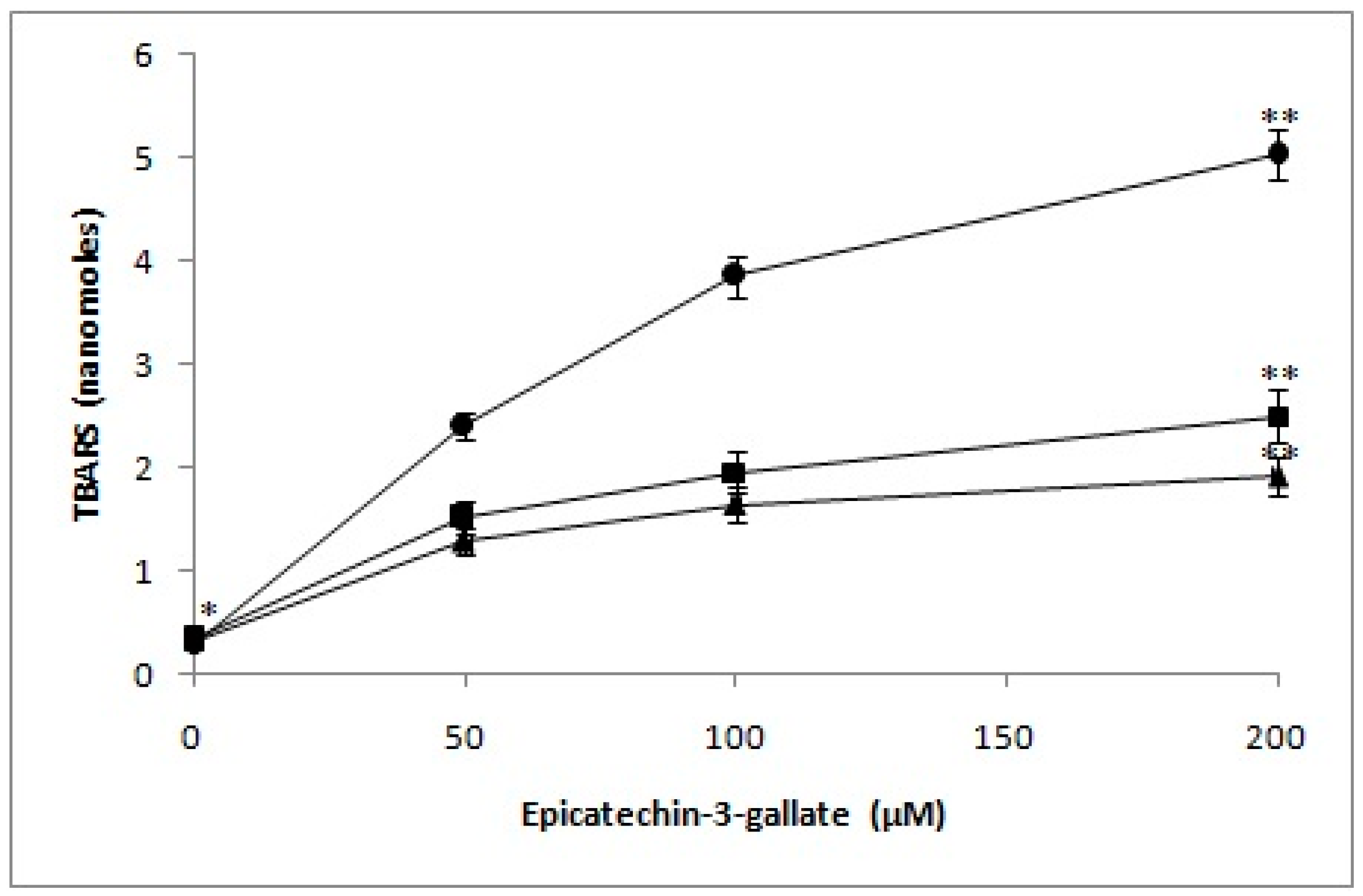
2.7. Determination of TBARS as a Measure of Oxidative Stress in Lymphocyte Nuclei by Epicatechin-3-Gallate in the Presence of Neocuproine and Thiourea
2.8. Epicatechin-3-Gallate Causes Inhibition of Cell Growth in Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Isolation of Lymphocytes
4.2. Viability Assessment of Lymphocytes
4.3. Treatment of Lymphocytes and Evaluation of DNA Breakage by Alkaline Single-Cell Gel Electrophoresis (Comet Assay)
4.4. Treatment of pBR322 DNA
4.5. Measurement of Intracellular ROS
4.6. Detection of H2O2 in the Incubation Medium by FOX Assay
4.7. Determination of TBARS
4.8. Cell Growth Inhibition as Studied by MTT Assay
4.9. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Mukhtar, H.; Das, M.; Khan, W.A.; Wang, Z.Y.; Bik, D.P.; Bickers, D.R. Exceptional activity of tannic acid among naturally occurring plant phenols in protecting against 7,12-dimethylbenz(a)anthracene-, benzo(a)pyrene-, 3-methylcholanthrene-, and N-methyl-N-nitrosourea-induced skin tumorigenesis in mice. Cancer Res. 1988, 48, 2361–2365. [Google Scholar] [PubMed]
- Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Antioxidant, gallic acid, induces apoptosis in HL-60RG cells. Biochem. Biophys. Res. Commun. 1994, 204, 898–904. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Feyes, D.K.; Nieminen, A.L.; Agarwal, R.; Mukhtar, H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl. Cancer Inst. 1997, 89, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.L.; Huang, T.S.; Lin, J.K. Curcumin, an antioxidant and antitumor promoter, induces apoptosis in human leukemia cells. Biochim. Biophys. Acta 1996, 1317, 95–100. [Google Scholar] [CrossRef]
- Clement, M.V.; Hirpara, J.L.; Chawdhury, S.H.; Pervaiz, S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998, 92, 996–1002. [Google Scholar] [PubMed]
- Said Ahmad, M.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of Cu(II): Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.S.; Hadi, S.M. Structural features of tannic acid important for DNA degradation in the presence of Cu(II). Mutagenesis 1998, 13, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef]
- Malik, A.; Azam, S.; Hadi, N.; Hadi, S.M. DNA degradation by water extract of green tea in the presence of copper ions: Implications for anticancer properties. Phytotherapyresearch 2003, 17, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; FarhanAsad, S.; Singh, S.; Hadi, S.M. DNA breakage by resveratrol and Cu(II): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000, 154, 29–37. [Google Scholar] [CrossRef]
- Kagawa, T.F.; Geierstanger, B.H.; Wang, A.H.; Ho, P.S. Covalent modification of guanine bases in double-stranded DNA. The 1.2-A Z-DNA structure of d(CGCGCG) in the presence of CuCl2. J. Biol. Chem. 1991, 266, 20175–20184. [Google Scholar] [PubMed]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Zhou, Y.; Zhang, H.; Demizu, Y.; Chen, Z.; Pelicano, H.; Chiao, P.J.; Achanta, G.; Arlinghaus, R.B.; Liu, J.; et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethylisothiocyanate. Cancer Cell 2006, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: Implications for anticancer properties. FEBS Lett. 2005, 579, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Shamim, U.; Hanif, S.; Ullah, M.F.; Azmi, A.S.; Bhat, S.H.; Hadi, S.M. Plant polyphenols mobilize nuclear copper in human peripheral lymphocytes leading to oxidatively generated DNA breakage: Implications for an anticancer mechanism. Free Radic. Res. 2008, 42, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Azmi, A.S.; Bhat, S.H.; Hanif, S.; Hadi, S.M. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: A putative mechanism for anticancer properties. FEBS Lett. 2006, 580, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Badwey, J.A.; Karnovsky, M.L. Active oxygen species and the functions of phagocytic leukocytes. Annu. Rev. Biochem. 1980, 49, 695–726. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechingallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Hadi, S.M. DNA breakage by tannic acid and Cu(II): Sequence specificity of the reaction and involvement of active oxygen species. Mutat. Res. 1994, 313, 39–48. [Google Scholar] [CrossRef]
- Smith, C.; Halliwell, B.; Aruoma, O.I. Protection by albumin against the pro-oxidant actions of phenolic dietary components. Food Chem. Toxicol. 1992, 30, 483–489. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Gutteridge, J.M. Oxygen radical damage to DNA by rifamycin SV and copper ions. Biochem. Pharmacol. 1987, 36, 3629–3633. [Google Scholar] [CrossRef]
- Khan, H.Y.; Zubair, H.; Faisal, M.; Ullah, M.F.; Farhan, M.; Sarkar, F.H.; Ahmad, A.; Hadi, S.M. Plant polyphenol induced cell death in human cancer cells involves mobilization of intracellular copper ions and reactive oxygen species generation: A mechanism for cancer chemopreventive action. Mol. Nutr. Food Res. 2014, 58, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Raffoul, J.J.; Kucuk, O.; Sarkar, F.H.; Hillman, G.G. Dietary agents in cancer chemoprevention and treatment. J. Oncol. 2012, 2012, 749310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Schell, J.B.; Ho, C.T.; Chen, K.Y. Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett. 1998, 129, 173–179. [Google Scholar] [CrossRef]
- Michels, G.; Watjen, W.; Weber, N.; Niering, P.; Chovolou, Y.; Kampkotter, A.; Proksch, P.; Kahl, R. Resveratrol induces apoptotic cell death in rat H4IIE hepatoma cells but necrosis in C6 glioma cells. Toxicology 2006, 225, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Gu, M.; Takahata, T.; Frederick, B.; Agarwal, C.; Siriwardana, S.; Agarwal, R.; Sclafani, R.A. Resveratrol selectively induces DNA damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin. Cancer Res. 2011, 17, 5402–5411. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Medina, I.; Ortega, A.; Carretero, J.; Bano, M.C.; Obrador, E.; Estrela, J.M. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic. Biol. Med. 2002, 33, 387–398. [Google Scholar] [CrossRef]
- Amin, A.R.; Wang, D.; Zhang, H.; Peng, S.; Shin, H.J.; Brandes, J.C.; Tighiouart, M.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Enhanced anti-tumor activity by the combination of the natural compounds (−)-epigallocatechin-3-gallate and luteolin: Potential role of p53. J. Biol. Chem. 2010, 285, 34557–34565. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [PubMed]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Boil. 2013, 3, 120144–120153. [Google Scholar] [CrossRef] [PubMed]
- Pool-Zobel, B.L.; Guigas, C.; Klein, R.; Neudecker, C.; Renner, H.W.; Schmezer, P. Assessment of genotoxic effects by lindane. Food Chem. Toxicol. 1993, 31, 271–283. [Google Scholar] [CrossRef]
- Czene, S.; Tiback, M.; Harms-Ringdahl, M. pH-dependent DNA cleavage in permeabilized human fibroblasts. Biochem. J. 1997, 323, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Ramanathan, R.; Das, N.P.; Tan, C.H. Effects of gamma-linolenic acid, flavonoids, and vitamins on cytotoxicity and lipid peroxidation. Free Radic. Biol. Med. 1994, 16, 43–48. [Google Scholar] [CrossRef]
- Tice, R.R.; Strauss, G.H. The single cell gel electrophoresis/comet assay: A potential tool for detecting radiation-induced DNA damage in humans. Stem Cells 1995, 13, 207–214. [Google Scholar] [PubMed]


| Dose | Comet Tail Length (µm) | % Inhibition of Tail Length (ECG Alone − Scavenger)/ECG Alone × 100 |
|---|---|---|
| Control (untreated) | 2.67 ± 0.18 | - |
| Epicatechin-3-gallate (50 µM) | 22.91 ± 0.81 # | - |
| +SOD (100 µg/mL) | 6.19 ± 0.46 * | 72.98 |
| +Catalase (100 µg/mL) | 9.01 ± 0.63 * | 60.67 |
| +Thiourea (1 mM) | 8.23 ± 0.68 * | 64.07 |
| Dose | Permeabilized Cells | Intact Cells | ||
|---|---|---|---|---|
| Comet Tail Length (µm) | % of Control | Comet Tail Length (µm) | % of Control | |
| Control | 2.83 ± 0.19 1 | - | 2.45 ± 0.15 1 | - |
| Epicatechin-3-gallate (50 µM) | 13.17 ± 0.79 2 | - | 22.16 ± 1.56 3 | - |
| +Neocuprione (50 µM) | 8.24 ± 0.21 3 | 37.43 | 10.48 ± 0.31 3 | 52.70 |
| +Bathocuprione (50 µM) | 8.96 ± 0.35 3 | 31.96 | 20.54 ± 1.04 3 | 7.31 |
| +Hisitidine (50 µM) | 12.60 ± 0.66 3 | 4.32 | 21.31 ± 1.52 3 | 3.83 |
| +Desferioxamine mesylate (50 µM) | 12.67 ± 0.68 3 | 3.79 | 20.78 ± 1.49 3 | 6.22 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; Oves, M.; Chibber, S.; Hadi, S.M.; Ahmad, A. Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy. Int. J. Mol. Sci. 2017, 18, 34. https://doi.org/10.3390/ijms18010034
Farhan M, Oves M, Chibber S, Hadi SM, Ahmad A. Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy. International Journal of Molecular Sciences. 2017; 18(1):34. https://doi.org/10.3390/ijms18010034
Chicago/Turabian StyleFarhan, Mohd, Mohammad Oves, Sandesh Chibber, Sheikh Mumtaz Hadi, and Aamir Ahmad. 2017. "Mobilization of Nuclear Copper by Green Tea Polyphenol Epicatechin-3-Gallate and Subsequent Prooxidant Breakage of Cellular DNA: Implications for Cancer Chemotherapy" International Journal of Molecular Sciences 18, no. 1: 34. https://doi.org/10.3390/ijms18010034







