Dual-Labeled Near-Infrared/99mTc Imaging Probes Using PAMAM-Coated Silica Nanoparticles for the Imaging of HER2-Expressing Cancer Cells
Abstract
:1. Introduction
2. Results
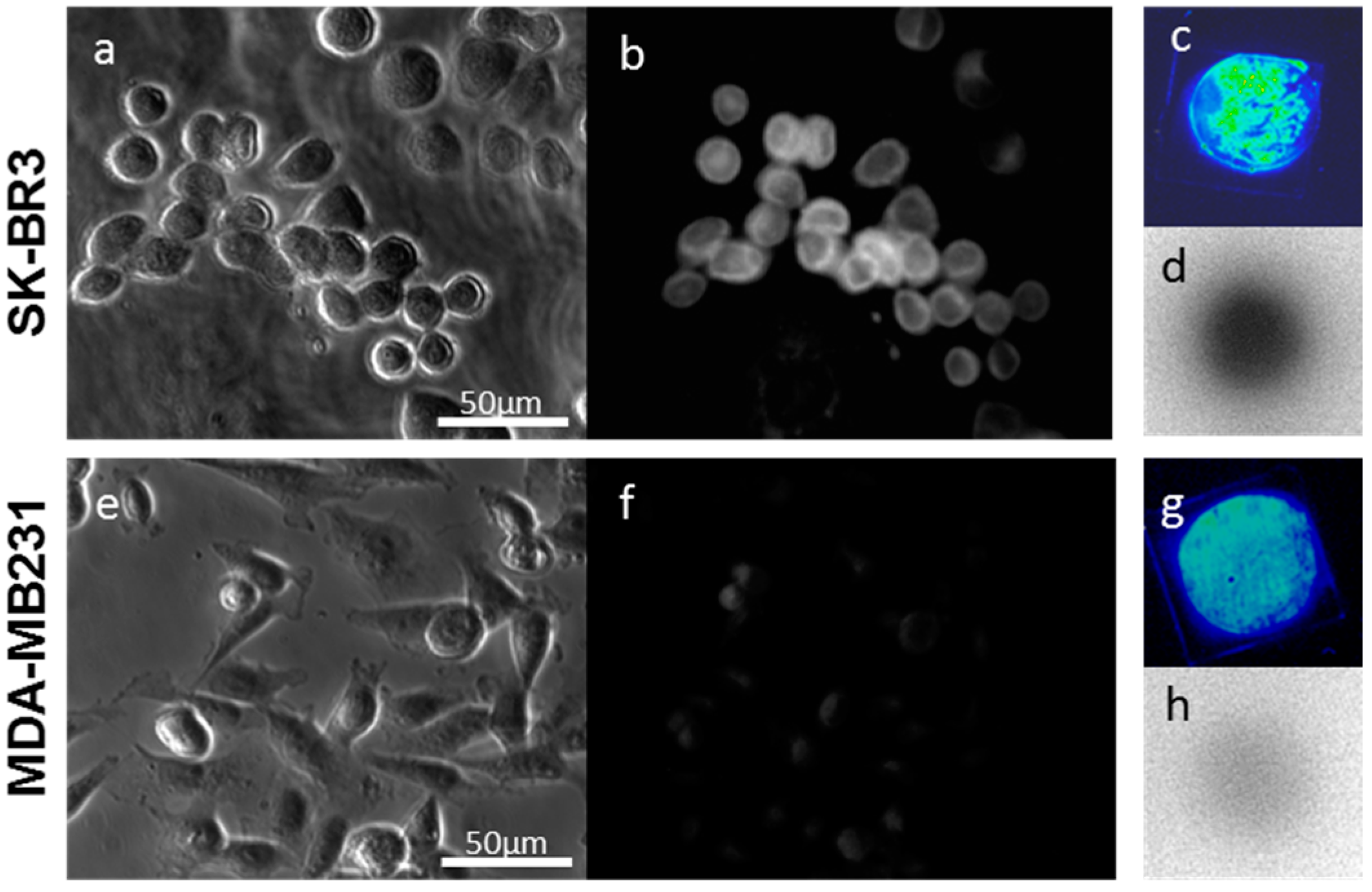
2.1. In Vitro
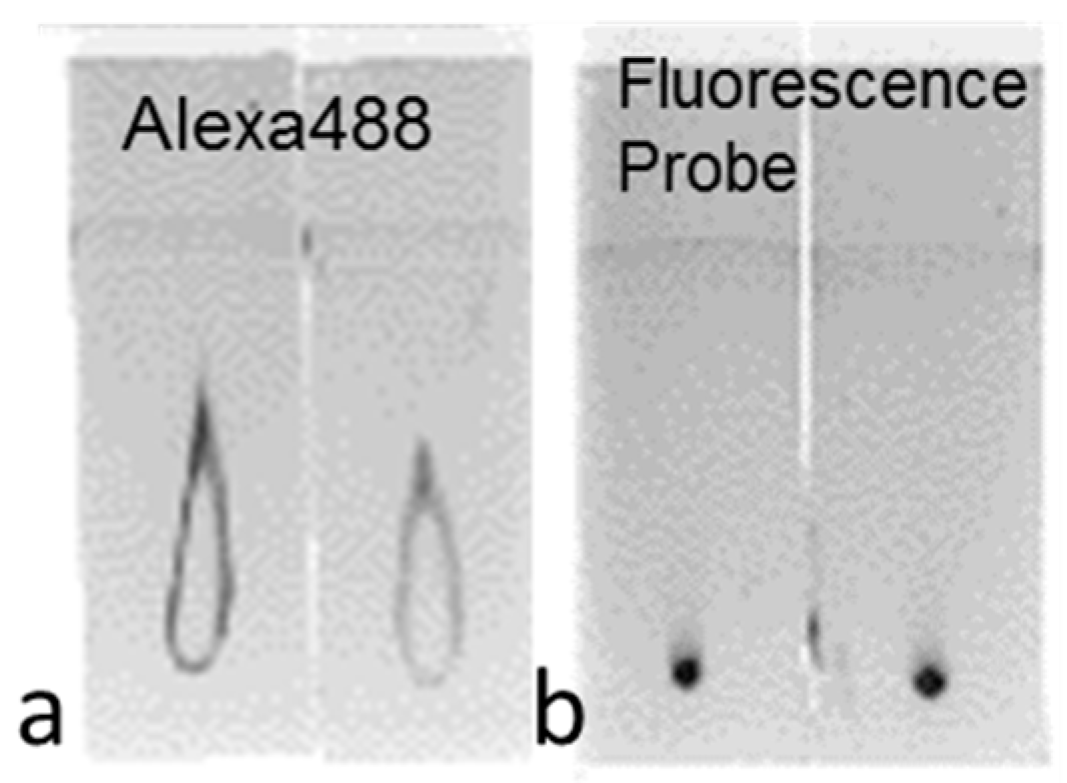
2.1.1. Imaging of Human Epidermal Growth Factor Receptor 2 (HER2)-Overexpressing Cells (SK-BR3) Using Fluorescent Probes
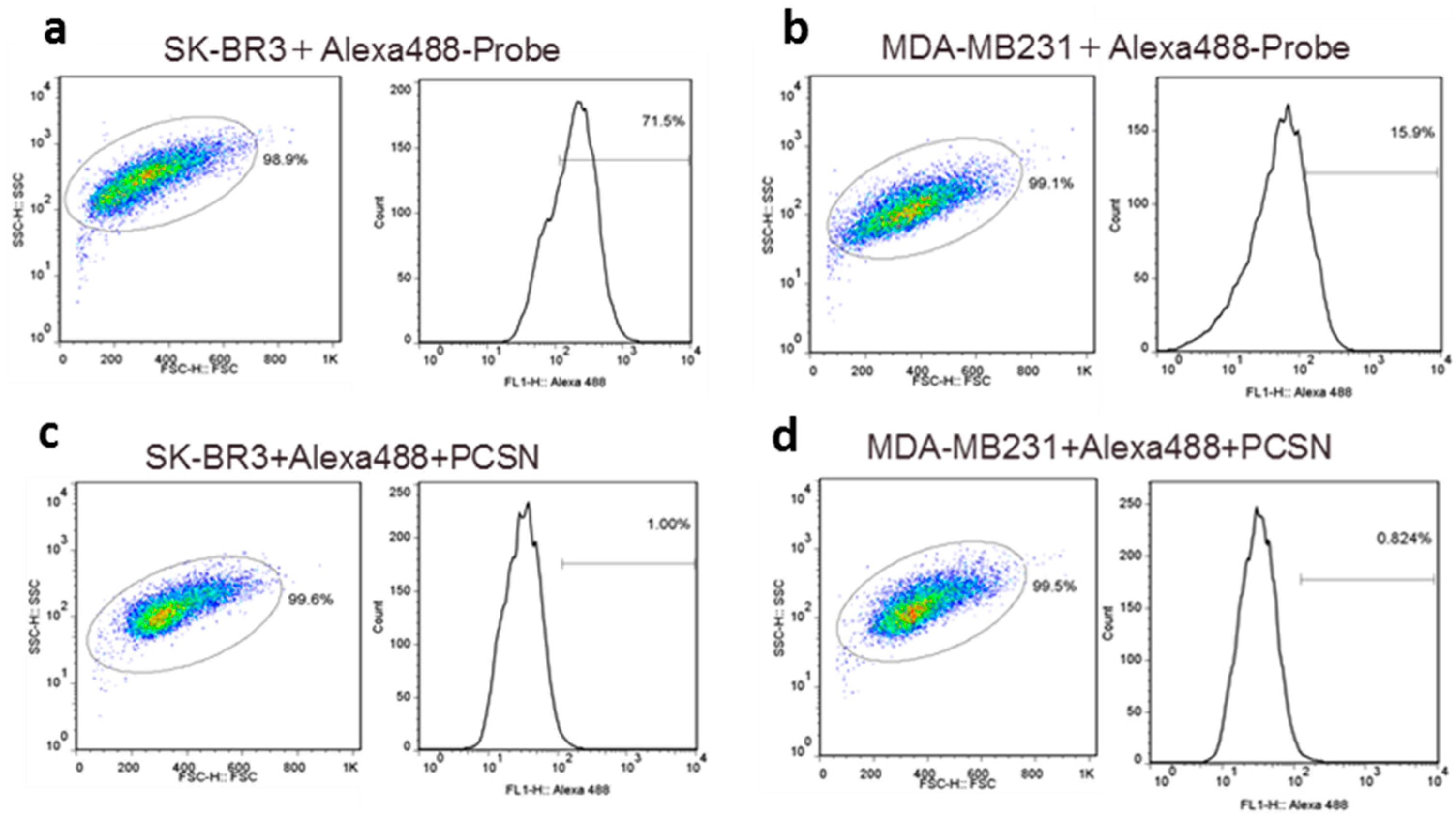
2.1.2. Imaging of HER2-Overexpressing Cells Using Dual-Imaging Probes
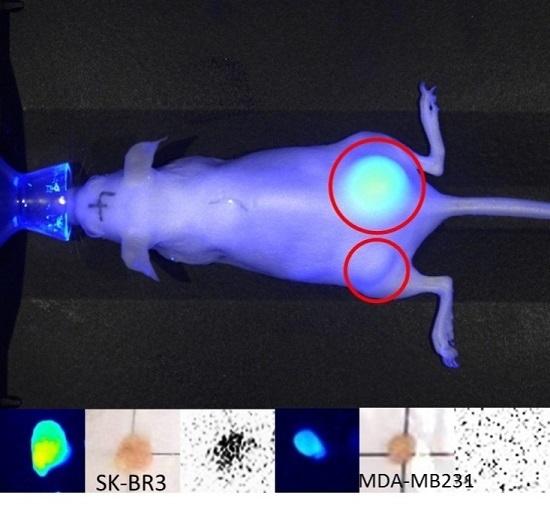
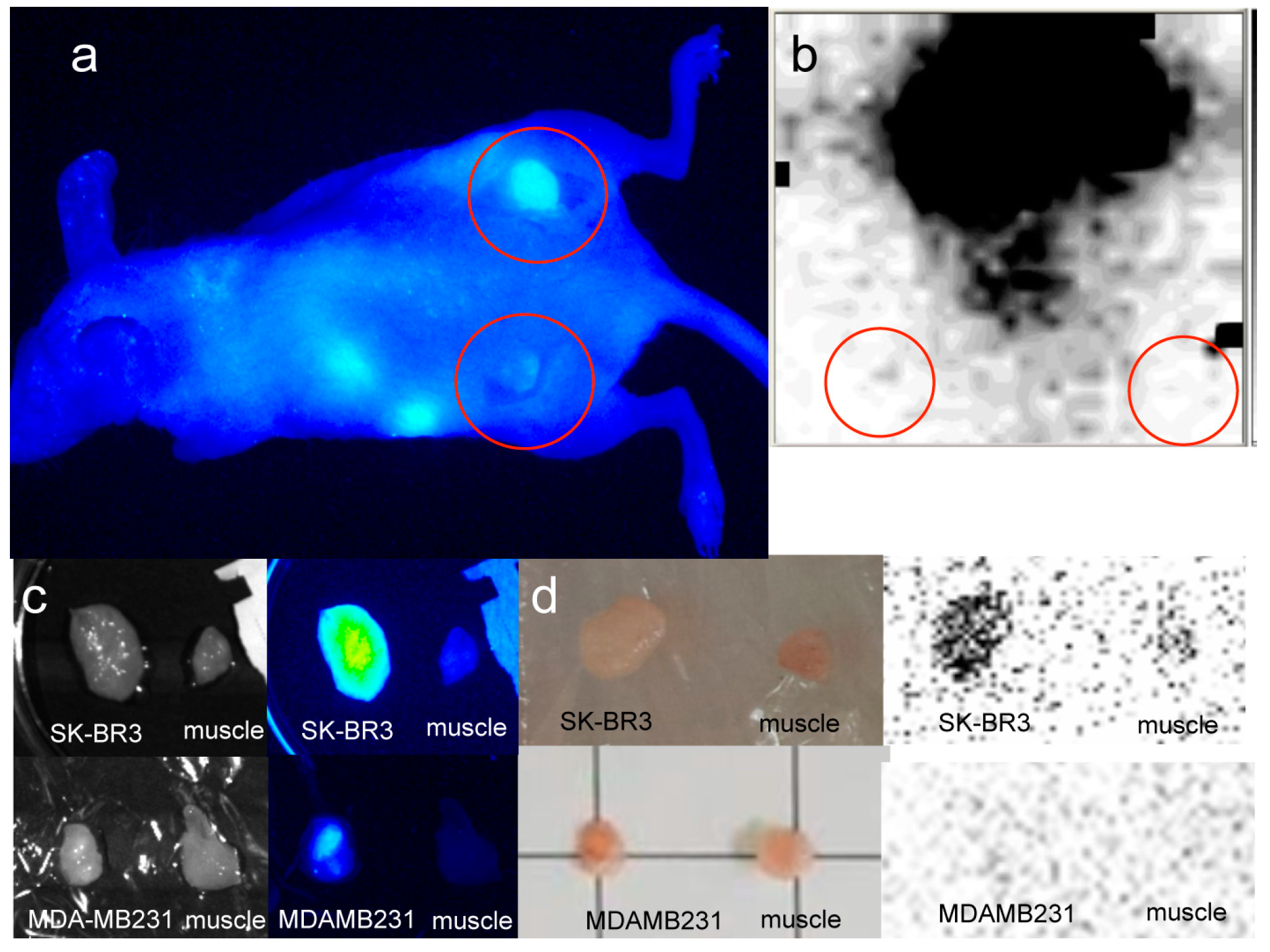
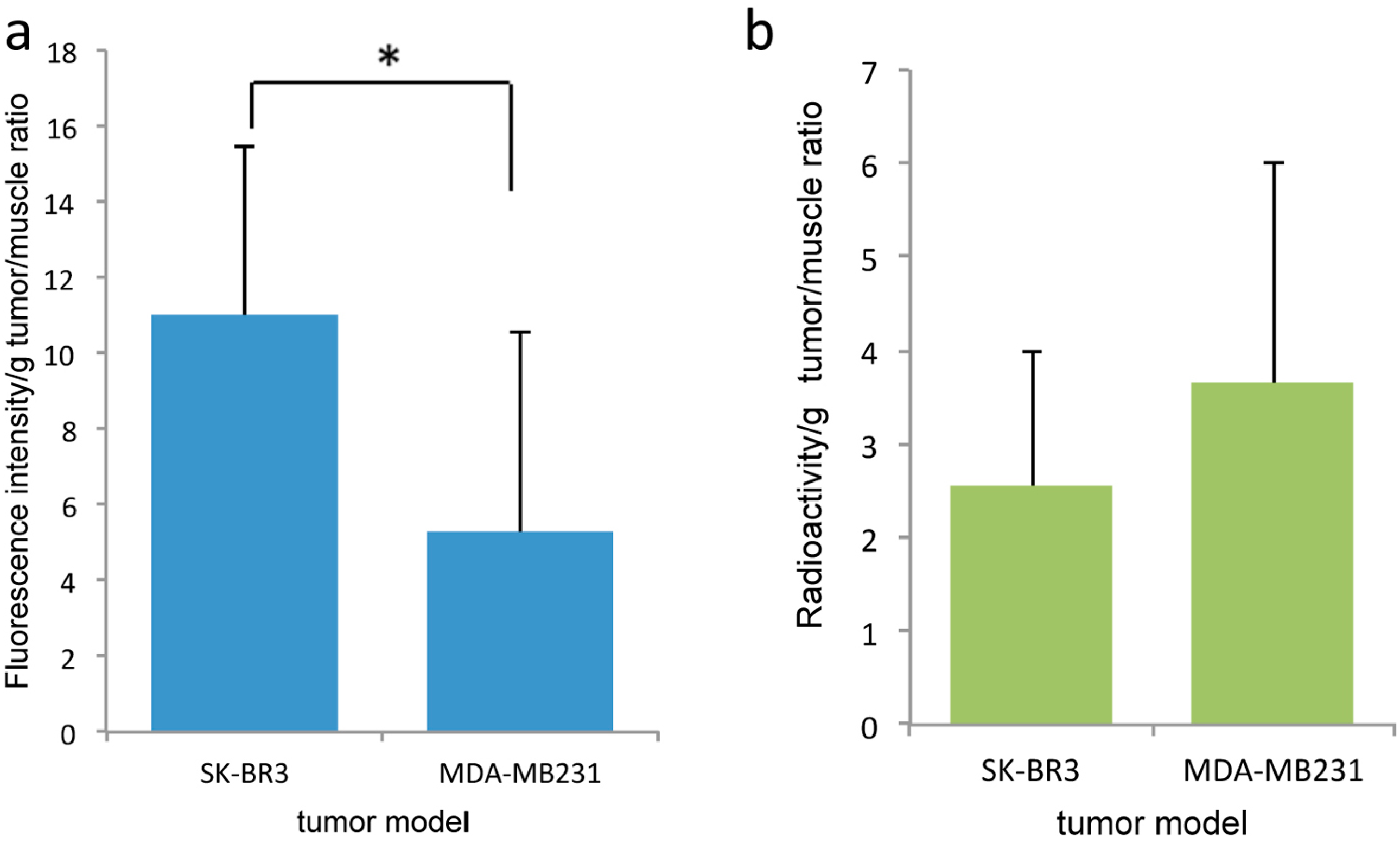
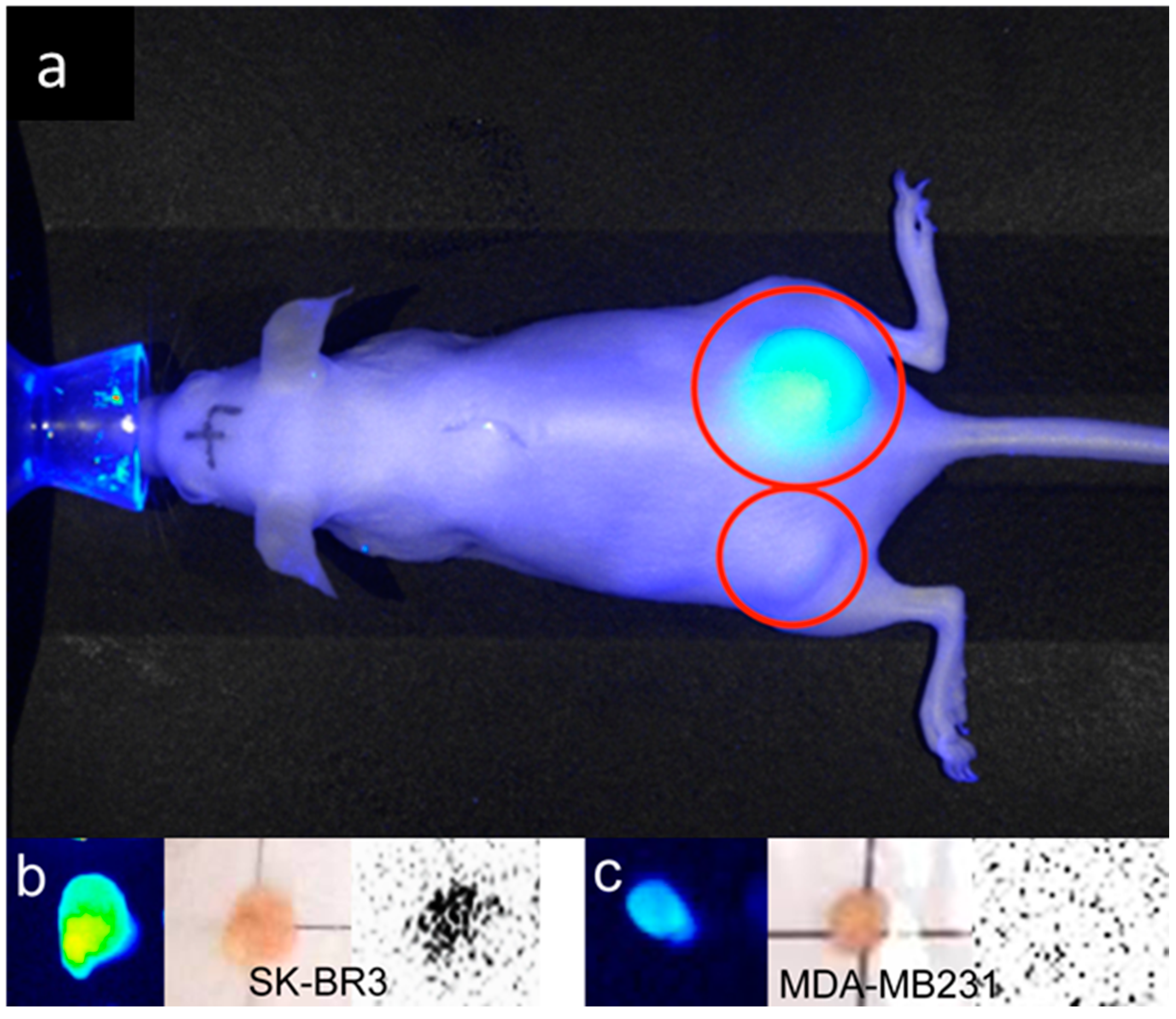
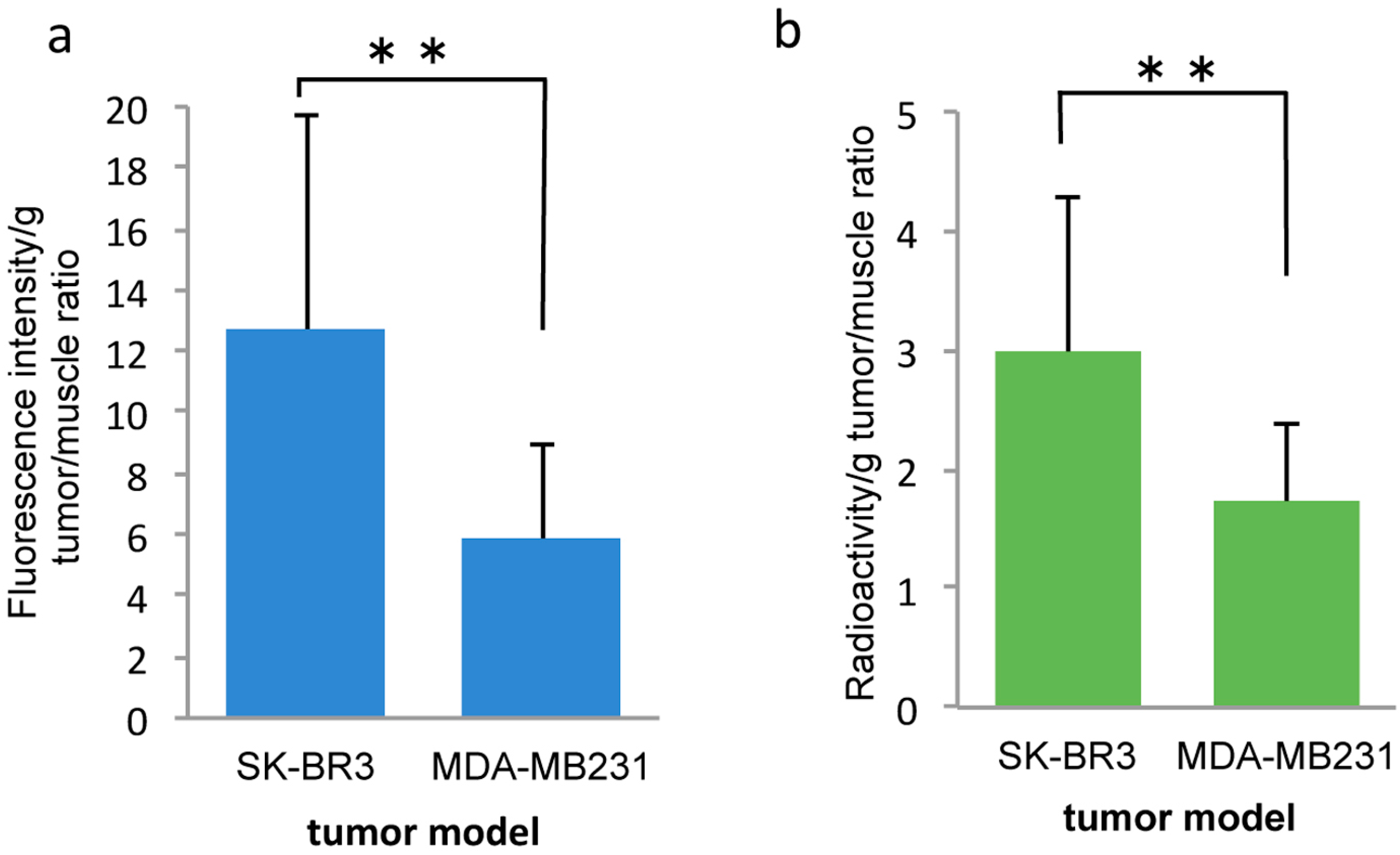
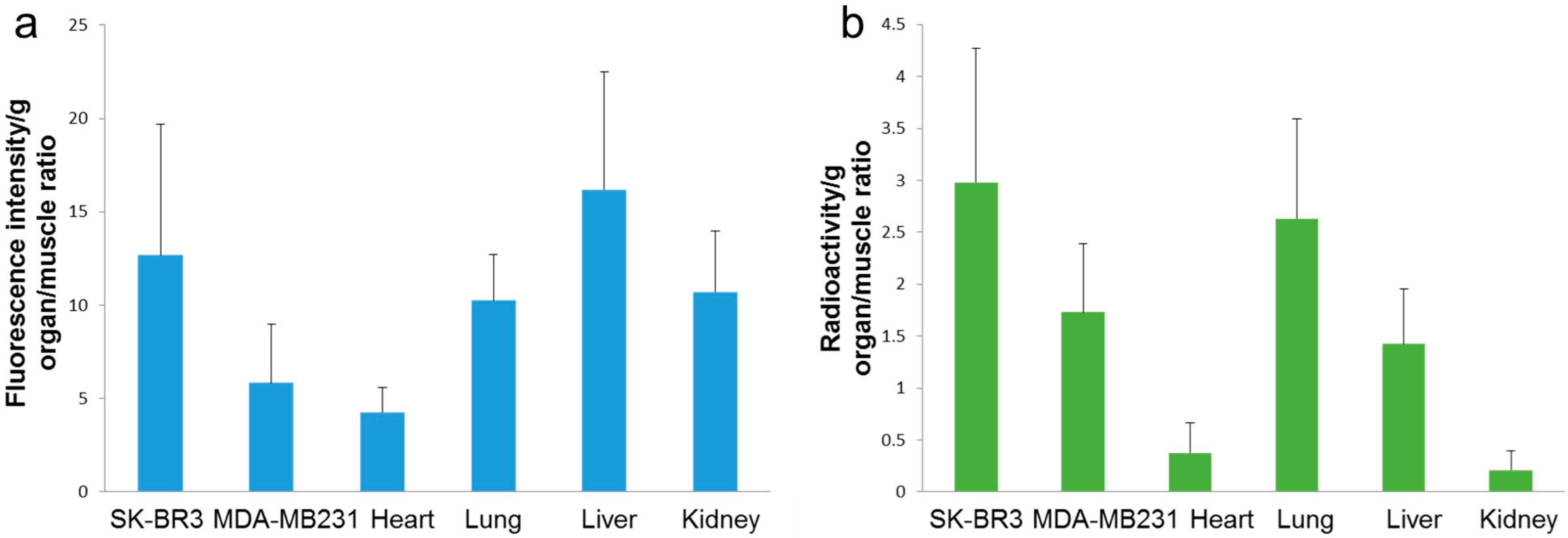
2.2. In Vivo
3. Discussion
4. Materials and Methods
4.1. Fluorescent Probes
4.2. Dual-Imaging Probes
4.3. Cell Culture
4.4. In Vitro, Imaging of HER2-Overexpressing Cells Using Fluorescent Probes
4.5. Flow Cytometry
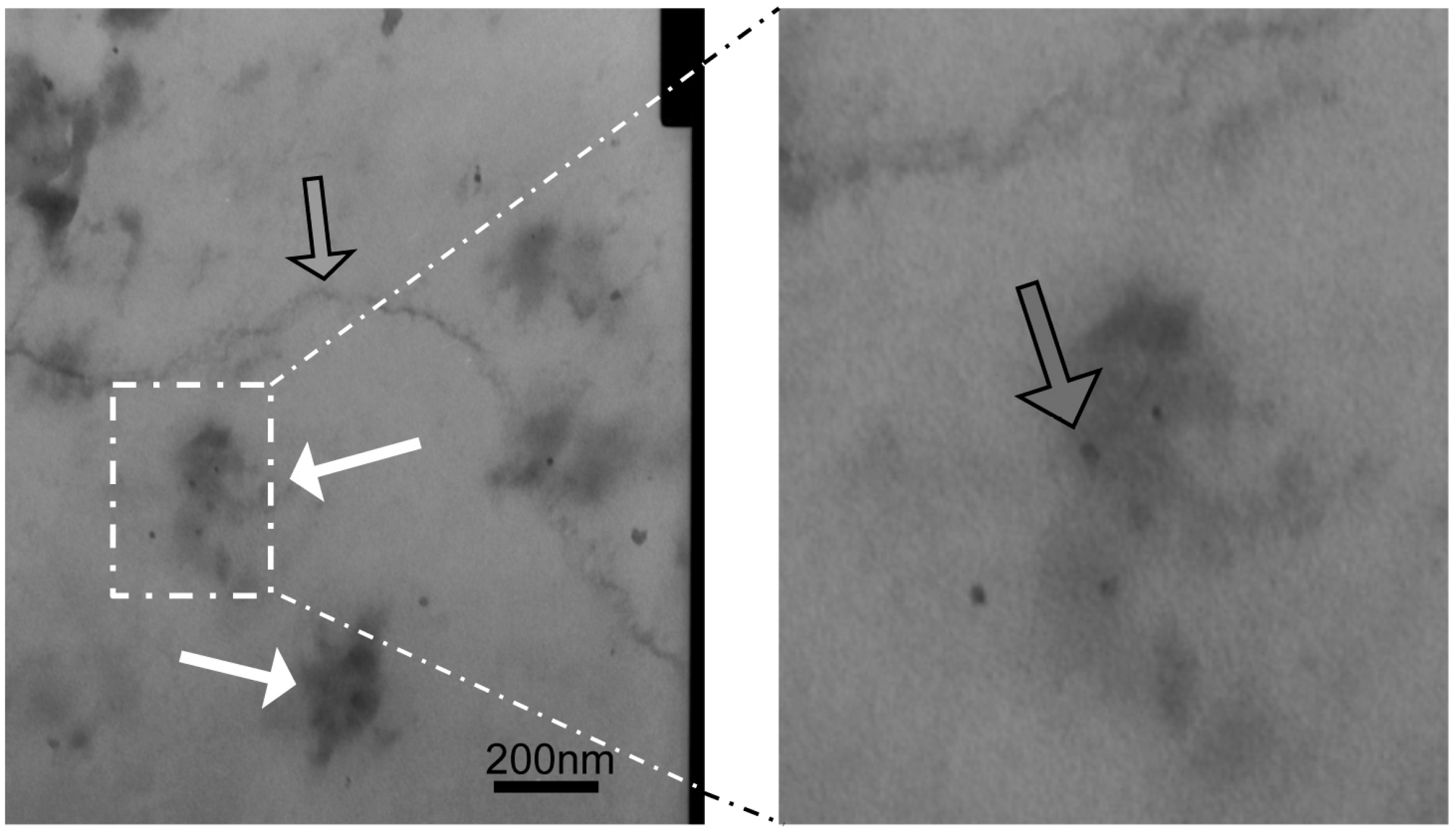
4.6. Transmission Electron Microscopy (TEM)
4.7. In Vitro, Imaging of HER2-Overexpressing Cells Using Dual-Imaging Probes
4.8. In Vivo, Tumor Model and Imaging of HER2-Overexpressing Cells Using Dual-Imaging Probes
4.9. Immunohistochemistry
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jokerst, J.V.; Gambhir, S.S. Molecular imaging with theranostic nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Morshed, R.A.; Auffinger, B.; Tobias, A.L.; Lesniak, M.S. Multifunctional nanoparticles for brain tumor imaging and therapy. Adv. Drug Deliv. Rev. 2014, 66, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; de Nicola, M.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimochi, M.; Hayama, K.; Toyama, M.; Sasagawa, I.; Tsubokawa, N. Dual-modality imaging with 99mTc and fluorescent indocyanine green using surface-modified silica nanoparticles for biopsy of the sentinel lymph node: An animal study. EJNMMI Res. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Leary, J.F. Nanoparticles for multimodal in vivo imaging in nanomedicine. Int. J. Nanomed. 2014, 9, 711–726. [Google Scholar]
- Mukerjee, A.; Ranjan, A.P.; Vishwanatha, J.K. Combinatorial nanoparticles for cancer diagnosis and therapy. Curr. Med. Chem. 2012, 19, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. Biology of HER2 and its importance in breast cancer. Oncology 2001, 61, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, T.J.; Sanders, M.E.; Abramson, V.G. Current HER2 testing recommendations and clinical relevance as a predictor of response to targeted therapy. Clin. Breast Cancer 2015, 15, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Martelotto, L.G.; Ng, C.K.; Piscuoglio, S.; Weigelt, B.; Reis-Filho, J.S. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014, 16, 210. [Google Scholar] [CrossRef] [PubMed]
- Capala, J.; Bouchelouche, K. Molecular imaging of HER2-positive breast cancer: A step toward an individualized ‘image and treat’ strategy. Curr. Opin. Oncol. 2010, 22, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Sandberg, D.; Sandstrom, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Astrom, G.; Lubberink, M.; Garske-Roman, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Huxford-Phillips, R.C.; Lin, W. Silica-based nanoprobes for biomedical imaging and theranostic applications. Chem. Soc. Rev. 2012, 41, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Bettoschi, A.; Garau, A.; Montis, R. Silica-based nanoparticles: A versatile tool for the development of efficient imaging agents. Chem. Soc. Rev. 2015, 44, 4645–4671. [Google Scholar] [CrossRef] [PubMed]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Linden, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Roy, I.; Ohulchanskky, T.Y.; Vathy, L.A.; Bergey, E.J.; Sajjad, M.; Prasad, P.N. In vivo biodistribution and clearance studies using multimodal organically modified silica nanoparticles. ACS Nano 2010, 4, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.S.; Phillips, E.; Montero, P.H.; Cheal, S.M.; Stambuk, H.; Durack, J.C.; Sofocleous, C.T.; Meester, R.J.; Wiesner, U.; Patel, S. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. 2013, 5, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gonen, M.; Kalaigian, H.; Schoder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, 260ra149. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.M.; Gilberti, R.M.; Knecht, D.A. The phagocytosis and toxicity of amorphous silica. PLoS ONE 2011, 6, e14647. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Mohamed, T.; Whitehead, D.; Azzawi, M. Biocompatibility of amorphous silica nanoparticles: Size and charge effect on vascular function, in vitro. Biotechnol. Appl. Biochem. 2011, 58, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- MacEwan, S.R.; Callahan, D.J.; Chilkoti, A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery. Nanomedicine 2010, 5, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.M.; Nance, E.; Kannan, S.; Tomalia, D.A. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J. Intern. Med. 2014, 276, 579–617. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 2009, 71, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.R.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Biologically optimized nanosized molecules and particles: More than just size. Bioconj. Chem. 2011, 22, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, G.E.; Joubran, C.; Arnould, R.; Lejeune, F.; Fruhling, J. Labelled polycyanoacrylate nanoparticles for human in vivo use. Appl. Radiat. Isot. 1993, 44, 1219–1224. [Google Scholar] [CrossRef]
- D'Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Hnatowich, D.J. Methods for MAG3 conjugation and 99mTc radiolabeling of biomolecules. Nat. Protoc. 2006, 1, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cheng, D.; Dou, S.; Chen, X.; Liang, M.; Pretorius, P.H.; Rusckowski, M.; Hnatowich, D.J. Replacing 99mTc with 111In improves MORF/cMORF pretargeting by reducing intestinal accumulation. Mol. Imaging Biol. 2009, 11, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.N.; Jiang, R.; Wang, F.; Takahashi, K.; Peterson, T.E.; Zemel, D.; Hao, C.M.; Fujita, H.; Harris, R.C.; Quarles, C.C.; et al. Assessment of renal function in mice with unilateral ureteral obstruction using 99mTc-MAG3 dynamic scintigraphy. BMC Nephrol. 2012, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, M.J.; Conrad, G.R.; Samal, M.; Watson, G.; Wanasundara, S.N.; Babyn, P.; Wesolowski, C.A. A simple method for determining split renal function from dynamic 99mTc-MAG3 scintigraphic data. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimer nanotechnology for enhanced formulation and controlled delivery of resveratrol. Ann. N. Y. Acad. Sci. 2015, 1348, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hudziak, R.M.; Lewis, G.D.; Winget, M.; Fendly, B.M.; Shepard, H.M.; Ullrich, A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol. Cell. Biol. 1989, 9, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Subik, K.; Lee, J.F.; Baxter, L.; Strzepek, T.; Costello, D.; Crowley, P.; Xing, L.; Hung, M.C.; Bonfiglio, T.; Hicks, D.G.; et al. The Expression Patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by Immunohistochemical analysis in breast cancer cell lines. Breast Cancer Basic Clin. Res. 2010, 4, 35–41. [Google Scholar]
- Tsuchimochi, M.; Sakahara, H.; Hayama, K.; Funaki, M.; Ohno, R.; Shirahata, T.; Orskaug, T.; Maehlum, G.; Yoshioka, K.; Nygard, E. A prototype small CdTe γ camera for radioguided surgery and other imaging applications. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Tsuchimochi, M.; Hayama, K.; Oda, T.; Togashi, M.; Sakahara, H. Evaluation of the efficacy of a small CdTe γ-camera for sentinel lymph node biopsy. J. Nucl. Med. 2008, 49, 956–962. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, H.; Tsuchimochi, M.; Hayama, K.; Kawase, T.; Tsubokawa, N. Dual-Labeled Near-Infrared/99mTc Imaging Probes Using PAMAM-Coated Silica Nanoparticles for the Imaging of HER2-Expressing Cancer Cells. Int. J. Mol. Sci. 2016, 17, 1086. https://doi.org/10.3390/ijms17071086
Yamaguchi H, Tsuchimochi M, Hayama K, Kawase T, Tsubokawa N. Dual-Labeled Near-Infrared/99mTc Imaging Probes Using PAMAM-Coated Silica Nanoparticles for the Imaging of HER2-Expressing Cancer Cells. International Journal of Molecular Sciences. 2016; 17(7):1086. https://doi.org/10.3390/ijms17071086
Chicago/Turabian StyleYamaguchi, Haruka, Makoto Tsuchimochi, Kazuhide Hayama, Tomoyuki Kawase, and Norio Tsubokawa. 2016. "Dual-Labeled Near-Infrared/99mTc Imaging Probes Using PAMAM-Coated Silica Nanoparticles for the Imaging of HER2-Expressing Cancer Cells" International Journal of Molecular Sciences 17, no. 7: 1086. https://doi.org/10.3390/ijms17071086