Sperm Proteases that May Be Involved in the Initiation of Sperm Motility in the Newt, Cynops pyrrhogaster
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acrosomal Protease in C. pyrrhogaster Sperm
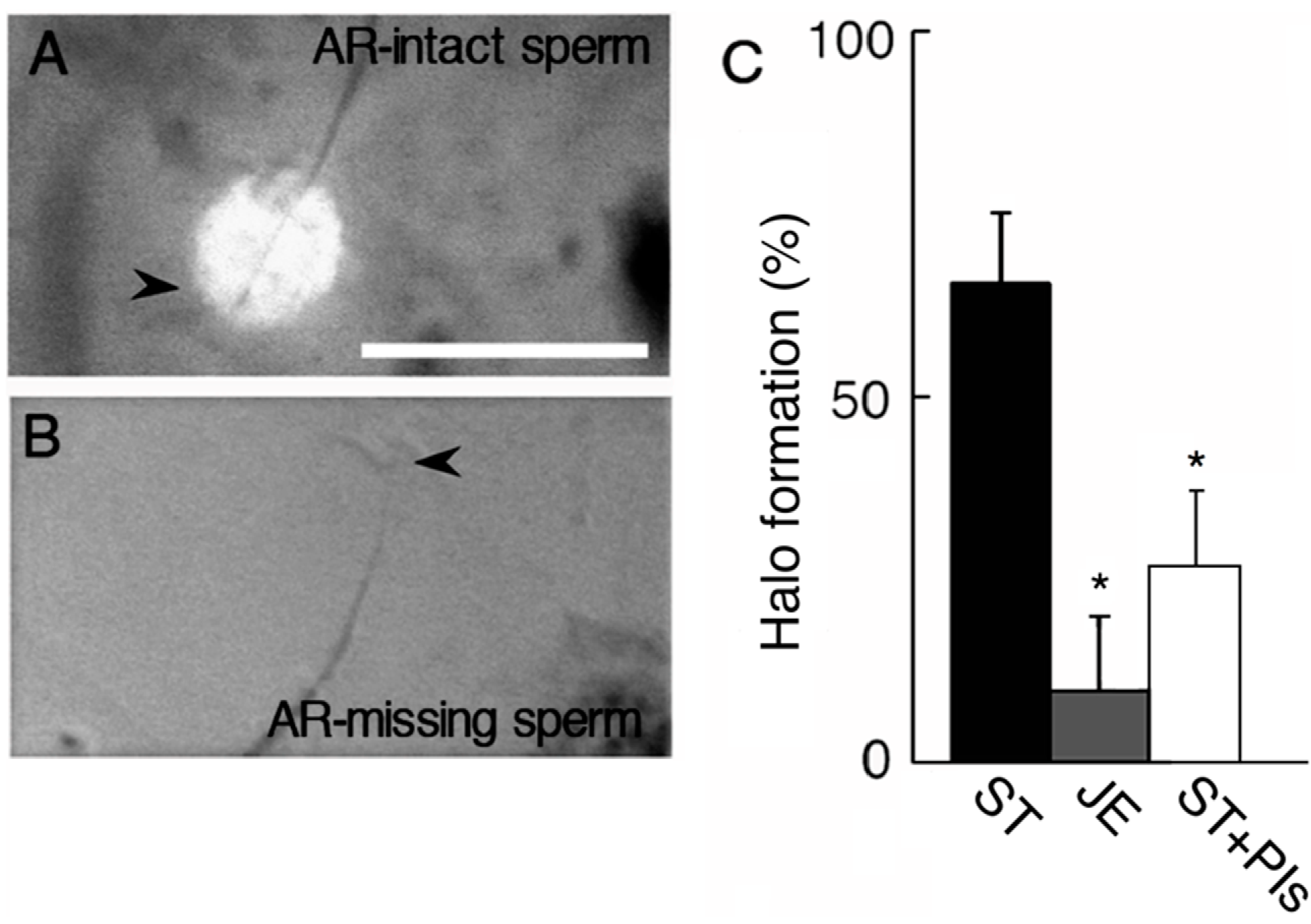

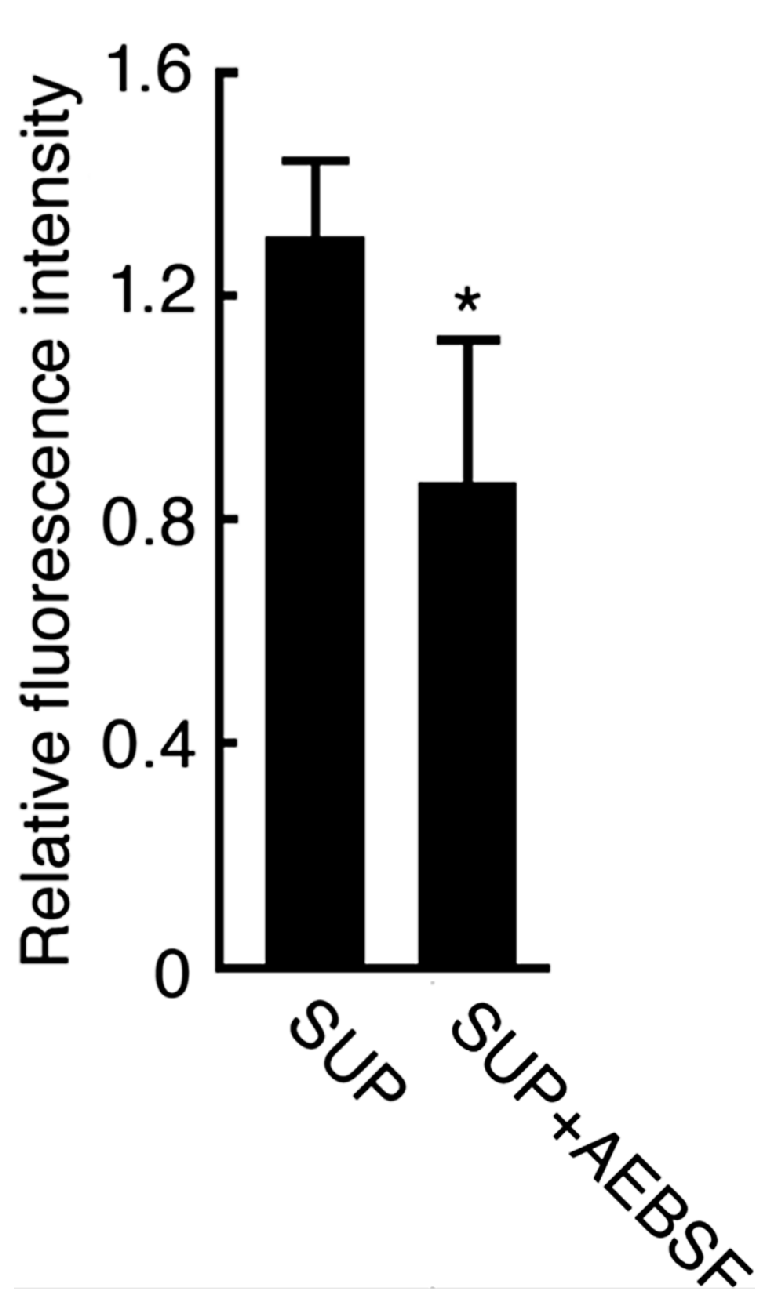
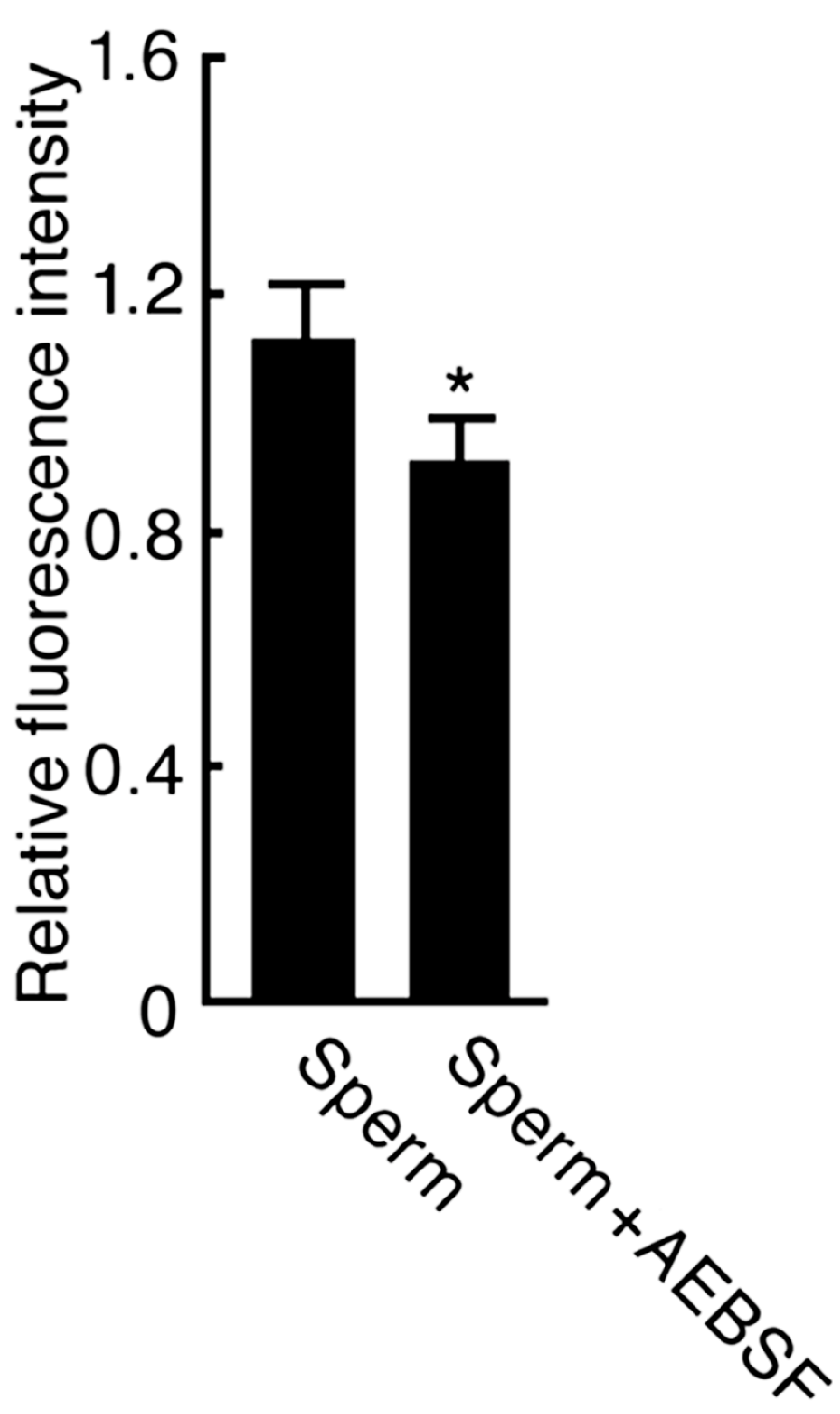
2.2. Effect of AEBSF (4-(2-Aminoethyl) benzenesulfonyl fluoride)-Sensitive Acrosomal Protease in AR (Acrosome Reaction)-Induced Sperm
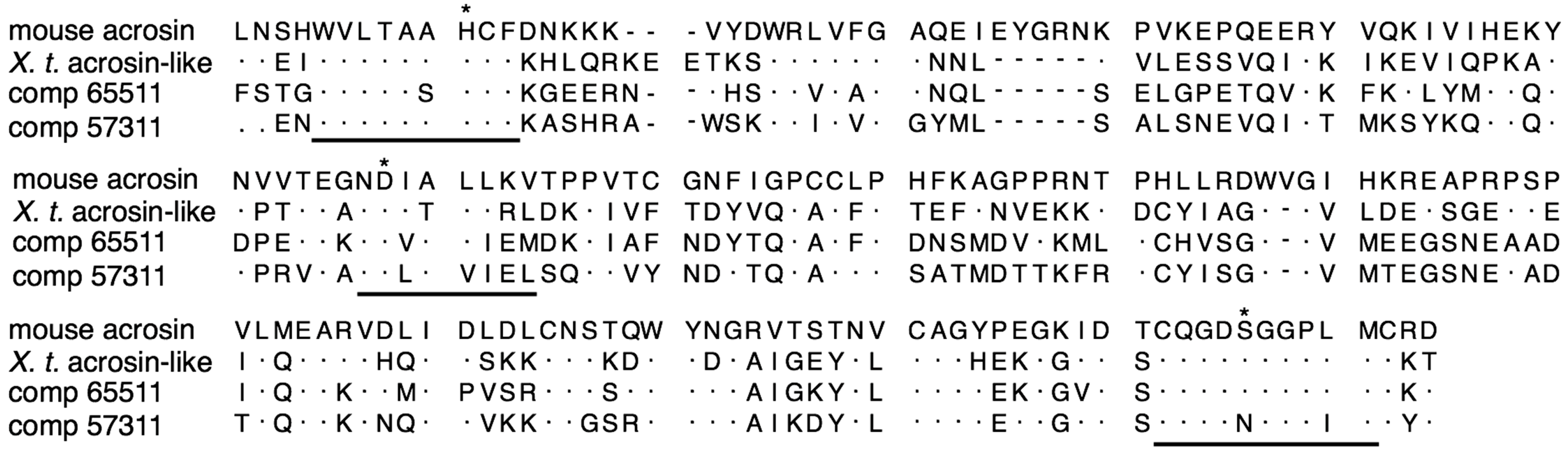
2.3. In Silico Detection of Acrosins and 20S Proteasomes Possibly Expressed in Sperm
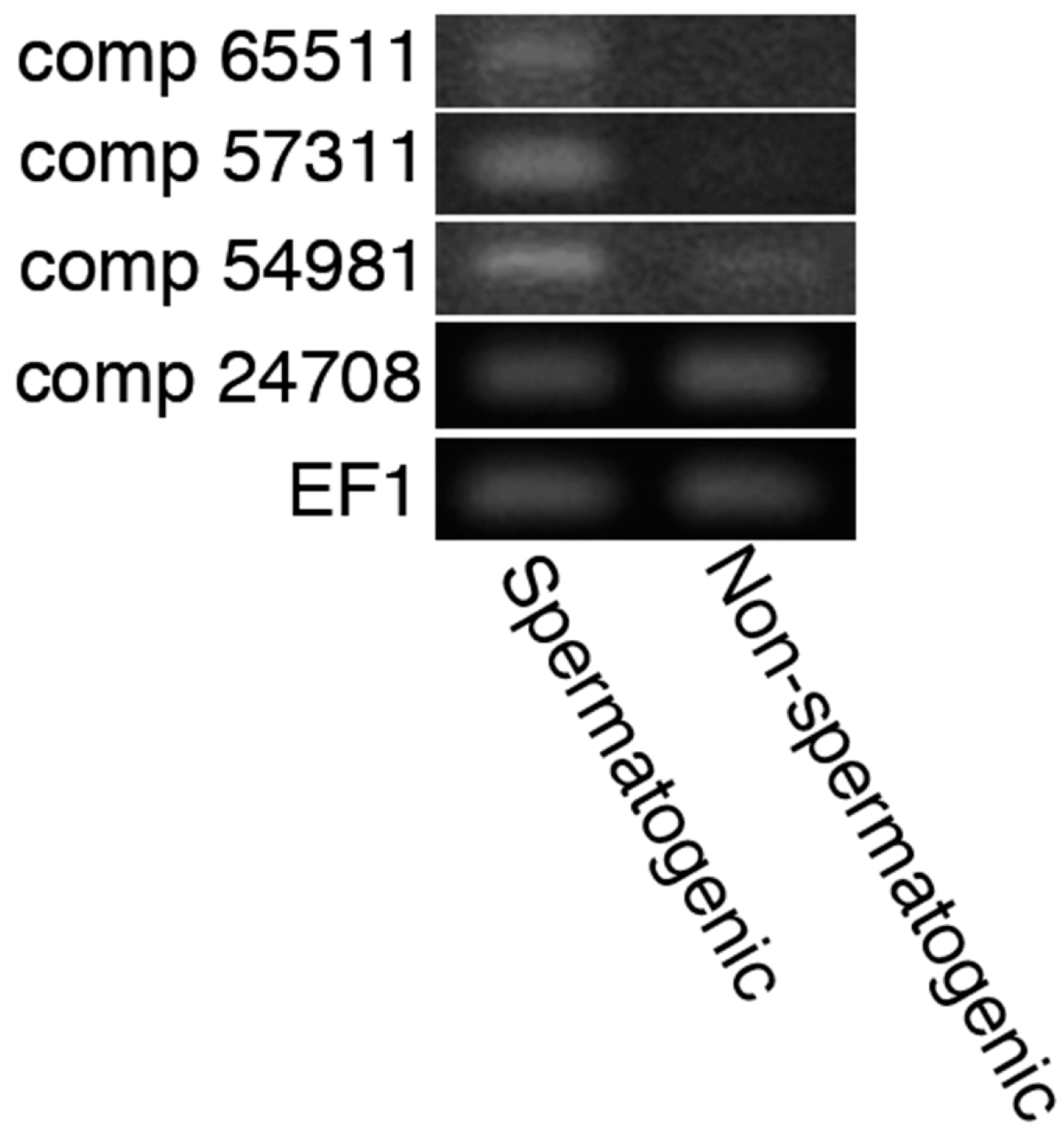
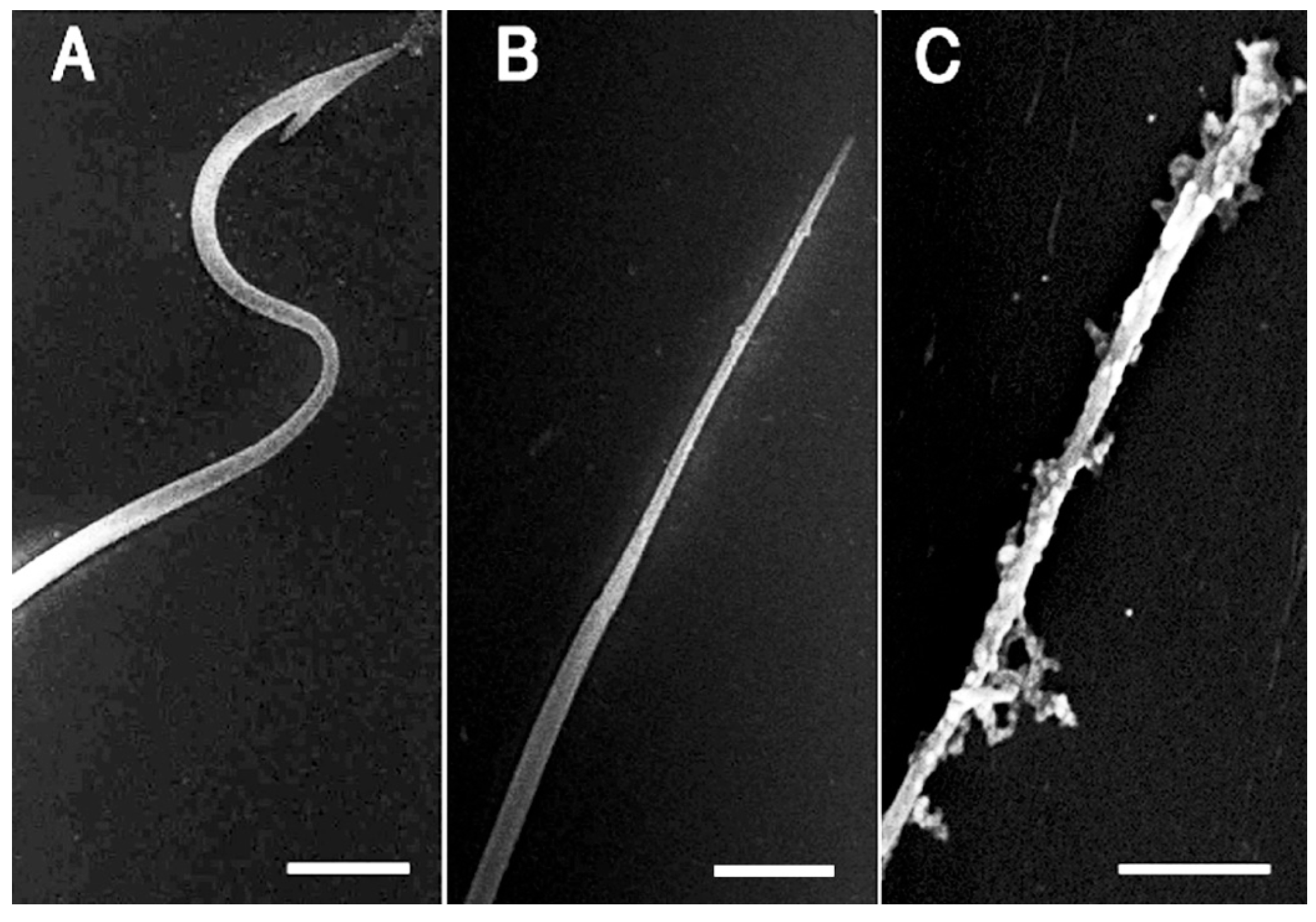
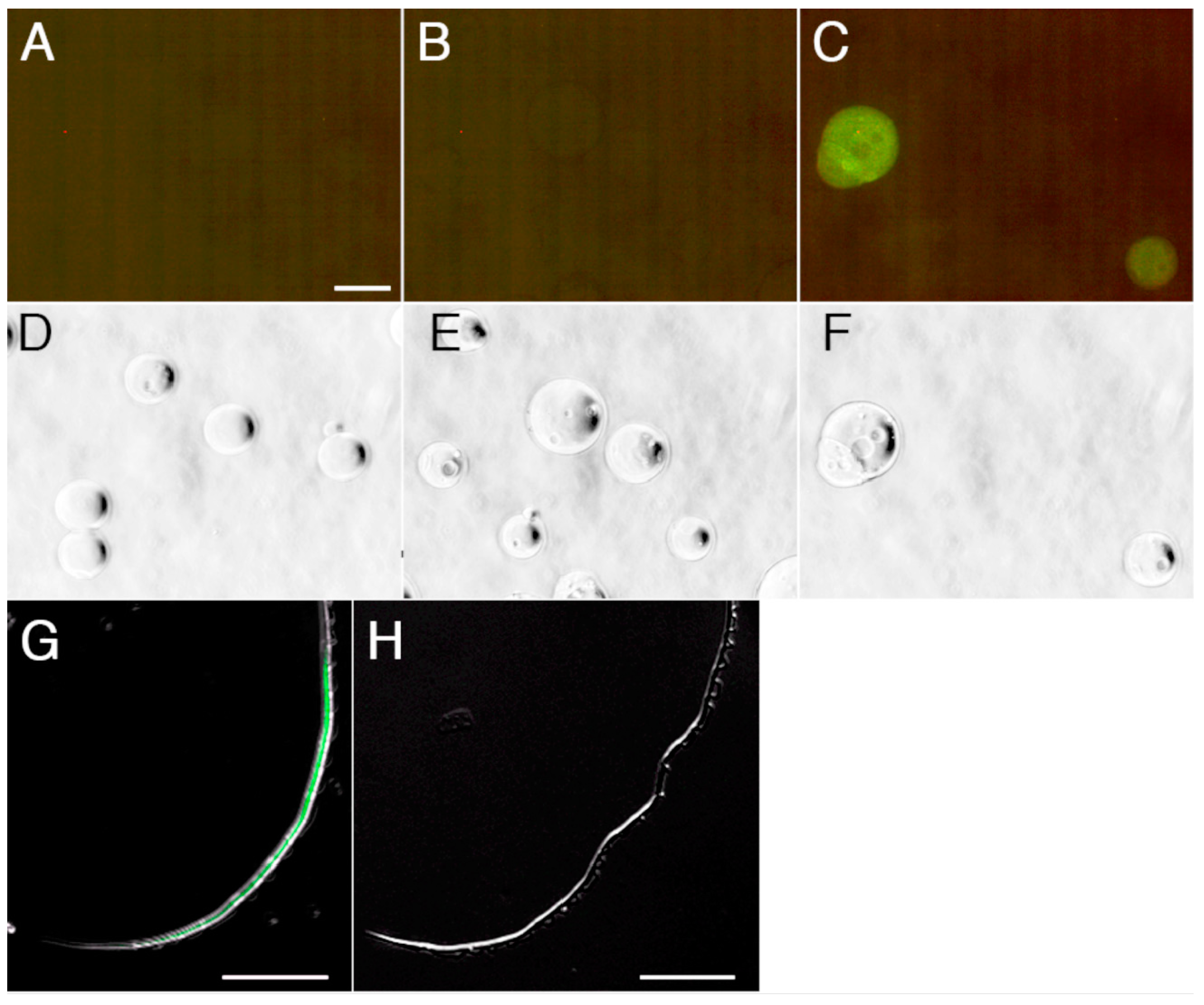
2.4. Detection and Visualization of Sperm Surface Protease

3. Experimental Section
3.1. Gametes
3.2. Solutions
3.3. Preparation of JE (Egg Jelly Extract)
3.4. Detection of Protease Activity
3.5. Scanning Electron Microscopy
3.6. RNA-seq and De Novo Transcriptome Assembly
3.7. RT-PCR
3.8. Fluorescence Staining of the Sperm
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ukita, M.; Itoh, T.; Watanabe, A.; Onitake, K. Substances for the initiation of sperm motility in egg-jelly of the Japanese newt, Cynops pyrrhogaster. Zool. Sci. 1999, 16, 793–802. [Google Scholar] [CrossRef]
- Watanabe, T.; Ito, T.; Watanabe, A.; Onitake, K. Characteristics of sperm motility induced on the egg-jelly in the internal fertilization of the newt, Cynops pyrrhogaster. Zool. Sci. 2003, 20, 345–352. [Google Scholar]
- Watanabe, T.; Kubo, H.; Takeshima, S.; Nakagawa, M.; Ohta, M.; Kamimura, S.; Takayama-Watanabe, E.; Watanabe, A.; Onitake, K. Identification of the sperm motility-initiating substance in the newt, Cynops pyrrhogaster, and its possible relationship with the acrosome reaction during internal fertilization. Int. J. Dev. Biol. 2010, 54, 591–597. [Google Scholar]
- Allison, A.C.; Hartree, E.F. Lysosomal enzymes in the acrosome and their possible role in fertilization. J. Reprod. Fertil. 1970, 21, 501–515. [Google Scholar] [CrossRef]
- Green, J.D.; Summers, R.G. Ultrastructual demonstration of trypsin-like protease in acrosomes of sea urchin sperm. Science 1980, 209, 398–400. [Google Scholar]
- Mack, S.; Bhattacharyya, A.K.; Joyce, C.; van der Ven, H.; Zaneveld, L.J.D. Acrosomal enzymes of human spermatozoa before and after in vitro capacitation. Biol. Reprod. 1983, 28, 1032–1042. [Google Scholar]
- Sawada, H.; Yokosawa, H.; Someno, T.; Saino, T.; Ishii, S. Evidence for the participation of two sperm proteases, spermosin and acrosin, in fertilization of the ascidian, Halocynthia roretzi: Inhibitory effects of leupeptin analogs on enzyme activities and fertilization. Dev. Biol. 1984, 105, 246–249. [Google Scholar]
- Polakoske, K.L.; McRoie, R.A.; Williams, W.L. Boar Acrosin I. Purification and preliminary characterization of a proteinase from boar sperm acrosomes. J. Biol. Chem. 1973, 248, 8178–8182. [Google Scholar]
- Honda, A.; Siruntawineti, J.; Baba, T. Role of acrosomal matrix proteases in sperm-zona pellucida interactions. Hum. Reprod. Update 2002, 8, 405–412. [Google Scholar] [CrossRef]
- Ho, J.J.L.; Meizel, S. Biochemical characterization of an acian spermtozoan acrosin and comparison of its properties to those of bovine trypsin and mammalian acrosins. Comp. Biochem. Physiol. 1976, 54B, 213–218. [Google Scholar]
- Hoshi, M.; Moriya, T.; Aoyagi, T.; Umezawa, H.; Mohri, H.; Nagai, Y. Effects of hydrolase inhibitors on fertilization of sea urchins: I. protease inhibitors. Gamete Res. 1979, 2, 107–109. [Google Scholar] [CrossRef]
- Sousa, M.; Moradas-Ferreira, P.; Azevedo, C. Presence of a trypsin-like protease in starfish sperm acrosome. J. Exp. Zool. 1992, 261, 349–354. [Google Scholar] [CrossRef]
- Miyamoto, H.; Chang, M.C. Effects of protease inhibitors on the fertilizing capacity of hamster spermatozoa. Biol. Reprod. 1973, 9, 533–537. [Google Scholar]
- Green, J.D.; Summers, R.G. Effects of protease inhibitors on sperm-related events in sea urchin fertilization. Dev. Biol. 1982, 92, 139–144. [Google Scholar] [CrossRef]
- Sawada, H.; Sakai, N.; Abe, Y.; Tanaka, E.; Takahashi, Y.; Fujino, J.; Kodama, E.; Takizawa, S.; Yokosawa, H. Extracellular ubiquitination and proteasome-mediated degradation of the ascidian sperm receptor. Proc. Natl. Acad. Sci. USA 2002, 99, 1223–1228. [Google Scholar] [CrossRef]
- Sasanami, T.; Sugiura, K.; Tokumoto, T.; Yoshizaki, N.; Dohra, H.; Nishio, S.; Mizushima, S.; Hiyama, G.; Matsuda, T. Sperm proteasome degrades egg envelope glycoprotein ZP1 during fertilization of Japanese quail (Coturnix japonica). Reproduction 2012, 144, 423–431. [Google Scholar] [CrossRef]
- Baba, T.; Azuma, S.; Kashiwabara, S.; Toyoda, Y. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 1994, 269, 31845–31849. [Google Scholar]
- Sawada, H. Ascidian sperm lysine system. Zool. Sci. 2002, 19, 139–151. [Google Scholar]
- Yamagata, K.; Murayama, K.; Okabe, M.; Toshimori, K.; Nakanishi, T.; Kashiwabata, S.; Baba, T. Acrosin accelerates the dispersal of sperm acrosomal proteins during acrosome reaction. J. Biol. Chem. 1998, 273, 10470–10474. [Google Scholar]
- Iwao, Y.; Miki, A.; Kobayashi, M.; Onitake, K. Activation of Xenopus eggs by an extract of Cynops sperm. Dev. Growth Differ. 1994, 36, 469–479. [Google Scholar]
- Markwardt, F.; Drawert, J.; Walsmann, P. Synthetic low molecular weight inhibitors of serum kallikrein. Biochem. Pharmacol. 1974, 23, 2247–2256. [Google Scholar] [CrossRef]
- Sasaki, T.; Kamimura, S.; Takai, H.; Watanabe, A.; Onitake, K. The activity for the induction of the sperm acrosome reaction localizes in the outer layer and exists in the high-molecular-weight components of the egg-jelly of the newt, Cynops pyrrhogaster. Zygote 2002, 10, 1–9. [Google Scholar]
- Hiyoshi, W.; Sasaki, T.; Takayama-Watanabe, E.; Takai, H.; Watanabe, A.; Onitake, K. Egg jelly of the newt, Cynops pyrrhogaster contains a factor essential for sperm binding to the vitelline envelope. J. Exp. Zool. 2007, 307A, 301–311. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakazawa, H.; Watanabe, A.; Onitake, K. The outermost layer of egg-jelly is crucial to successful fertilization in the newt, Cynops pyrrhogaster. J. Exp. Zool. 2006, 305A, 1010–1017. [Google Scholar] [CrossRef]
- Takayama-Watanabe, E.; Ochiai, H.; Tanino, S.; Watanabe, A. Contribution of different Ca2+ channels to the acrosome reaction-mediated initiation of sperm motility in the newt Cynops pyrrhogaster. Zygote 2013. [Google Scholar] [CrossRef]
- Takahashi, T.; Kutsuzawa, M.; Shiba, K.; Takayama-Watanabe, E.; Inaba, K.; Watanabe, A. Distinct Ca2+ channels maintain a high motility state of the sperm that may be needed for penetration of egg jelly of the newt, Cynops pyrrhogaster. Dev. Growth Differ. 2013, 55, 657–667. [Google Scholar] [CrossRef]
- Takayama-Watanabe, E.; Takahashi, T.; Yokoe, M.; Watanabe, A. Acrosome reaction-mediated motility initiation that is critical for the internal fertilization of urodele amphibians. In Sexual Reproduction in Animals and Plants; Sawada, H., Inoue, N., Iwano, M., Eds.; Springer: Tokyo, Japan, 2014; pp. 97–103. [Google Scholar]
- Itoh, T.; Kamimura, S.; Watanabe, A.; Onitake, K. Egg-jelly structure promotes efficiency of internal fertilization in the newt, Cynops pyrrhogaster. J. Exp. Zool. 2002, 292, 314–322. [Google Scholar]
- Rypniewski, W.R.; Perrakis, A.; Vorgias, C.E.; Wilson, K.W. Evolutionary divergence and conservation of trypsin. Protein Eng. 1994, 7, 57–64. [Google Scholar] [CrossRef]
- Yokota, N.; Kataoka, Y.; Hashii, N.; Kawasaki, N; Sawada, H. Sperm-specific C-terminal processing of the proteasome PSMA1/alpha6 subunit. Biochem. Biophys. Res. Commun. 2011, 410, 809–815. [Google Scholar] [CrossRef]
- IMORI. Available online: http://antler.is.utsunomiya-u.ac.jp/imori (accessed on 27 August 2014).
- Mizuno, J.; Watanabe, A.; Onitake, K. Initiation of sperm motility in the newt, Cynops pyrrhogaster, is induced by a heat-stable component of egg-jelly. Zygote 1999, 7, 329–334. [Google Scholar]
- Islam, M.R.; Nakamura, K.; Casco-Robles, M.M.; Kunahong, A.; Inami, W.; Toyama, F.; Maruo, F.; Chiba, C. The newt reprograms mature RPE cells into a unique multipotent state for retinal regeneration. Sci. Rep. 2014. [Google Scholar] [CrossRef]
- RNA-Seq De Novo Assembly Using Trinity. Available online: http://trinityrnaseq.sourceforge.net (accessed on 27 August 2014).
- Yazawa, T.; Nakayama, N.; Fujimoto, K.; Matsuda, Y.; Abe, K.; Kitano, T.; Abe, S.; Yamamoto, T. Abnormal spermatogenesis at low temperatures in the Japanease red-bellied newt, Cynops pyrrhogaster: Possible biological significance of the cessation of spermatocytogenesis. Mol. Reprod. Dev. 2003, 66, 60–66. [Google Scholar] [CrossRef]
- Miyata, H.; Thaler, C.D.; Haimo, L.T.; Cardullo, R.A. Protease activation and the signal transduction pathway regulating motility in sperm from the water strider Aquarius remigis. Cytoskeleton 2012, 69, 207–220. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yokoe, M.; Sano, M.; Shibata, H.; Shibata, D.; Takayama-Watanabe, E.; Inaba, K.; Watanabe, A. Sperm Proteases that May Be Involved in the Initiation of Sperm Motility in the Newt, Cynops pyrrhogaster. Int. J. Mol. Sci. 2014, 15, 15210-15224. https://doi.org/10.3390/ijms150915210
Yokoe M, Sano M, Shibata H, Shibata D, Takayama-Watanabe E, Inaba K, Watanabe A. Sperm Proteases that May Be Involved in the Initiation of Sperm Motility in the Newt, Cynops pyrrhogaster. International Journal of Molecular Sciences. 2014; 15(9):15210-15224. https://doi.org/10.3390/ijms150915210
Chicago/Turabian StyleYokoe, Misato, Makoto Sano, Honami Shibata, Daisuke Shibata, Eriko Takayama-Watanabe, Kazuo Inaba, and Akihiko Watanabe. 2014. "Sperm Proteases that May Be Involved in the Initiation of Sperm Motility in the Newt, Cynops pyrrhogaster" International Journal of Molecular Sciences 15, no. 9: 15210-15224. https://doi.org/10.3390/ijms150915210
APA StyleYokoe, M., Sano, M., Shibata, H., Shibata, D., Takayama-Watanabe, E., Inaba, K., & Watanabe, A. (2014). Sperm Proteases that May Be Involved in the Initiation of Sperm Motility in the Newt, Cynops pyrrhogaster. International Journal of Molecular Sciences, 15(9), 15210-15224. https://doi.org/10.3390/ijms150915210



