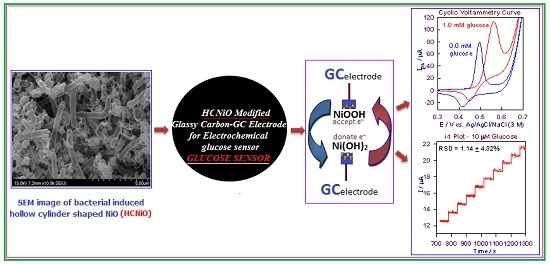
Bacteria-Templated NiO Nanoparticles/Microstructure for an Enzymeless Glucose Sensor
Abstract
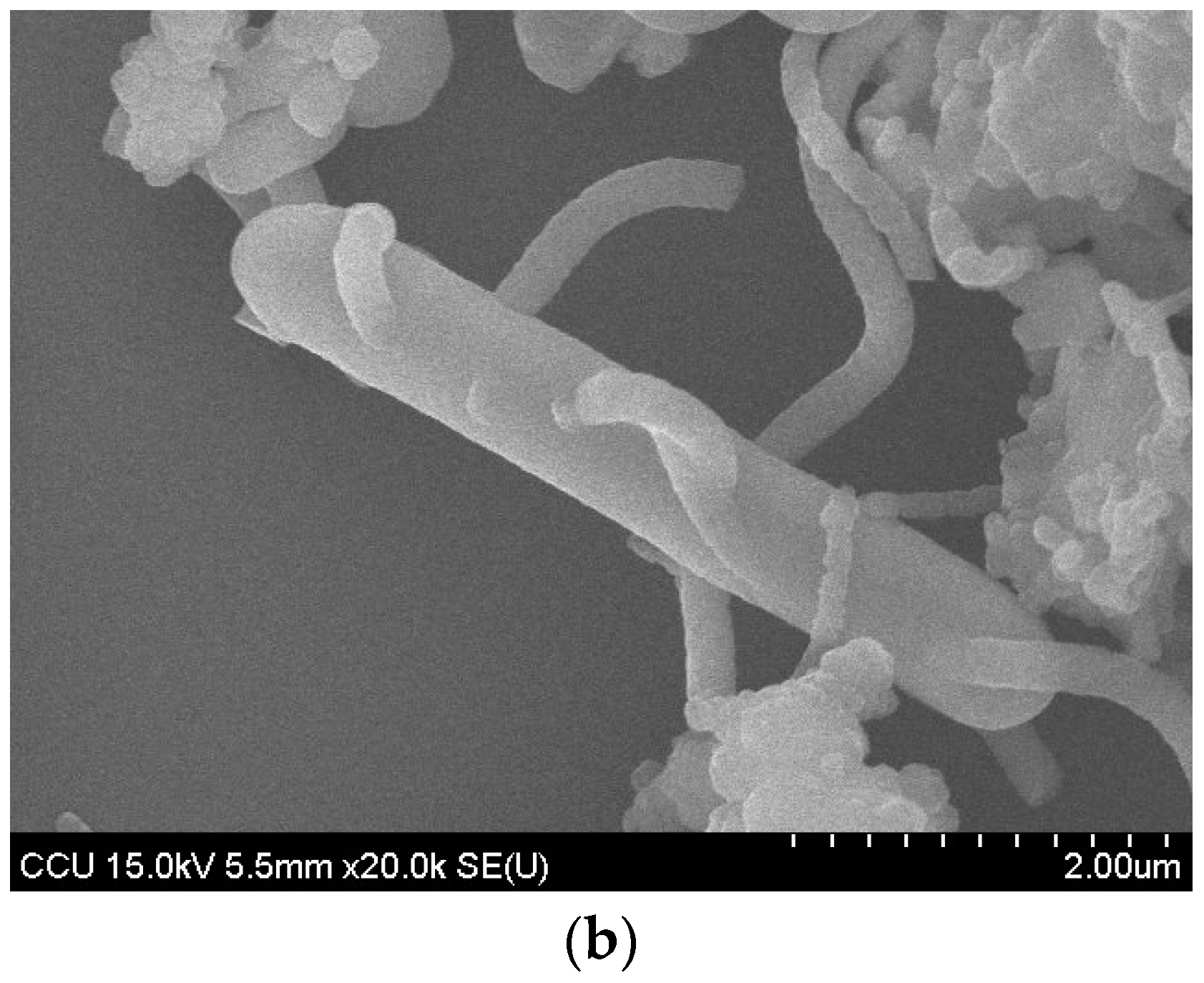
:1. Introduction
2. Results and Discussions
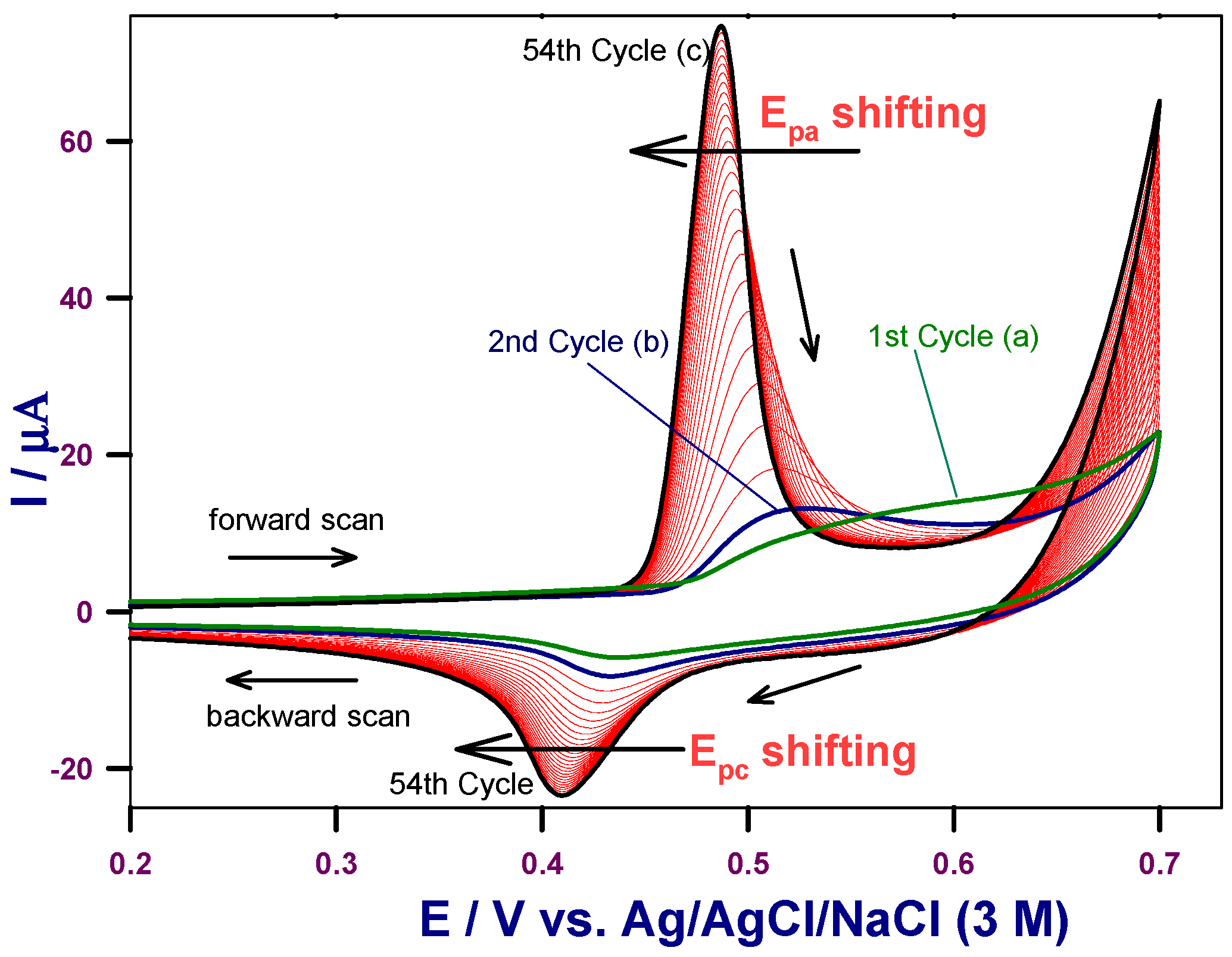
2.1. Electrochemical Properties of the HCNiO/GC-Modified Electrode
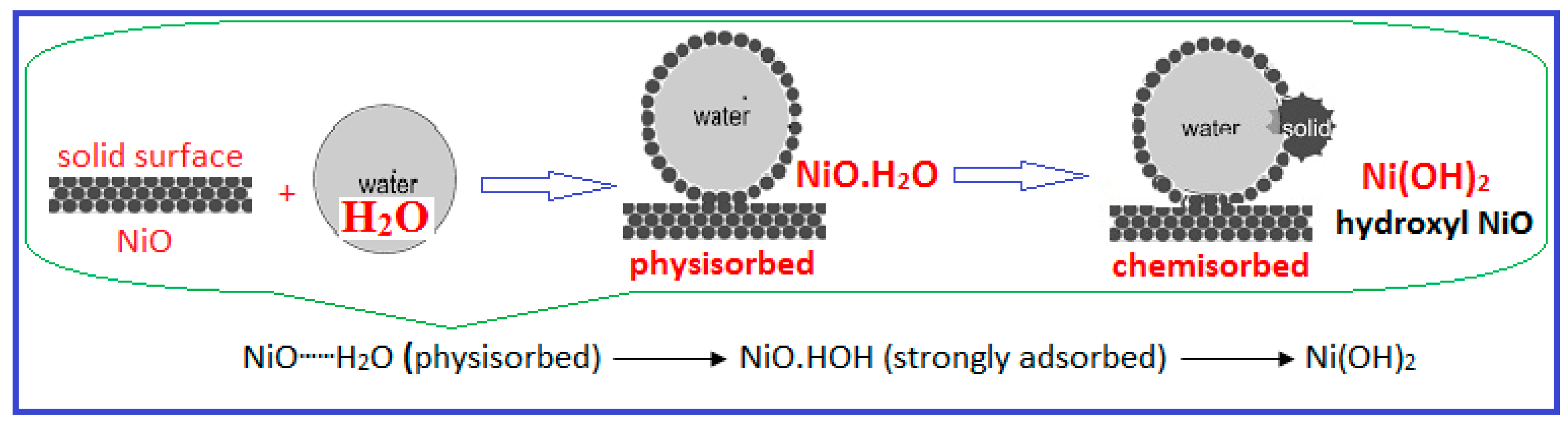
2.2. Electrochemical Surface Characterization of the HCNiO/GC Modified Electrode
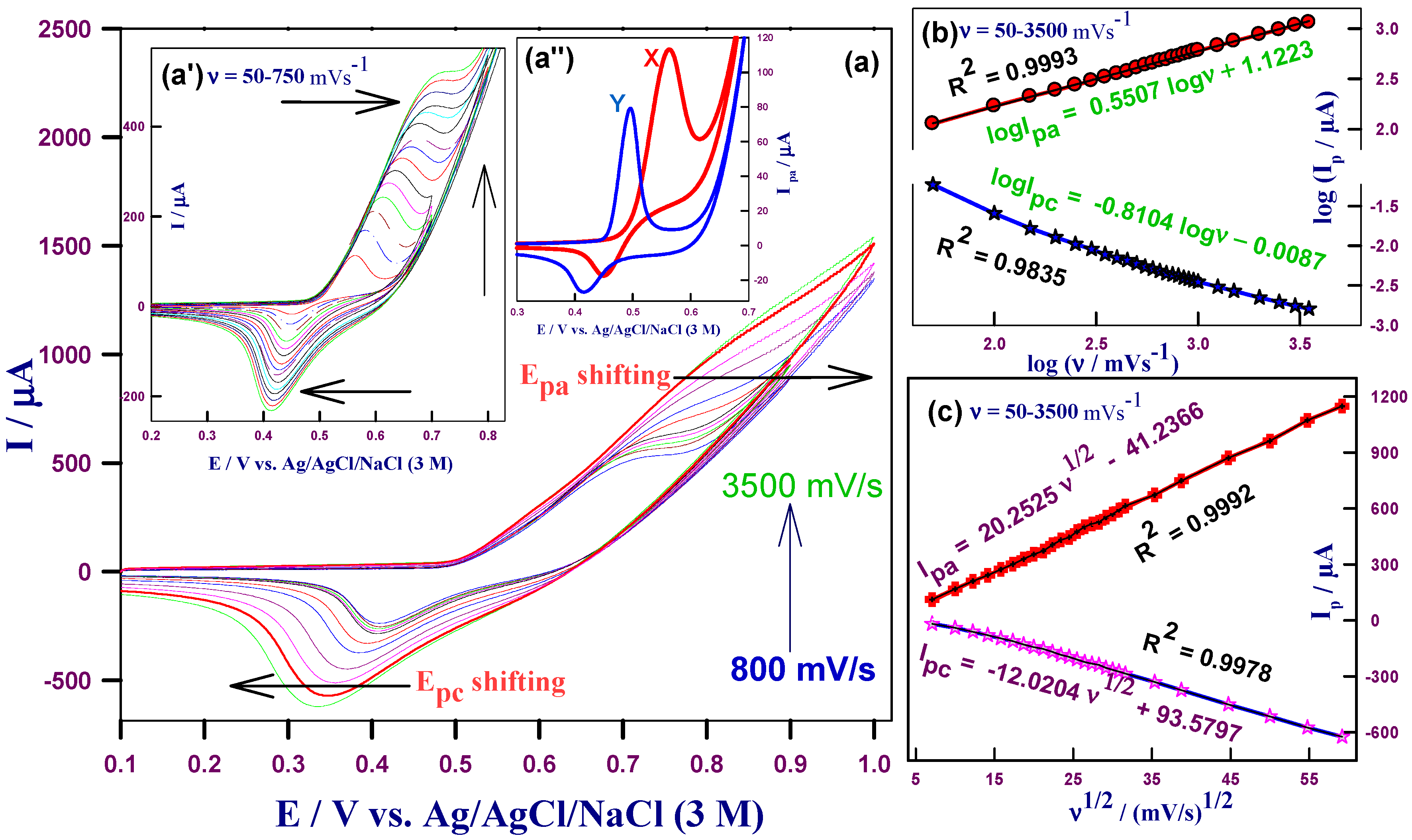
2.2.1. Effect of Scan Rates in the Blank Supporting Electrolyte
2.2.2. Effect of Scan Rates and Electrocatalytic Effect in the Basic Glucose Solution
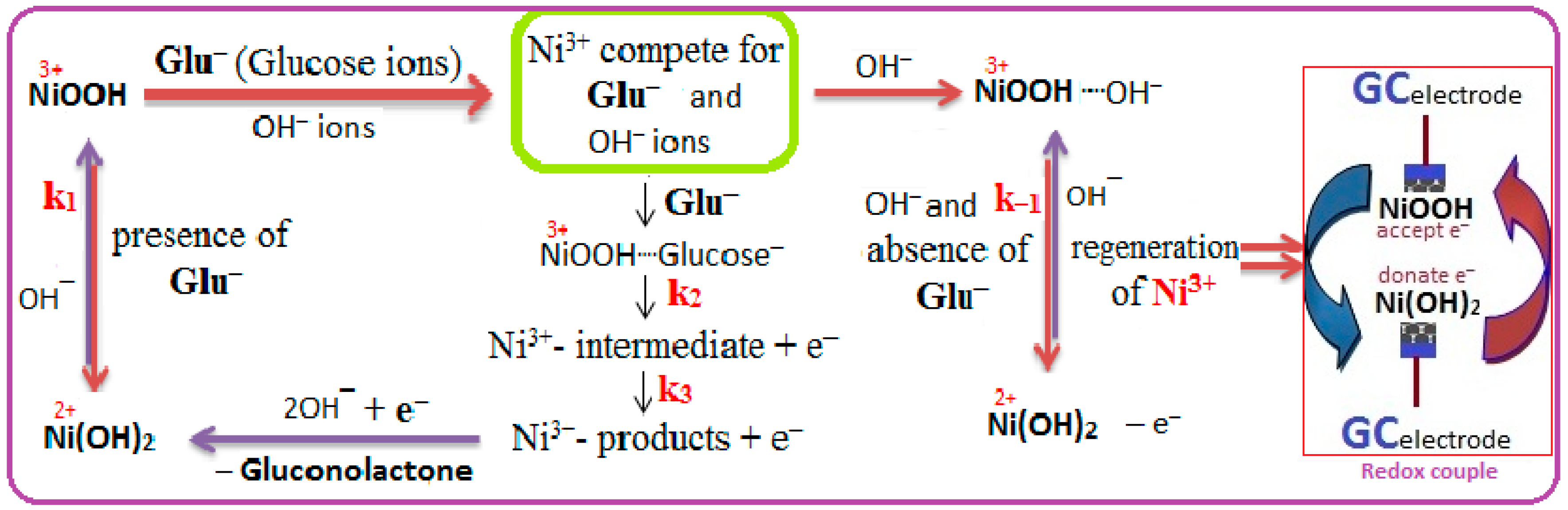
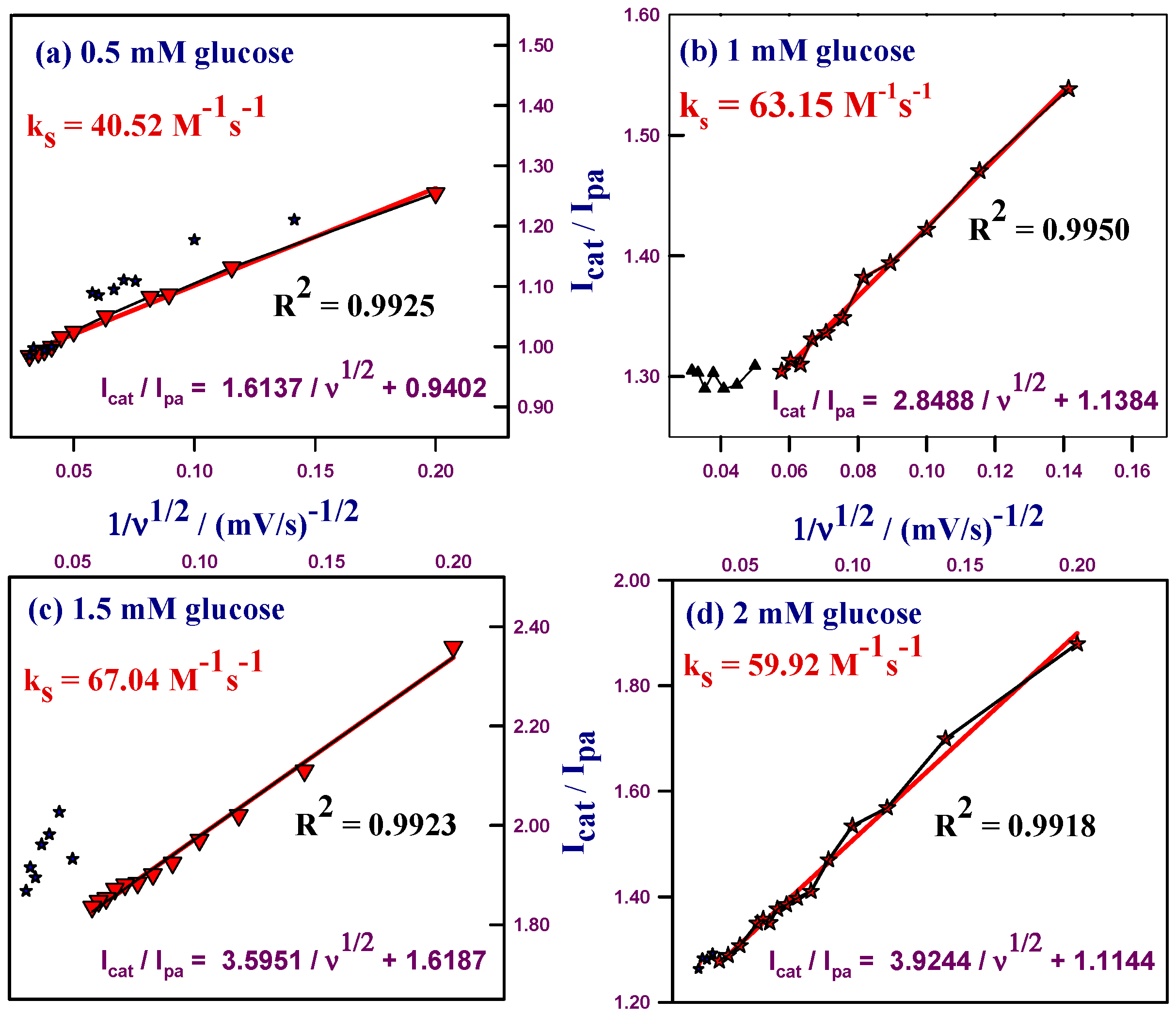
2.2.3. Kinetic Studies of HCNiO/GC Electrode in the Basic Glucose Solution
2.3. Electrocatalytic Determination of Glucose by a HCNiO/GC Electrode
2.3.1. Cyclic Voltammetric (CV) Method for the Detection of Glucose
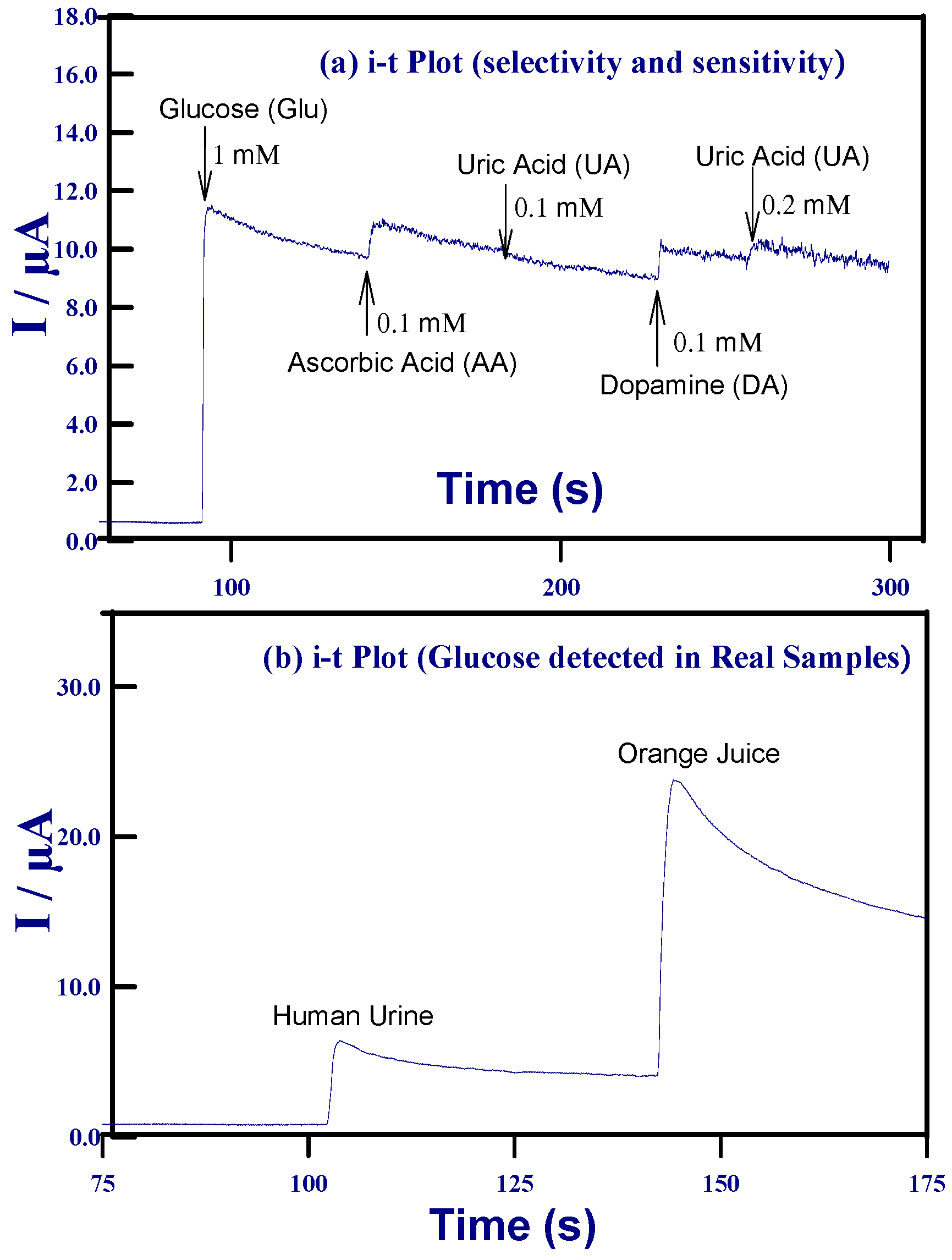
2.3.2. Amperometric i–t Detection of Glucose
3. Materials and Methods
3.1. Reagents
3.2. Electrochemical Measurements
3.3. Preparation of Hollow Cylinder NiO Nanostructured Material
3.4. HCNiO/GC Electrode Preparation and Its Activation by CV Cycle
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Deplanche, K.; Macaskie, L.E. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol. Bioeng. 2008, 99, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of Nanoclusters and Formation of Submicron Crystallites Assisted by Lactobacillus Strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Haefeli, C.; Franklin, C.; Hardy, K. Plasmid-determined silver resistance in Pseudomonas stutzeri isolated from a silver mine. J. Bacteriol. 1984, 158, 389–392. [Google Scholar] [PubMed]
- Holmes, J.D.; Smith, P.R.; Gowing, R.E.; Richardson, D.J.; Russell, D.A.; Sodeau, J.R. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch. Microbiol. 1995, 163, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Philipse, A.P.; Maas, D. Magnetic colloids from magnetotactic bacteria: chain formation and colloidal stability. Langmuir 2002, 18, 9977–9984. [Google Scholar] [CrossRef]
- Casella, I.G.; Cataldi, T.R.I.; Salvi, A.M.; Desimoni, E. Electrocatalytic oxidation and liquid chromatographic detection of aliphatic alcohols at a nickel-based glassy carbon modified electrode. Anal. Chem. 1993, 65, 3143–3150. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical glucose sensors and their applications in diabetes management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; He, J. Nonenzymatic Glucose Sensors. Prog. Chem. 2015, 27, 585–593. [Google Scholar]
- Ahmad, R.; Vaseem, M.; Tripathy, N.; Hahn, Y.B. Wide linear-range detecting nonenzymatic glucose biosensor based on CuO nanoparticles inkjet-printed on electrodes. Anal. Chem. 2013, 85, 10448–10454. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.; Sutariya, P.G.; Menon, S.K. A non enzymatic glucose biosensor based on an ultrasensitive calix[4]arene functionalized boronic acid gold nanoprobe for sensing in human blood serum. Analyst 2013, 138, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Lu, Q.; Wang, J.; Li, Y.; Gao, F. Glucose-assisted synthesis of copper micropuzzles and their application as nonenzymatic glucose sensors. Chem. Commun. 2010, 46, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, Z.H.; Khun, K.; Beni, V.; Willander, M. Non-Enzymatic Glucose Sensor Based on the Novel Flower Like Morphology of Nickel Oxide. Soft Nanosci. Lett. 2013, 3, 46–50. [Google Scholar] [CrossRef]
- Fang, B.; Gu, A.; Wang, G.; Wang, W.; Feng, Y.; Zhang, C.; Zhang, X. Silver Oxide Nanowalls Grown on Cu Substrate as an Enzymeless Glucose Sensor. ACS Appl. Mater. Interfaces 2009, 1, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Thomas, D.F.; Chen, A. Nonenzymatic Electrochemical Glucose Sensor Based on Nanoporous PtPb Networks. Anal. Chem. 2008, 80, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Jia-Hong, H.; Qiang, X.; Zhong-Rong, S.; Hai-Yan, K. Study on non-enzymatic glucose sensor based on a Ag2O nanoparticles self-assembled Ag electrode. In Proceedings of the 2010 International Conference on Electrical and Control Engineering, Wuhan, China, 25–27 June 2010; pp. 2109–2111.
- Lei, J.; Liu, Y.; Wang, X.; Hua, P.; Peng, X. Au/CuO nanosheets composite for glucose sensor and CO oxidation. RSC Adv. 2015, 5, 9130–9137. [Google Scholar] [CrossRef]
- Thiagarajan, S.; Chen, S.M. Preparation and characterization of PtAu hybrid film modified electrodes and their use in simultaneous determination of dopamine, ascorbic acid and uric acid. Talanta 2007, 74, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, F.; Chen, Z. Electrochemical glucose sensor based on one-step construction of gold nanoparticle–chitosan composite film. Sens. Actuators B 2009, 138, 539–544. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Yang, W.; Zhou, M.; Hu, X. Facile One-Step Microwave-Assisted Route towards Ni Nanospheres/Reduced Graphene Oxide Hybrids for Non-Enzymatic Glucose Sensing. Sensors 2012, 12, 4860–4869. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, B.; Zhang, T. A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochim. Acta 2013, 102, 104–107. [Google Scholar] [CrossRef]
- Kumar, D.R.; Manoj, D.; Santhanalakshmi, J. Au–ZnO bullet-like heterodimer nanoparticles: synthesis and use for enhanced nonenzymatic electrochemical determination of glucose. RSC Adv. 2014, 4, 8943–8952. [Google Scholar] [CrossRef]
- Park, B.; Cairns, E.J. Electrochemical performance of TiO2 and NiO as fuel cell electrode additives. Electrochem. Commun. 2011, 13, 75–77. [Google Scholar] [CrossRef]
- Needham, S.A.; Wang, G.X.; Liu, H.K. Synthesis of NiO nanotubes for use as negative electrodes in lithium ion batteries. J. Power Sources 2006, 159, 254–257. [Google Scholar] [CrossRef]
- Pang, H.; Lu, Q.; Zhang, Y.; Li, Y.; Gao, F. Selective synthesis of nickel oxide nanowires and length effect on their electrochemical properties. Nanoscale 2010, 2, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, Y.; Wakabayashi, N.; Yamazaki, J.; Yamada, S.; Kimishima, Y.; Komatsu, E.; Tajima, H. Magnetic properties of NiO nanoparticles. Phys. B 2003, 329, 862–863. [Google Scholar] [CrossRef]
- Ren, S.; Yang, C.; Sun, C.; Hui, Y.; Dong, Z.; Wang, J.; Su, X. Novel NiO nanodisks and hollow nanodisks derived from Ni(OH)2 nanostructures and their catalytic performance in epoxidation of styrene. Mater. Lett. 2012, 80, 23–25. [Google Scholar] [CrossRef]
- Fernando, L.M.; Merca, F.E.; Paterno, E.S. Biogenic synthesis of gold nanoparticles by plant-growth-promoting bacteria isolated from philippine soils. Philipp. Agric. Sci. 2013, 96, 129–136. [Google Scholar]
- Millo, C.; Ader, M.; Dupraz, S.; Guyot, F.; Thaler, C.; Foy, E.; Ménez, B. Carbon isotope fractionation during calcium carbonate precipitation induced by ureolytic bacteria. Geochim. Cosmochim. Acta 2012, 98, 107–124. [Google Scholar] [CrossRef]
- Chahal, N.; Rajor, A.; Siddique, R. Calcium carbonate precipitation by different bacterial strains. Afr. J. Biotechnol. 2011, 10, 8359–8372. [Google Scholar]
- Xing, W.; Li, F.; Yan, Z.F.; Lu, G.Q. Synthesis and electrochemical properties of mesoporous nickel oxide. J. Power Sources 2004, 134, 324–330. [Google Scholar] [CrossRef]
- Lyons, M.; Brandon, M. The Oxygen Evolution Reaction on Passive Oxide Covered Transition Metal Electrodes in Aqueous Alkaline Solution. Part 1-Nickel. Int. J. Electrochem. Sci. 2008, 3, 1386–1424. [Google Scholar]
- Kuroda, Y.; Hamano, H.; Mori, T.; Yoshikawa, Y.; Nagao, M. Specific Adsorption Behavior of Water on a Y2O3 Surface. Langmuir 2000, 16, 6937–6947. [Google Scholar] [CrossRef]
- Kumary, V.A.; Nancy, T.E.M.; Divya, J.; Sreevalsan, K. Nonenzymatic Glucose Sensor: Glassy Carbon Electrode Modified with Graphene-Nickel/Nickel Oxide Composite. Int. J. Electrochem. Sci. 2013, 8, 2220–2228. [Google Scholar]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.L. Nano nickel oxide modified non- enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.S.; Yang, C.H.; Wan, M.J. Morphological and structural studies of nanoporous nickel oxide films fabricated by anodic electrochemical deposition Techniques. Electrochim. Acta 2008, 54, 155–161. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Gao, R. Ti/TiO2 Nanotube Array/Ni Composite Electrodes for Nonenzymatic Amperometric Glucose Sensing. J. Phys. Chem. C 2010, 114, 4408–4413. [Google Scholar] [CrossRef]
- Salimi, A.; Abdi, K.; Khayatiyan, R. Preparation and electrocatalytic oxidation properties of a nickel pentacyanonitrosylferrate modified carbon composite electrode by two-step sol–gel technique: improvement of the catalytic activity. Electrochim. Acta 2004, 49, 413–422. [Google Scholar] [CrossRef]
- Hamdan, M.S.; Nordin, N.; Amir, S.F.M.; Riyanto; Othman, M.R. Electrochemical Behaviour of Ni and Ni-PVC Electrodes for the Electroxidation of Ethanol. Sains Malays. 2011, 40, 1421–1427. [Google Scholar]
- Kibria, A.K.M.F.; Tarafdar, S.A. Electrochemical studies of a nickel–copper electrode for the oxygen evolution reaction (OER). Int. J. Hydrog. Energy 2002, 27, 879–884. [Google Scholar] [CrossRef]
- Ender, B.; Pakize, C. Electrochemical behaviuor of the antibiotic drug Novobiocin sodium on a mercury electrode. Croat. Chem. Acta 2009, 82, 573–582. [Google Scholar]
- Danaee, I.; Jafarian, M.; Forouzandeh, F.; Gobal, F.; Mahjani, M.G. Impedance spectroscopy analysis of glucose electro-oxidation on Ni-modified glassy carbon electrode. Electrochim. Acta 2008, 53, 6602–6609. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, C.; Li, M.; Jiao, K. Flow-injection analysis of glucose without enzyme based on electrocatalytic oxidation of glucose at a nickel electrode. Talanta 2007, 71, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Ojani, R.; Raoof, J.B.; Afagh, P.S. Electrocatalytic oxidation of some carbohydrates by poly(1-naphthylamine)/nickel modified carbon paste electrode. J. Electroanal. Chem. 2004, 571, 1–8. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.G.; Heli, H.; Gobal, F.; Heydarpoor, M. Electrocatalytic oxidation of methane at nickel hydroxide modified nickel electrode in alkaline solution. Electrochem. Commun. 2003, 5, 184–188. [Google Scholar] [CrossRef]
- Elahi, M.Y.; Heli, H.; Bathaie, S.Z.; Mousavi, M.F. Electrocatalytic oxidation of glucose at a Ni-curcumin modified glassy carbon electrode. J. Solid State Electrochem. 2007, 11, 273–282. [Google Scholar] [CrossRef]
- Muthuraman, G.; Pillai, K.C. Electrocatalysis in micellar media: Indirect reduction of allylchloride in cationic surfactant cetrimide containing aqueous solution. Bull. Electrochem. 1999, 15, 448–451. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical methods, Fundamentals and Applications, 2nd ed.; John Wiley: New York, NY, USA, 2001; pp. 96–108. [Google Scholar]
- Golabi, S.M.; Zare, H.R. Electrocatalytic oxidation of hydrazine at a chlorogenic acid (CGA) modified glassy carbon electrode. J. Electroanal. Chem. 1999, 465, 168–176. [Google Scholar] [CrossRef]
- Acelino, C.S.; Leonardo, L.P.; Nelson, R.S. Sugars Electrooxidation at Glassy Carbon Electrode Decorate with Multi-Walled Carbon Nanotubes with Nickel OxyHydroxide. Int. J. Electrochem. Sci. 2014, 9, 7746–7762. [Google Scholar]
- Noel, M.; Vasu, K.I. Cyclic Voltammetry and the Frontiers of Electrochemistry; Oxford and IBH publishing Co. Pvt. Ltd.: New Delhi, India, 1990; pp. 272–304. [Google Scholar]
- Shamsipur, M.; Najafi, M.; Hosseini, M.R.M. Highly improved electrooxidation of glucose at a nickel(II) oxide/multi-walled carbon nanotube modified glassy carbon electrode. Bioelectrochemistry 2010, 77, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Feng, M.J.; Quangui, G.; Quan, H.Y.; Feiyu, K. DNA dispersed graphene/NiO hybrid materials for highly sensitive non-enzymatic glucose sensor. Electrochim. Acta 2012, 73, 129–135. [Google Scholar]
- Atla, S.B.; Chen, C.Y.; Fu, C.W.; Chien, T.C.; Sun, A.C.; Huang, C.F.; Lo, C.J. Microbial induced synthesis of hollow cylinder and helical NiO micro/nanostructure. MRS Commun. 2014, 4, 121–127. [Google Scholar] [CrossRef]



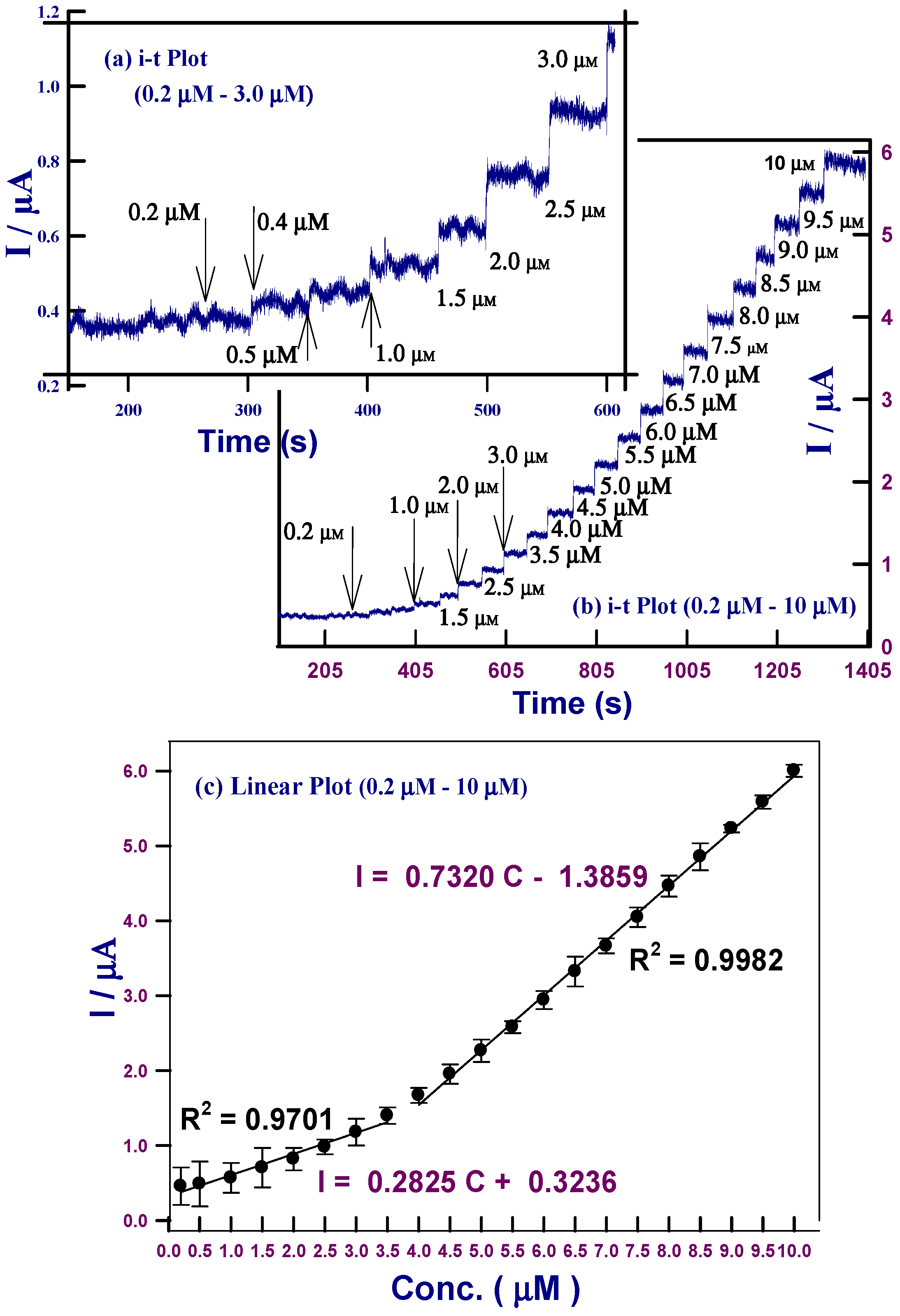


| Serial Number | Modified GC Electrodes | Linear Range (µM) | Sensitivity | LOD (µM) | Year | Reference |
|---|---|---|---|---|---|---|
| 1 | HCNiO/GC | 0.2–3.5 0.5–250 | 3978.9 µA·mM−1·cm−2 1232.4 µA·mM−1·cm−2 | 0.9 | 2016 | This work |
| 2 | Ni-rGO/GC | 1.0–10 | 937 µA·mM−1·cm−2 | - | 2012 | [23] |
| 3 | NiO/MWCNT/GC | 200–12,000 | - | 160 | 2010 | [55] |
| 4 | NiOOH/MWCNT/GC | 250–5600 | 1.5 × 10−5 µA·M−1 | 190 | 2014 | [53] |
| 5 | GNS/NiO/DNA-GC | 1–200 | 14.3 µA·mM−1·cm−2 | 2.5 | 2012 | [56] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaidyanathan, S.; Cherng, J.-Y.; Sun, A.-C.; Chen, C.-Y. Bacteria-Templated NiO Nanoparticles/Microstructure for an Enzymeless Glucose Sensor. Int. J. Mol. Sci. 2016, 17, 1104. https://doi.org/10.3390/ijms17071104
Vaidyanathan S, Cherng J-Y, Sun A-C, Chen C-Y. Bacteria-Templated NiO Nanoparticles/Microstructure for an Enzymeless Glucose Sensor. International Journal of Molecular Sciences. 2016; 17(7):1104. https://doi.org/10.3390/ijms17071104
Chicago/Turabian StyleVaidyanathan, Settu, Jong-Yuh Cherng, An-Cheng Sun, and Chien-Yen Chen. 2016. "Bacteria-Templated NiO Nanoparticles/Microstructure for an Enzymeless Glucose Sensor" International Journal of Molecular Sciences 17, no. 7: 1104. https://doi.org/10.3390/ijms17071104