Interplay between Endothelin and Erythropoietin in Astroglia: The Role in Protection against Hypoxia
Abstract
:1. Introduction
2. Results
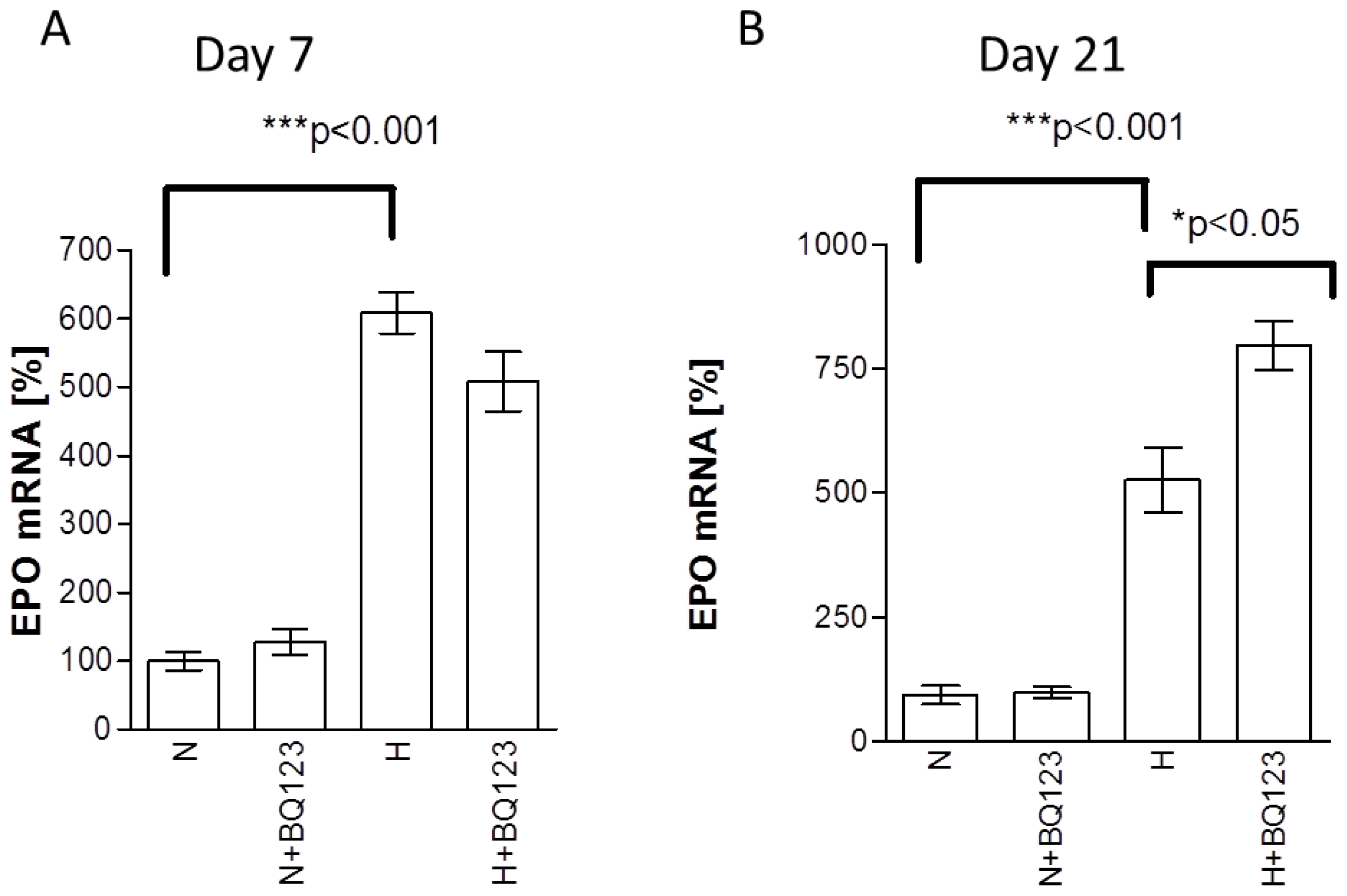
2.1. Influence of ETA Receptor Blockade on the Expression of EPO mRNA
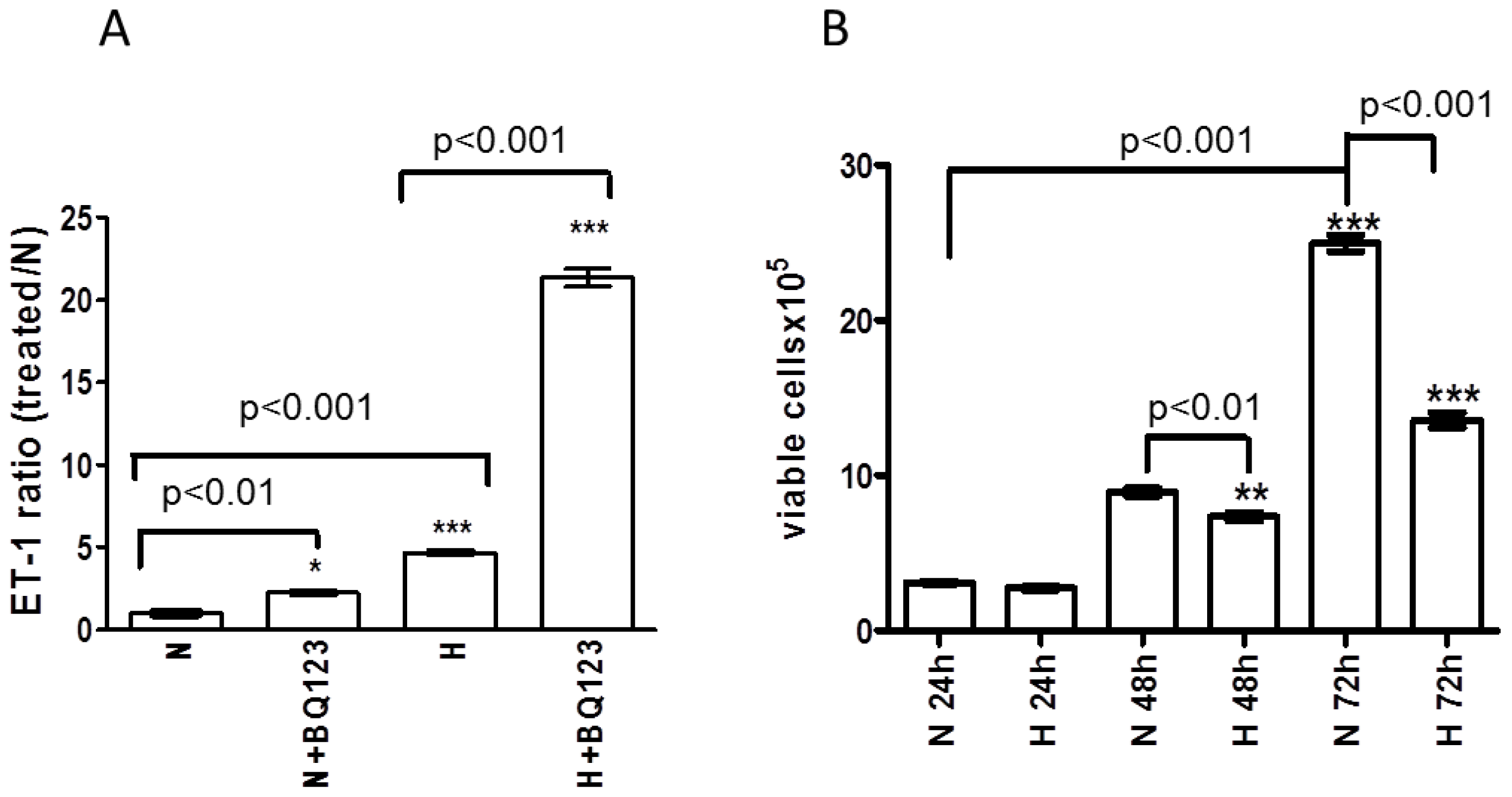
2.2. ETA Receptor Blockade Increases the Release of ET-1 Protein from Human Astrocytes
2.3. Influence of Prolonged Hypoxia on the Viability of Human Astrocytes
2.4. Influence of EPO on the Release of ET-1 Protein from Human Astrocytes
2.5. Effects of ETA Receptor Blockade on the Expression of EPO Receptor in Astrocytes and Neural Precursors
3. Discussion
4. Experimental Section
4.1. Rat Astroglial Primary Culture
4.2. Human Astrocytes (SV-40 FHAS) Culture
4.3. Hypoxia and Stimulation with BQ123 and EPO
4.4. Immunocytochemical Analysis
4.5. Real Time RT-PCR
4.6. Determination of ET-1 Protein
4.7. Statistical Analyses
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsL.D., M.S. and C.H.G. conceived and designed the study; L.M., B.P. and R.B. performed the experiments; R.S., L.M. and L.D. wrote the manuscript; M.S., C.H.G., B.P. and R.B. reviewed and edited the manuscript.
References
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 32, 411–415. [Google Scholar]
- Gandhi, C.R.; Nemoto, E.M.; Watkins, S.C.; Subbotin, V.M. An endothelin receptor antagonist TAK-044 ameliorates carbon tetrachloride-induced acute liver injury and portal hypertension in rats. Liver 1998, 18, 39–48. [Google Scholar]
- Sakurai, T.; Yanagisawa, M.; Inoue, A.; Ryan, U.S.; Kimura, S.; Mitsui, I.; Goto, K.; Masaki, T. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem. Biophys. Res. Commun 1991, 175, 44–47. [Google Scholar]
- Boulanger, C.; Luscher, T.F. Release of from the porcine aorta. Inhibition by endotheliumderived nitric oxide. J. Clin. Investig 1990, 85, 587–590. [Google Scholar]
- Lin, H.Y.; Kaji, E.H.; Winkel, G.K.; Ives, H.E.; Lodish, H.F. Cloning and functional expression of a vascular smooth muscle endothelin 1 receptor. Proc. Natl. Acad. Sci. USA 1991, 88, 3185–3189. [Google Scholar]
- Simonson, M.S. Endothelins: Multifunctional renal peptides. Physiol. Rev 1993, 73, 375–411. [Google Scholar]
- Kobari, M.; Fukuuchi, Y.; Tomita, M.; Tanahashi, N.; Konno, S.; Takeda, H. Dilatation of cerebral microvessels mediated by endothelin ETB receptor and nitric oxide in cats. Neurosci Lett 1994, 176, 157–160. [Google Scholar]
- Luo, J.; Grammas, P. Endothelin-1 is elevated in Alzheimer’s disease brain microvessels and is neuroprotective. J. Alzheimers Dis 2010, 21, 887–896. [Google Scholar]
- Bychkov, R.; Glowinski, J.; Giaume, C. Sequential and opposite regulation of two outward K+ currents by ET-1 in cultured striatal astrocytes. Am. J. Physiol. Cell Physiol 2001, 281, C1373–C1384. [Google Scholar]
- Taberner, O.A.; Giaume, C.; Medina, J.M. Endothelin-1 regulates glucose utilization in cultured astrocytes by controlling intercellular communication through gap junctions. Glia 1996, 16, 187–195. [Google Scholar]
- Leonova, J.; Thorleif, T.; Åberg, N.D.; Eriksson, P.S.; Rönnbäck, L.; Hansson, E. Endothelin-1 decreases glutamate uptake in primary cultured rat astrocytes. Am. J. Physiol. Cell Physiol 2001, 281, C1495–C1503. [Google Scholar]
- Sasaki, Y.; Takimoto, M.; Oda, K.; Fruh, T.; Takai, M.; Okada, T.; Hori, S. Endothelin evokes efflux of glutamate in cultures of rat astrocytes. J. Neurochem 1997, 68, 2194–2200. [Google Scholar]
- Blomstrand, F.; Giaume, C.; Hansson, E.; Rönnbäck, L. Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling. Am. J. Physiol. Cell Physiol 1999, 277, C616–C627. [Google Scholar]
- Giaume, C.; Cordier, J.; Glowinski, J. Endothelins inhibit junctional permeability in cultured mouse astrocytes. Eur. J. Neurosci 1992, 4, 877–881. [Google Scholar]
- Venance, L.; Stella, N.; Glowinski, J.; Giaume, C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J. Neurosci 1997, 17, 1981–1992. [Google Scholar]
- Ehrenreich, H.; Anderson, R.W.; Ogino, Y.; Rieckmann, P.; Costa, T.; Wood, G.P.; Coligan, J.E.; Kehrl, J.H.; Fauci, A.S. Selective autoregulation of endothelins in primary astrocyte cultures: Endothelin receptor-mediated potentiation of endothelin-1 secretion. New Biol 1991, 3, 135–141. [Google Scholar]
- Ehrenreich, H.; Costa, T.; Clouse, K.A.; Pluta, R.M.; Ogino, Y.; Coligan, J.E.; Burd, P.R. Thrombin is a regulator of astrocytic endothelin-1. Brain Res 1993, 600, 201–207. [Google Scholar]
- Kleeberg, J.; Petzold, G.C.; Major, S.; Dirnagl, U.; Dreier, J.P. ET-1 induces cortical spreading depression via activation of the ETA receptor/phospholipase C pathway in vivo. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1339–H1346. [Google Scholar]
- Agnati, L.F.; Zoli, M.; Benfenati, F.; Bagini, G.; Zini, I.; Hallstrom, A.; Ungerstedt, U.; Toffano, G.; Fuxe, K. A new model of focal brain ischemia based on the intracerebral injection of endothelin-1. Ital. J. Neurol. Sci 1991, 12, 49–53. [Google Scholar]
- Gartshore, G.; Patterson, J.; Macrae, I.M. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp. Neurol 1997, 147, 353–360. [Google Scholar]
- Sato, M.; Noble, L.J. Involvement of the endothelin receptor subtype A in neuronal pathogenesis after traumatic brain injury. Brain Res 1998, 809, 39–49. [Google Scholar]
- Ehrenreich, H.; Nau, T.R.; Dembowski, C.; Hasselblatt, M.; Barth, M.; Hahn, A.; Schilling, L.; Siren, A.L.; Bruck, W. Endothelin B receptor deficiency is associated with an increased rate of neuronal apoptosis in the dentate gyrus. Neuroscience 2000, 95, 993–1001. [Google Scholar]
- Kohzuma, M.; Kataoka, Y.; Koizumi, S.; Shibaguchi, H.; Nakashima, M.N.; Yamashita, K.; Niwa, M.; Taniyama, K. ETB receptor involvement in stimulatory and neurotoxic action of endothelin on dopamine neurones. Neuroreport 1994, 5, 2653–2656. [Google Scholar]
- Yagami, T.; Ueda, K.; Asakura, K.; Kuroda, T.; Hata, S.; Sakaeda, T.; Kambayashi, Y.; Fujimoto, M. Effects of endothelin B receptor agonists on amyloid b protein 25–35-induced neuronal cell death. Brain Res 2002, 948, 72–81. [Google Scholar]
- Bachmann, S.; le Hir, M.; Eckardt, K.U. Co-localization of erythropoietin mRNA and ecto-5′-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J. Histochem. Cytochem 1993, 41, 335–341. [Google Scholar]
- Siren, A.L.; Knerlich, F.; Poser, W.; Gleiter, C.H.; Bruck, W.; Ehrenreich, H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. (Berl.) 2001, 101, 271–276. [Google Scholar]
- Digicaylioglu, M.; Kaul, M.; Fletcher, L.; Dowen, R.; Lipton, S.A. Erythropoietin protects cerebrocortical neurons from HIV-1/gp120-induced damage. Neuroreport 2004, 15, 761–763. [Google Scholar]
- Sattler, M.B.; Merkler, D.; Maier, K.; Stadelmann, C.; Ehrenreich, H.; Bahr, M.; Diem, R. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ 2004, 11(Suppl 2), S181–S192. [Google Scholar]
- Chong, Z.Z.; Li, F.; Maiese, K. Erythropoietin requires NF-κB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr. Neurovasc. Res 2005, 2, 387–399. [Google Scholar]
- Danielyan, L.; Mueller, L.; Proksch, B.; Kabisch, D.; Weller, M.; Wiesinger, H.; Buniatian, G.H.; Gleiter, C.H. Similar protective effects of BQ123 and erythropoietin on survival of neural cells and generation of neurons upon hypoxic injury. Eur. J. Cell Biol 2005, 84, 907–913. [Google Scholar]
- Esneault, E.; Pacary, E.; Eddi, D.; Freret, T.; Tixier, E.; Toutain, J.; Touzani, O.; Schumann-Bard, P.; Petit, E.; Roussel, S.; et al. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab 2008, 28, 1552–1563. [Google Scholar]
- Danielyan, L.; Schäfer, R.; Schulz, A.; Ladewig, T.; Lourhmati, A.; Buadze, M.; Schmitt, A.L.; Verleysdonk, S.; Kabisch, D.; Koeppen, K.; et al. Survival, neuron-like differentiation and functionality of mesenchymal stem cells in neurotoxic environment: The critical role of erythropoietin. Cell Death Differ 2009, 16, 1599–1614. [Google Scholar]
- Danielyan, L.; Gembizki, O.; Proksch, B.; Weinmann, M.; Morgalla, M.; Wiesinger, H.; Buniatian, G.H.; Gleiter, C.H. The blockade of endothelin A receptor protects astrocytes against hypoxic injury: Common effects of BQ123 and erythropoietin on the rejuvenation of the astrocyte population. Eur. J. Cell Biol 2005, 84, 567–579. [Google Scholar]
- Slowinski, T.; Schulz, N.; Ruschitzka, F.T.; Quaschning, T.; Bauer, C.; Theuring, F.; Neumayer, H.H.; Gassmann, M.; Hocher, B. Pattern of prepro-endothelin-1 expression revealed by reporter-gene activity in kidneys of erythropoietin-overexpressing mice. Clin. Sci. (Lond.) 2002, 103(Suppl 48), 44S–47S. [Google Scholar]
- Quaschning, T.; Ruschitzka, F.; Stallmach, T.; Shaw, S.; Morawietz, H.; Goettsch, W.; Hermann, M.; Slowinski, T.; Theuring, F.; Hocher, B.; et al. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. FASEB J 2003, 17, 259–261. [Google Scholar]
- Earley, S.; Nelin, L.D.; Chicoine, L.G.; Walker, B.R. Hypoxia-induced pulmonary endothelin-1 expression is unaltered by nitric oxide. J. Appl. Physiol 2002, 92, 1152–1158. [Google Scholar]
- Ritthaler, T.; Göpfert, T.; Firth, J.D.; Ratcliffe, P.J.; Krämer, B.K.; Kurtz, A. Influence of hypoxia on hepatic and renal endothelin gene expression. Pflug. Arch 1996, 431, 587–593. [Google Scholar]
- McWhinnie, R.; Pechkovsky, D.V.; Zhou, D.; Lane, D.; Halayko, A.J.; Knight, D.A.; Bai, T.R. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol 2007, 292, L278–L286. [Google Scholar]
- Banes-Berceli, A.K.; Ketsawatsomkron, P.; Ogbi, S.; Patel, B.; Pollock, D.M.; Marrero, M.B. Angiotensin II and endothelin-1 augment the vascular complications of diabetes via JAK2 activation. Am. J. Physiol. Heart Circ. Physiol 2007, 293, H1291–H1299. [Google Scholar]
- Rafiee, P.; Shi, Y.; Su, J.; Pritchard, K.A., Jr.; Tweddell, J.S.; Baker, J.E. Erythropoietin protects the infant heart against ischemia-reperfusion injury by triggering multiple signaling pathways. Basic Res. Cardiol 2005, 100, 187–197. [Google Scholar]
- Cheng, T.H.; Shih, N.L.; Chen, C.H.; Lin, H.; Liu, J.C.; Chao, H.H.; Liou, J.Y.; Chen, Y.L.; Tsai, H.W.; Chen, Y.S.; et al. Role of mitogen-activated protein kinase pathway in reactive oxygen species-mediated endothelin-1-induced β-myosin heavy chain gene expression and cardiomyocyte hypertrophy. J. Biomed. Sci 2005, 12, 123–133. [Google Scholar]
- Gonzalez, F.F.; McQuillen, P.; Mu, D.; Chang, Y.; Wendland, M.; Vexler, Z.; Ferriero, D.M. Erythropoietin enhances long-term neuroprotection and neurogenesis in neonatal stroke. Dev. Neurosci 2007, 29, 321–330. [Google Scholar]
- Agnello, D.; Bigini, P.; Villa, P.; Mennini, T.; Cerami, A.; Brines, M.L.; Ghezzi, P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 2002, 952, 128–134. [Google Scholar]
- Ehrenreich, H.; Fischer, B.; Norra, C.; Schellenberger, F.; Stender, N.; Stiefel, M.; Sirén, A.L.; Paulus, W.; Nave, K.A.; Gold, R.; et al. Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 2007, 130, 2577–2588. [Google Scholar]
- Xue, Y.Q.; Zhao, L.R.; Guo, W.P.; Duan, W.M. Intrastriatal administration of erythropoietin protects dopaminergic neurons and improves neurobehavioral outcome in a rat model of Parkinson’s disease. Neuroscience 2007, 146, 1245–1258. [Google Scholar]
- Rakai, B.D.; Antle, M.C. Lesion size and behavioral deficits afterendothelin-1-induced ischemia are not dependent on time of day. J. Stroke Cerebrovasc. Dis 2013, 22, 397–405. [Google Scholar]
- Ho, M.C.; Lo, A.C.; Kurihara, H.; Yu, A.C.; Chung, S.S.; Chung, S.K. Endothelin-1 protects astrocytes from hypoxic/ischemic injury. FASEB J 2001, 15, 618–626. [Google Scholar]
- Morga, E.; Faber, C.; Heuschling, P. Stimulation of endothelin B receptor modulates the inflammatory activation of rat astrocytes. J. Neurochem 2000, 74, 603–612. [Google Scholar]
- Hellwig-Bürgel, T.; Stiehl, D.P.; Wagner, A.E.; Metzen, E.; Jelkmann, W. Review: Hypoxia-inducible factor-1 (HIF-1): A novel transcription factor in immune reactions. J. Interf. Cytokine Res 2005, 25, 297–310. [Google Scholar]
- Ameln, H.; Gustafsson, T.; Sundberg, C.J.; Okamoto, K.; Jansson, E.; Poellinger, L.; Makino, Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J 2005, 19, 1009–1011. [Google Scholar]
- Desai, D.; He, S.; Yorio, T.; Krishnamoorthy, R.R.; Prasanna, G. Hypoxia augments TNF-α-mediated endothelin-1 release and cell proliferation in human optic nerve head astrocytes. Biochem. Biophys. Res. Commun 2004, 318, 642–648. [Google Scholar]
- Danielyan, L.; Lourhmati, A.; Verleysdonk, S.; Kabisch, D.; Proksch, B.; Thiess, U.; Umbreen, S.; Schmidt, B.; Gleiter, C.H. Angiotensin receptor type 1 blockade in astroglia decreases hypoxia-induced cell damage and TNF α release. Neurochem. Res 2007, 32, 1489–1498. [Google Scholar]
- Iwasaki, S.; Homma, T.; Matsuda, Y.; Kon, V. Endothelin receptor subtype B mediates autoinduction of endothelin-1 in rat mesangial cells. J. Biol. Chem 1995, 270, 6997–7003. [Google Scholar]
- Khamaisi, M.; Dahan, R.; Hamed, S.; Abassi, Z.; Heyman, S.N.; Raz, I. Role of protein kinase C in the expression of endothelin converting enzyme-1. Endocrinology 2008, 150, 1440–1449. [Google Scholar]
- Benigni, A.; Remuzzi, G. Endothelin antagonists. Lancet 1999, 353, 133–138. [Google Scholar]
- Nakamura, S.; Naruse, M.; Naruse, K.; Demura, H.; Uemura, H. Immunocytochemical localization of endotheIin in cultured bovine endothelial cells. Histochentistry 1990, 94, 475–477. [Google Scholar]
- Nakamura, S.; Naruse, M.; Naruse, K.; Shioda, S.; Nakai, Y.; Uemura, H. Colocalization of immunoreactive endothelin-1 and neurohypophysial hormones in the axons of the neural lobe of the rat pituitary. Endocrinology 1993, 132, 530–533. [Google Scholar]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar]
- Lourhmati, A.; Buniatian, G.H.; Paul, C.; Verleysdonk, S.; Buecheler, R.; Buadze, M.; Proksch, B.; Schwab, M.; Gleiter, C.H.; Danielyan, L. Age-dependent astroglial vulnerability to hypoxia and glutamate: The role for erythropoietin. PLoS One 2013, 8, e77182. [Google Scholar]
- Ehrenreich, H.; Hasselblatt, M.; Dembowski, C.; Cepek, L.; Lewczuk, P.; Stiefel, M.; Rustenbeck, H.H.; Breiter, N.; Jacob, S.; Knerlich, F.; et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol. Med 2002, 8, 495–505. [Google Scholar]



© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schäfer, R.; Mueller, L.; Buecheler, R.; Proksch, B.; Schwab, M.; Gleiter, C.H.; Danielyan, L. Interplay between Endothelin and Erythropoietin in Astroglia: The Role in Protection against Hypoxia. Int. J. Mol. Sci. 2014, 15, 2858-2875. https://doi.org/10.3390/ijms15022858
Schäfer R, Mueller L, Buecheler R, Proksch B, Schwab M, Gleiter CH, Danielyan L. Interplay between Endothelin and Erythropoietin in Astroglia: The Role in Protection against Hypoxia. International Journal of Molecular Sciences. 2014; 15(2):2858-2875. https://doi.org/10.3390/ijms15022858
Chicago/Turabian StyleSchäfer, Richard, Lars Mueller, Reinhild Buecheler, Barbara Proksch, Matthias Schwab, Christoph H. Gleiter, and Lusine Danielyan. 2014. "Interplay between Endothelin and Erythropoietin in Astroglia: The Role in Protection against Hypoxia" International Journal of Molecular Sciences 15, no. 2: 2858-2875. https://doi.org/10.3390/ijms15022858




