Adult Stem Cell Transplantation: Is Gender a Factor in Stemness?
Abstract
:1. Introduction
2. Disparities in Disease Prevalence in Men and Women
3. Variations among Male and Female Cells
4. Dissimilarities between Male- and Female-Derived Stem Cells
5. Characterization of Gender-Specific Donor Cells for Transplantation
5.1. Transplantable Qualities of Endometrial Cells
5.2. Transplantable Qualities of Sertoli Cells
6. Experimental and Clinical Applications of Gender-Specific Stem Cells
6.1. Endometrial Cell Transplantation Studies
6.2. Sertoli Cell Transplantation Studies
| Stem Cell Features | Sertoli Cell | Mentrual Blood Cells |
|---|---|---|
| Cell fate | Phenotype retained | Neural phenotype |
| Therapeutic molecules | Immunosuppressant factors | Trophic factors |
| Disease target | Parkinsonʼs disease | Stroke, limb ishemia |
| Functional outcome | Better co-graft survival | Reduced host cell loss |
| Cell therapy use | Localized immunosuppressioin or by-stander effects | Cell replacement or by-stander effects |
| Limitations | Pre-pubertial harvest | Menstruation period harvest |
7. Practical Aspects of Personalized Medicine with Emphasis on Gender-Specific Stem Cells
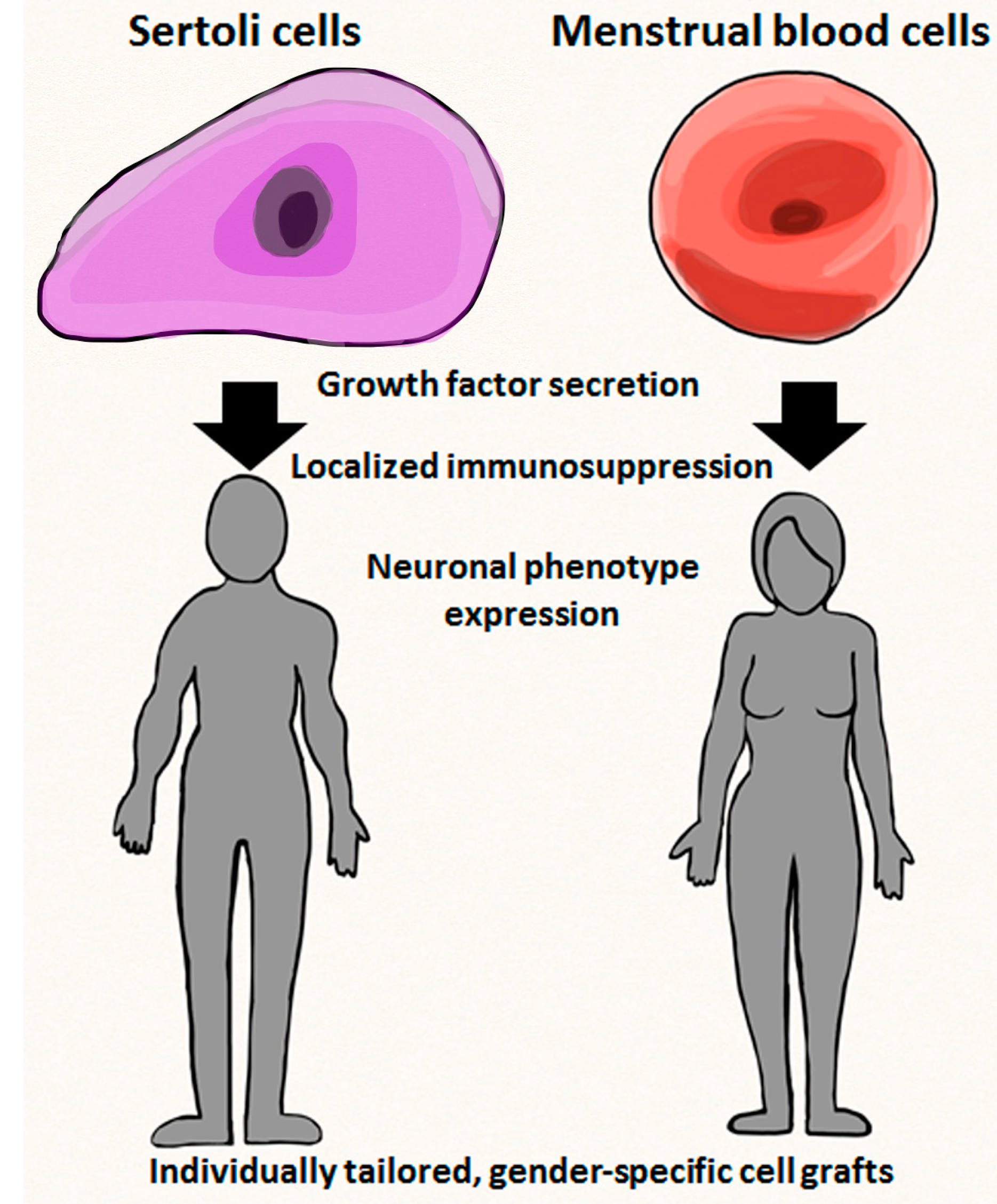
8. Limitations Regarding Gender-Specific Donor Cells
9. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gargett, C.E.; Schwab, K.E.; Zillwood, R.M.; Nguyen, H.P.; Wu, D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol. Reprod. 2009, 80, 1136–1145. [Google Scholar]
- Xu, J.; Kenneth, D.; Kochanek, M.A.; Sherry, L.; Murphy, B.S. Deaths, final data for 2007. Natl. Vital Stat. Rep. 2010, 58, 1–134. [Google Scholar]
- Richard Green, A.; Odergen, T.; Ashwood, T. Animal models of stroke: Do they have value for discovering neuroprotective agents? Trends Pharmacol. Sci. 2003, 24, 402–408. [Google Scholar] [CrossRef]
- Chavez, J.C.; Hurko, O.; Barone, F.C.; Feuerstein, G.Z. Pharmacologic interventions for stroke: Looking beyond the thrombolysis time window into the penumbra with biomarkers, not a stopwatch. Stroke 2010, 40, e558–e563. [Google Scholar]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Davalos, A.; Guidetti, D.; Laurrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T.; et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef]
- Ovbiagele, B.; Nguyen-Huynh, M.N. Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics 2011, 8, 319–329. [Google Scholar]
- Miller, J.; Hartwell, C.; Lewandowski, C. Stroke treatment using intravenous and intra-arterial tissue plasminogen activator. Curr. Treat. Options Cardiovasc. Med. 2012, 14, 273–283. [Google Scholar] [CrossRef]
- Hess, D.C.; Borlongan, C.V. Cell-based therapy in ischemic stroke. Expert. Rev. Neurother. 2008, 8, 1193–1201. [Google Scholar] [CrossRef]
- Eissa, A.; Krass, I.; Bajorek, B.V. Optimizing the management of acute ischemic stroke: A review of the utilization of intravenous recombinant tissue plasminogen activator (tPA). J. Clin. Pharm. Ther. 2012, 37, 620–629. [Google Scholar] [CrossRef]
- Kriz, J. Inflammation in ischemic brain injury, timing is important. Crit. Rev. Neurobiol. 2006, 18, 145–157. [Google Scholar]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Emsley, C.A.; Smith, C.J.; Tyrrell, P.J.; Hopkins, S.J. Inflammation in acute ischemic stroke and its relevance to stroke critical care. Neurocrit. Care 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Takano, T.; Oberbeim, N.; Cotrina, M.L.; Nedergaard, M. Astrocytes and ischemic injury. Stroke 2009, 40, S8–S12. [Google Scholar]
- Park, D.H.; Eve, D.J.; Musso, J., 3rd.; Klasko, S.K.; Borlongan, C.V.; Sanberg, P.R. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009, 18, 693–701. [Google Scholar] [CrossRef]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef]
- Boucherie, C.; Hermans, E. Adult stem cell therapies for neurological disorders: Benefits beyond neuronal replacement? J. Neurosci. Res. 2009, 87, 1509–1521. [Google Scholar]
- Fujiwara, Y.; Tanaka, N.; Ishida, O.; Fujimoto, Y.; Murakami, T.; Kajihara, H.; Yasunaga, Y.; Ochi, M. Intravenously injected neural progenitor cells of transgenic rats can migrate to the injured spinal cord and differentiate into neurons, astrocytes and oligodendrocytes. Neurosci. Lett. 2004, 366, 287–291. [Google Scholar] [CrossRef]
- Hill, W.D.; Hess, D.C.; Martin-Studdard, A.; Carothers, J.J.; Zheng, J.; Hale, D.; Maeda, M.; Fagan, S.C.; Carroll, J.E.; Conway, S.J. SDF-1 (CXCL12) is upregulated in the ischemic penumbra following stroke: association with bone marrow cell homing to injury. J. Neuropathol. Exp. Neurol. 2004, 63, 84–96. [Google Scholar]
- Daar, A.S.; Bhatt, A.; Court, E.; Singer, P.A. Stem cell research and transplantation: Science leading ethics. Transplant. Proc. 2004, 36, 2504–2506. [Google Scholar] [CrossRef]
- Takagi, Y.; Nishimura, M.; Morizane, A.; Takahashi, J.; Nozaki, K.; Hayashi, J.; Hashimoto, N. Survival and differentiation of neural progenitor cells derived from embryonic cells and transplanted into ischemic brain. J. Neurosurg. 2004, 103, 304–310. [Google Scholar]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef]
- Patel, A.N.; Park, E.; Kuzman, M.; Benetti, F.; Silva, F.J.; Allickson, J.G. Multipotent menstrual blood stromal stem cells: Isolation, characterization and differentiation. Cell Transplant. 2008, 17, 303–311. [Google Scholar] [CrossRef]
- Hida, N.; Nishiyama, N.; Miyoshi, S.; Kira, S.; Segawa, K.; Uyama, T.; Mori, T.; Miyado, K.; Ikegami, Y.; Cui, C.; et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 2008, 26, 1695–1704. [Google Scholar]
- Borlongan, C.V.; Kaneko, Y.; Maki, M.; Yu, S.J.; Ali, M.; Allickson, J.G.; Sanberg, C.D.; Kuzmin-Nichols, N.; Sanberg, P.R. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells 2010, 19, 439–451. [Google Scholar] [CrossRef]
- Murphy, M.P.; Wang, H.; Patel, A.N.; Kambhampati, S.; Angle, N.; Chan, K.; Marleau, A.M.; Pysznika, A.; Carrier, E.; Ichim, T.E.; et al. Allogeneic endometrial regenerative cells, an “off the shelf solution” for critical limb ischemia? J. Transl. Med. 2008, 6, 45. [Google Scholar] [CrossRef]
- Wolff, E.F.; Gao, X.B.; Andrews, Z.B.; Du, H.; Elsworth, J.D.; Taylor, H.S. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J. Cell. Mol. Med. 2011, 15, 747–755. [Google Scholar]
- Borlongan, C.V.; Cameron, D.F.; Saporta, S.; Sandberg, P.R. Intracerebral transplantation of testis-derived SC promotes functional recovery in female rats with 6-hydroxydopamine-induced hemiparkinsonism. Exp. Neurol. 1997, 148, 388–392. [Google Scholar] [CrossRef]
- Saporta, S.; Cameron, D.F.; Borlongan, C.V.; Sanberg, P.R. Survival of rat and porcine Sertoli cell transplants in the rat striatum without cyclosporine-A immunosuppression. Exp. Neurol. 1997, 146, 299–304. [Google Scholar]
- Yamori, Y.; Nagaoka, A.; Okamoto, K. Importance of genetic factors in stroke: An evidence obtained by selective breeding of stroke-prone and stroke-resistant SHR. Circ. J. 1974, 38, 1095–1100. [Google Scholar]
- Sudlow, C.L.; Warlow, C.P. Comparable studies of the incidence of stroke and its pathological types: Results from an international collaboration. Stroke 1997, 28, 491–499. [Google Scholar]
- Bairey Merz, C.N.; Mark, S.; Boyan, B.D.; Jacobs, A.K.; Shah, P.K.; Taylor, D.; Marban, E. Proceedings from the scientific symposium: Sex differences in cardiovascular disease and implications for therapies. J. Womens Health 2010, 19, 1059–1072. [Google Scholar]
- Vitale, C.; Fini, M.; Speziale, G.; Chierchia, S. Gender differences in the cardiovascular effects of sex hormones. Fundam. Clin. Pharmacol. 2010, 24, 675–685. [Google Scholar]
- Chiang, N.; Hurwitz, S.; Ridker, P.M.; Serhan, C.N. Aspirin has a gender-dependent impact on antiinflammatory 15-epi-lipoxin A4 formation: a randomized human trial. Arterioscler. Thromb. Vasc. Biol. 2006, 26, e14–e17. [Google Scholar]
- Chiang, N.; Serhan, C.N. New mechanism for an old drug: Aspirin triggers anti-inflammatory lipid mediators with gender implications. Compr. Ther. 2006, 32, 150–157. [Google Scholar]
- Kumar, A.; Vishvakarma, N.K.; Bharti, A.C.; Singh, S.M. Gender-specific antitumor action of aspirin in a murine model of a T-cell lymphoma bearing host. Blood Cells Mol. Dis. 2012, 48, 137–144. [Google Scholar] [CrossRef]
- Duckles, S.P.; Krause, D.N. Mechanisms of cerebrovascular protection: Oestrogen, inflammation, and mitochondria. Acta Physiol. 2011, 203, 149–154. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef]
- Bund, D.; Buhmann, R.; Gökmen, F.; Zorn, J.; Kolb, H.J.; Schmetzer, H.M. Minor histocompatibility antigen UTY as target for gGaft-versus-leukemia and graft-versus-haematopoiesis in the canine model. Scand. J. Immunol. 2013, 77, 39–53. [Google Scholar]
- Herson, P.S.; Hurn, P.D. Gender and the injured brain. Prog. Brain Res. 2010, 186, 177–187. [Google Scholar] [CrossRef]
- Lang, J.T.; McCullough, L.D. Pathways to ischemic neuronal cell death: Are sex differences relevant? J. Transl. Med. 2008, 6, 33. [Google Scholar] [CrossRef]
- McCullough, L.D.; Zeng, Z.; Blizzard, K.K.; Debchoudhury, I.; Hurn, P.D. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: Male toxicity, female protection. J. Cereb. Blood Flow Metab. 2005, 25, 502–512. [Google Scholar]
- Yuan, M.; Siegel, C.; Zeng, Z.; Li, J.; Liu, F.; McCullough, L.D. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp. Neurol. 2009, 217, 210–218. [Google Scholar] [CrossRef]
- Renolleau, S.; Fau, S.; Goyenvalle, C.; Joly, L.M.; Chavier, D.; Jacotot, E.; Mariani, J.; Charriaut-Marlangue, C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: A role for gender. J. Neurochem. 2007, 100, 1062–1071. [Google Scholar]
- Liu, M.; Hurn, P.D.; Roselli, C.E.; Alkayed, N.J. Role of P450 aromatase in sex-specific astrocytic cell death. J. Cereb. Blood Flow Metab. 2007, 27, 135–141. [Google Scholar] [CrossRef]
- Ajmo, C.T., Jr.; Vernon, D.O.; Collier, L.; Hall, A.A.; Garbuzova-Davis, S.; Willing, A.; Pennypacker, K.R. The Spleen Contributes to Stroke-Induced Neurodegeneration. J. Neurosci. Res. 2008, 86, 2227–2234. [Google Scholar]
- Dirnagl, U.; Klehmet, J.; Braun, J.S.; Harms, H.; Meisel, C.; Ziemssen, T.; Prass, K.; Meisel, A. Stroke-induced immunodepression: Experimental evidence and clinical relevance. Stroke 2007, 38, 770–773. [Google Scholar]
- Offner, H.; Vandenbark, A.A.; Hurn, P.D. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience 2009, 158, 1098–1111. [Google Scholar]
- Zhang, B.; Subramanian, S.; Dziennis, S.; Jia, J.; Uchida, M.; Akiyoshi, K.; Migliati, E.; Lewis, A.D.; Vandenbrak, A.A.; Offner, H.; et al. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immunol. 2010, 184, 4087–4094. [Google Scholar] [CrossRef]
- Chiappetta, O.; Gliozzi, M.; Siviglia, E.; Amantea, D.; Morrone, L.A.; Berliocchi, L.; Bagetta, G.; Corasaniti, M.T. Evidence to implicate early modulation of interleukin-1β expression in the neuroprotection afforded by 17β-estradiol in male rats undergone transient middle cerebral artery occlusion. Int. Rev. Neurobiol. 2007, 82, 357–372. [Google Scholar] [CrossRef]
- Li, J.; Siegel, M.; Yuan, M.; Zeng, Z.; Finnucan, L.; Persky, R.; Hurn, P.D.; McCullough, L.D. Estrogen enhances neurogenesis and behavioral recovery after stroke. J. Cereb. Blood Flow Metab. 2011, 31, 413–425. [Google Scholar]
- Selvamani, A.; Sohrabji, F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 2010, 30, 6852–6861. [Google Scholar]
- Yuan, J.; Yu, J-X.; Ge, J. Sexual dimorphism on the neurogenic potential of rhesus monkeys mesenchymal stem cells. Biochem. Biophys. Res. Comm. 2010, 396, 394–400. [Google Scholar] [CrossRef]
- Waldron, J.; McCourty, A.; Lecanu, L. Aging differentially affects male and female neural stem cell neurogenic properties. Stem Cells Cloning 2010, 3, 119–127. [Google Scholar]
- Nakada, D.; Oquro, H.; Levi, B.P.; Ryan, N.; Kitano, A.; Saitoh, Y.; Takeichi, M.; Wendt, G.R.; Morrison, S.J. Oestrogen increases haematopoietic stem-cell self-renewal in females during pregnancy. Nature 2014, 505, 555–558. [Google Scholar] [CrossRef]
- Stern, M.; Brand, R.; de Witte, T.; Sureda, A.; Rocha, V.; Passweg, J.; Baldomero, H.; Niederwiesse, D.; Gratwohl, A. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am. J. Transplant. 2008, 8, 2149–2157. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, M.; Herring, C.M.; Markel, T.A.; Meldrum, K.K.; Lillemoe, K.D.; Meldrum, D.R. Gender differences in injury induced mesenchymal stem cell apoptosis and VEGF, TNF, IL-6 expression: Role of the 55 kDa TNF receptor (TNFR1). J. Mol. Cell Cardiol. 2007, 42, 142–149. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Markel, T.A.; Wang, M.; Lahm, T.; Lillemoe, K.D.; Meldrum, D.R. In the adult mesenchymal stem cell population, source gender is a biologically relevant aspect of protective power. Surgery 2007, 142, 215–221. [Google Scholar] [CrossRef]
- Zenovich, A.G.; Panoskaltsis-Mortari, A.; Caron, G.J.; Kolb, A.G.; Fremming, R.; Nelson, W.D.; Taylor, D.A. Sex-based differences in vascular repair with bone marrow cell therapy: Relevance of regulatory and Th2-type cytokines. Transplant. Proc. 2008, 40, 641–643. [Google Scholar]
- Nelson, W.D.; Zenovich, A.G.; Ott, H.C.; Stolen, C.; Caron, G.J.; Panoskaltsis-Mortari, A.; Barnes, S.A., 3rd.; Xin, X.; Taylor, D.A. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ. Res. 2007, 101, 1319–1327. [Google Scholar] [CrossRef]
- Waldron, J.; McCourty, A.; Lecanu, L. Neural stem cell sex dimorphism in aromatase (CYP19) expression: A basis for differential neural fate. Stem Cells Cloning 2010, 3, 175–182. [Google Scholar]
- Cavaco, J.E.; Laurentino, S.S.; Barros, A.; Sousa, M.; Socorro, S. Estrogen receptors α and β in human testis: Both isoforms are expressed. Syst. Biol. Reprod. Med. 2009, 55, 137–144. [Google Scholar]
- Berensztein, E.B.; Baquedano, M.S.; Gonzalez, C.R.; Saraco, N.I.; Rodriguez, J.; Ponzio, R.; Rivarola, M.A.; Belgorosky, A. Expression of aromatase, estrogen receptor α and β, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr. Res. 2006, 60, 740–744. [Google Scholar]
- Bonagura, T.W.; Zhou, H.; Babischkin, J.S.; Pepe, G.J.; Albrecht, E.D. Expression of P-450 aromatase, estrogen receptor α and β, and α-inhibin in the fetal baboon testis after estrogen suppression during the second half of gestation. Endocrine 2011, 39, 75–82. [Google Scholar] [CrossRef]
- Critchley, H.O.; Brenner, R.M.; Henderson, T.A.; Williams, K.; Nayak, N.R.; Slayden, O.D.; Millar, M.R.; Saunders, P.T.K. Estrogen receptor β, but not estrogen receptor α, is present in the vascular endothelium of the human and nonhuman primate endometrium. J. Clin. Endocrinol. Metab. 2001, 86, 1370–1378. [Google Scholar]
- Shreihofer, D.A.; Ma, Y. Estrogen receptors and ischemic neuroprotection: Who, what, where, and when? Brain Res. 2013, 1514, 107–122. [Google Scholar]
- Waldron, J.; Lecanu, L. Age and sex differences in neural stem cell transplantation: A descriptive study rats. Stem Cells Cloning 2011, 24, 25–37. [Google Scholar]
- Lecanu, L. Sex, the underestimated potential determining factor in brain tissue repair strategy. Stem Cells Dev. 2011, 20, 2031–2035. [Google Scholar]
- Shamekh, R.; el-Badri, N.S.; Saporta, S.; Pascual, C.; Sanberg, P.R.; Cameron, D.F. Sertoli cells induce systemic donor-specific tolerance in xenogenic transplantation model. Cell Transplant. 2006, 15, 45–53. [Google Scholar]
- Golat, B.T.; Cameron, D.F. Sertoli cells enhance formation of capillary-like structures in vitro. Cell Transplant. 2008, 17, 1135–1144. [Google Scholar] [CrossRef]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Meng, X.; Ichim, T.E.; Zhong, J.; Rogers, A.; Yin, Z.; Jackson, J.; Wang, H.; Ge, W.; Bogin, V.; Chan, K.W.; et al. Endometrial regenerative cells: A novel stem cell population. J. Transl. Med. 2007, 5, 57. [Google Scholar] [CrossRef]
- Allickson, J.G.; Sanchez, A.; Yeflmenko, N.; Borlongan, C.V.; Sanberg, P.R. Recent studies assessing the proliferative capability of a novel adult stem cell identified in menstrual blood. Open Stem Cell J. 2011, 3, 4–10. [Google Scholar] [CrossRef]
- Cui, C.H.; Uyama, T.; Miyado, K.; Terai, M.; Kyo, S.; Kiyono, T.; Umezawa, A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell. 2007, 18, 1586–1594. [Google Scholar] [CrossRef]
- Chan, R.W.; Schwab, K.E.; Gargett, C.E. Clonogenicity of human endometrial epithelial and stromal cells. Biol. Reprod. 2004, 70, 1738–1750. [Google Scholar] [CrossRef]
- Rodrigues, C.D.; Asensi, K.D.; Vairo, L.; Azevedo-Pereira, R.L.; Silva, R.; Rondinelli, E.; Goldenberg, R.C.; Campos de Carvalho, A.C.; Urmenyi, T.P. Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transplant. 2012, 21, 2215–2224. [Google Scholar]
- Oliveira Rodrigues, M.C.; Voltarelli, J.; Sanberg, P.R.; Allickson, J.G.; Kuzmin-Nichols, N.; Garbuzova-Davis, S.; Borlongan, C. Recent progress in cell therapy for basal ganglia disorders with emphasis on menstrual cell transplantation in stroke. Neurosci. Biobehav. Rev. 2012, 36, 177–190. [Google Scholar]
- Ketema, M.; Kraft, M.; Secades, P.; Janssen, H.; Sonnenberg, A. Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Am. Soc. Cell Biol. 2013, 24, 2454–2466. [Google Scholar]
- Wan, H.T.; Mruk, D.D.; Wong, C.K.; Cheng, C.Y. Perfluoroooctanesulfonate (PFOS) perturbs male rate Sertoli cell blood–testis barrier function by affecting F-actin organization via p-FAK–Tyr (407): An in vitro study. Endocrinology 2014, 155, 249–262. [Google Scholar] [CrossRef]
- Emerich, D.F.; Hemendinger, R.; Halberstadt, C.R. The testicular-derived Sertoli cell: Cellular immunoscience to enable transplantation. Cell Transplant. 2003, 12, 335–349. [Google Scholar]
- Rodriguez, A.I.; Willing, A.E.; Saporta, S.; Cameron, D.F.; Sanburg, P.R. Effects of Sertoli cell transplants in a 3-nitropropionic acid model of early Huntington’s disease: A preliminary study. Neurotox. Res. 2003, 5, 443–450. [Google Scholar] [CrossRef]
- Selawry, H.P.; Cameron, D.F. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant. 1993, 2, 123–129. [Google Scholar]
- Shamekh, R.; Newcomb, J.; Mallery, J.; Cassady, C.J.; Saporta, S.; Cameron, D.F. Survival of rat or mouse ventral mesencephalon neurons after o-transplantation with rat Sertoli cells in the mouse striatum. Cell Transplant. 2005, 14, 551–564. [Google Scholar] [CrossRef]
- Skinner, M. Sertoli cell secreated regulatory factors. In Sertoli Cell Biology; Skinner, M., Griswold, M., Eds.; Elsevier Academic Press: San Diego, CA, USA, 2005; pp. 95–120. [Google Scholar]
- Skinner, M.K. General Considerations. In The Sertoli Cell Cache River Press; Russell, L.D., Griswold, M.D., Eds.; River Press: Clearwater, FL, USA, 2003; pp. 237–247. [Google Scholar]
- Li, Y.; Xu, W.; Liu, H.; Fan, P.; Wang, X.; Ding, X.; Tian, X.; Feng, X.; Pan, X.; Zheng, J.; et al. Combined strategy of endothelial cells coating, Sertoli cells coculture and infusion improves vascularization and rejection protection of islet graft. PLoS One 2013, 8, e56696. [Google Scholar]
- Bellgrau, D.; Gold, D.; Selawry, H.; Moore, J.; Franzusoff, A.; Duke, R.C. A role for CD-95 ligand in preventing graft rejection. Nature 1995, 377, 630–632. [Google Scholar]
- Sanberg, P.R.; Saporta, S.; Borlongan, C.V.; Othberg, A.L.; Allen, R.C.; Cameron, D.F. The testis-derived cultured Sertoli cell as a natural fas-L secreting cell for immunosuppressive cellular therapy. Cell Transplant. 1997, 62, 191–193. [Google Scholar]
- Davidoff, M.S.; Middendorff, R.; Koeva, Y.; Pusch, W.; Jezek, D.; Muller, D. Glial cell line-derived neurotrophic factor (GDNF) and its receptors GFRα-1 and GFRα-2 in the human testis. Ital. J. Anat. Embryol. 2001, 106, 173–180. [Google Scholar]
- Hofmann, M.C. GDNF signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell. Endocrinol. 2008, 288, 95–103. [Google Scholar] [CrossRef]
- Dufour, J.M.; Rajotte, R.V.; Korbutt, G.S.; Emerich, D.F. Harnessing the immunomodulatory properties of Sertoli cells to enable xenotransplantation in type I diabetes. Immunol. Investig. 2003, 32, 275–297. [Google Scholar]
- Buganim, Y.; Itskovich, E.; Hu, Y.; Cheng, A.W.; Ganz, K.; Sarkar, S.; Fu, D.; Welstead, G.G.; Page, D.C.; Jaenissch, R. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell 2012, 3, 373–386. [Google Scholar]
- Zhong, Z.; Patel, A.N.; Ichim, T.E.; Ichim, T.E.; Riordan, N.H.; Wang, H.; Min, W.; Woods, E.J.; Reid, M.; Mansilla, R.; et al. Feasibility investigation of allogeneic endometrial regenerative cells. J. Transl. Med. 2009, 7, 15. [Google Scholar]
- Fan, X.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar]
- Drago, H.; Marín, G.H.; Sturla, F.; Roque, G.; Mártire, K.; Díaz Aquino, V.; Lamonega, R.; Gardiner, C.; Ichim, T.; Riordan, N.; et al. The next generation of burns treatment, intelligent films and matrix, controlled enzymatic debridement, and adult stem cells. Transplant. Proc. 2010, 42, 345–349. [Google Scholar]
- Sanberg, P.R.; Borlongan, C.V.; Saporta, S.; Cameron, D.F. Sertoli cells: An alternative cell source for neural transplantation in Parkinson’s disease. Exp. Neurol. 1995, 135, 169. [Google Scholar]
- Sanberg, P.R.; Borlongan, C.V.; Saporta, S.; Cameron, D.F. Testis-derived Sertoli cells survive and provide localized immunoprotection for xenografts in rat brain. Nat. Biotechnol. 1996, 14, 1692–1695. [Google Scholar]
- Sanberg, P.R.; Othberg, A.I.; Borlongan, C.V.; Saporta, S.; Anton, A.; Freeman, T.B.; Cahill, D.W.; Allen, R.C.; Cameron, D.F. Transplantation of testis-derived Sertoli cells into the mammalian brain. Transplant. Proc. 1997, 29, 1926–1928. [Google Scholar] [CrossRef]
- Abo-Elmaksoud, A.; Sinowatz, F. Expression and localization of growth factors and their receptors in the mammalian testis. Part I: Fibroblast growth factors and insulin-like growth factors. Anat. Histol. Embryol. 2005, 34, 319–334. [Google Scholar]
- Reddy, N.; Kasukurthi, K.B.; Mahla, R.S.; Pawar, R.M.; Goel, S. Expression of vascular endothelial growth factor (VEGF) transcript and protein in the testis of several vertebrates including endangered species. Theriogenology 2012, 77, 608–614. [Google Scholar] [CrossRef]
- Ferreira, L.M.; Mostajo-Radji, M.A. How induced pluripotent stem cells are redefining personalized medicine. Gene 2013, 520, 1–6. [Google Scholar] [CrossRef]
- Zhang, M.J.; Liu, B.; Xia, W.; Sun, Z.Y.; Lu, K.H. Could cells from menstrual blood be a new source for cell-based therapies? Med. Hypotheses 2009, 72, 252–254. [Google Scholar]
- Itman, C.; Wong, C.; Hunyadi, B.; Ernst, M.; Jans, D.A.; Loveland, K.L. Smad3 dosage determines androgen responsiveness and sets the pace of postnatal testis development. Endocrinology 2011, 152, 2076–2089. [Google Scholar] [CrossRef]
- Tarulli, G.A.; Stanton, P.G.; Meachem, S.J. Is the adult Sertoli cell terminally differentiated? Biol. Reprod. 2012, 87, 1–11. [Google Scholar]
- Halley, K.; Dyson, E.L.; Kaur, G; Mital, P.; Uong, P.M.; Dass, B.; Crowell, S.N.; Dufours, J.M. Delivery of a therapeutic protein by immune-privileged Sertoli cells. Cell Transplant. 2010, 19, 1645–1657. [Google Scholar] [CrossRef]
- Nurmio, M.; Keros, V.; Lähteenmäki, P.; Salmi, T.; Kallajoki, M.; Jahnukainen, K. Effect of childhood acute lymphoblastic leukemia therapy on spermatogonia populations and future fertility. J. Clin. Endocrinol. Metab. 2009, 94, 2119–2122. [Google Scholar] [CrossRef]
- Izadyar, F.; Spierenberg, G.T.; Creemers, L.B.; den Ouden, K.; de Rooij, D.G. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction 2002, 124, 85–94. [Google Scholar]
- Ryser, S.; Glauser, D.; Vigier, M.; Zhang, Y.Q.; Tachini, P.; Schlegel, W.; Durand, P.; Irminger-Finger, I. Gene expression profiling of rat spermatogonial and sertoli cells reveals signaling pathways from stem cells to niche and testicular cancer cells to surrounding stroma. BMC Genomics 2011, 12, 29. [Google Scholar] [CrossRef] [Green Version]
- Brinster, C.J.; Ryu, B.Y.; Avarbock, M.R.; Karagenc, L.; Brinster, R.L.; Orwig, K.E. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol. Reprod. 2003, 69, 412–420. [Google Scholar] [CrossRef]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Nat. Acad. Sci. USA 2001, 98, 6186–6191. [Google Scholar] [CrossRef]
- McLean, D.J.; Friel, P.J.; Johnston, D.S.; Griswold, M.D. Characterization of spermatogonial stem cell maturation and differentiation in neonatal mice. Biol. Reprod. 2003, 69, 2085–2091. [Google Scholar]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar]
- Nakagawa, T.; Nabeshima, Y.; Yoshida, S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell 2007, 12, 195–206. [Google Scholar]
- Drummond, A.E.; Britt, K.L.; Dyson, M.; Jones, M.E.; Kerr, J.B.; O’Donnell, L.; Simpson, E.R.; Findlay, J.K. Ovarian steroid receptors and their role in ovarian function. Mol. Cell. Endocrinol. 2002, 191, 27–33. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tajiri, N.; Duncan, K.; Borlongan, M.C.; Pabon, M.; Acosta, S.; De la Pena, I.; Hernadez-Ontiveros, D.; Lozano, D.; Aguirre, D.; Reyes, S.; et al. Adult Stem Cell Transplantation: Is Gender a Factor in Stemness? Int. J. Mol. Sci. 2014, 15, 15225-15243. https://doi.org/10.3390/ijms150915225
Tajiri N, Duncan K, Borlongan MC, Pabon M, Acosta S, De la Pena I, Hernadez-Ontiveros D, Lozano D, Aguirre D, Reyes S, et al. Adult Stem Cell Transplantation: Is Gender a Factor in Stemness? International Journal of Molecular Sciences. 2014; 15(9):15225-15243. https://doi.org/10.3390/ijms150915225
Chicago/Turabian StyleTajiri, Naoki, Kelsey Duncan, Mia C. Borlongan, Mibel Pabon, Sandra Acosta, Ike De la Pena, Diana Hernadez-Ontiveros, Diego Lozano, Daniela Aguirre, Stephanny Reyes, and et al. 2014. "Adult Stem Cell Transplantation: Is Gender a Factor in Stemness?" International Journal of Molecular Sciences 15, no. 9: 15225-15243. https://doi.org/10.3390/ijms150915225




