Androgen Receptor Phosphorylation at Serine 308 and Serine 791 Predicts Enhanced Survival in Castrate Resistant Prostate Cancer Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Clinico-Pathological Detail
2.3. Protein Expression in the Castrate Resistant Tumours
2.3.1. Association of pAR791 Expression with Apoptosis and Proliferation
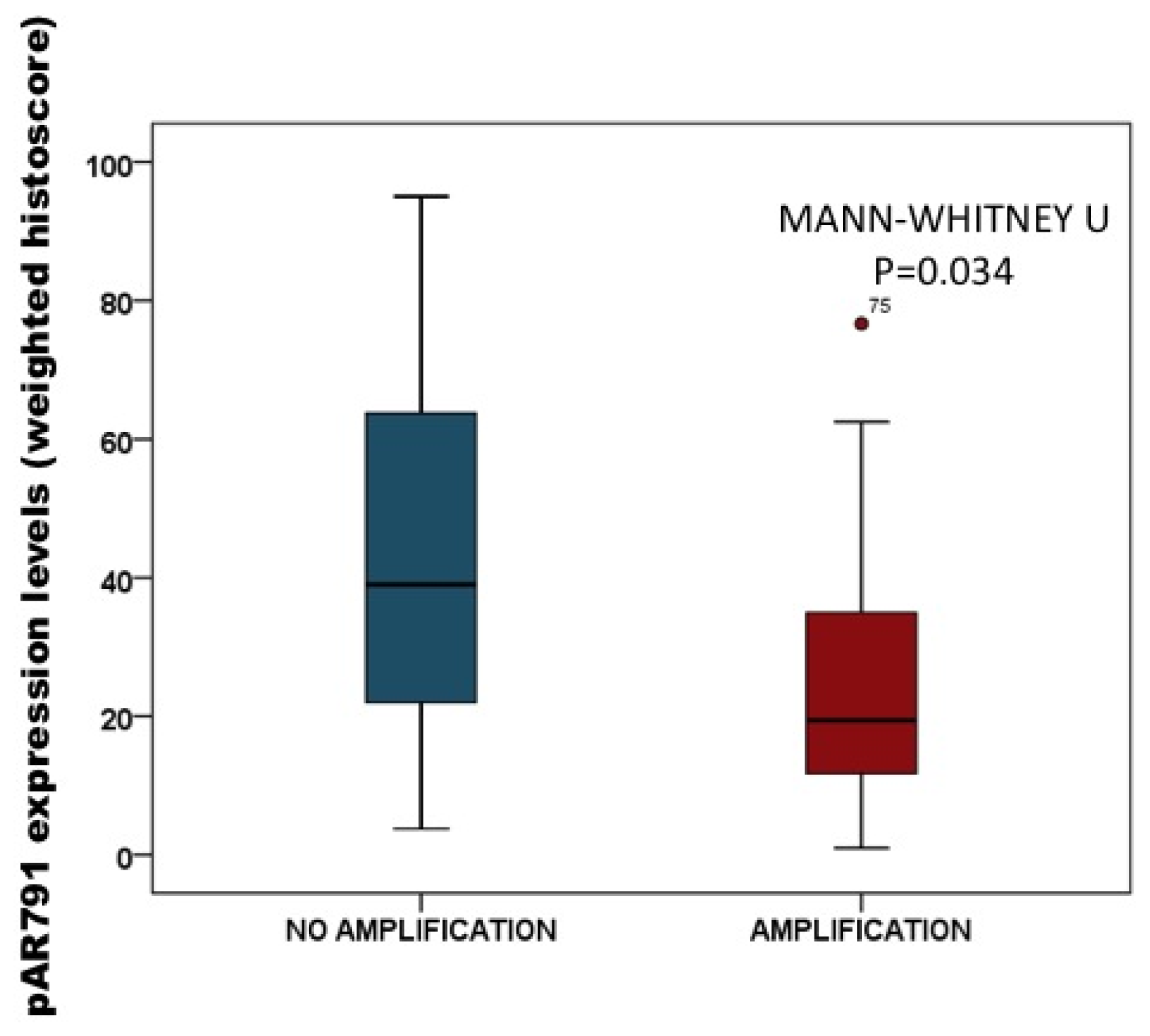
2.3.2. Association of pAR791 Expression with AR Gene Amplification
2.4. Discussion
3. Experimental Section
3.1. Patients
3.2. Tissue Microarray (TMA) Construction
3.3. Protein Expression Assessment
3.3.1. Antibody Specificity
3.3.2. Immunohistochemistry
3.3.3. Scoring Method
3.4. Statistical Analysis
4. Conclusions
Supplementary Information
ijms-14-16656-s001.pdfAcknowledgments
Conflicts of Interest
References
- Cancer Incidence for Common Cancers. Available online: http://www.cancerresearchuk.org/cancer-info/cancerstats/incidence/commoncancers/-Top (accessed on 8 January 2013).
- Cancer Mortality for Common Cancers. Available online: http://www.cancerresearchuk.org/cancer-info/cancerstats/mortality/cancerdeaths/-Top (accessed on 8 January 2013).
- Gioeli, D.; Ficarro, S.B.; Kwiek, J.J.; Aaronson, D.; Hancock, M.; Catling, A.D.; White, F.M.; Christian, R.E.; Settlage, R.E.; Shabanowitz, J.; et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem 2002, 277, 29304–29314. [Google Scholar]
- Ponguta, L.A.; Gregory, C.W.; French, F.S.; Wilson, E.M. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J. Biol. Chem 2008, 283, 20989–21001. [Google Scholar]
- Van Laar, J.H.; Berrevoets, C.A.; Trapman, J.; Zegers, N.D.; Brinkmann, A.O. Hormone-dependent androgen receptor phosphorylation is accompanied by receptor transformation in human lymph node carcinoma of the prostate cells. J. Biol. Chem 1991, 266, 3734–3738. [Google Scholar]
- Mahajan, N.P.; Liu, Y.; Majumder, S.; Warren, M.R.; Parker, C.E.; Mohler, J.L.; Earp, H.S.; Whang, Y.E. Activated Cdc42-associated kinase ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 2007, 104, 8438–8443. [Google Scholar]
- Callewaert, L.; Verrijdt, G.; Haelens, A.; Claessens, F. Differential effect of small ubiquitin-like modifier (Sumo)-Ylation of the androgen receptor in the control of cooperativity on selective versus canonical response elements. Mol. Endocrinol 2004, 18, 1438–1449. [Google Scholar]
- Fu, M.; Wang, C.; Wang, J.; Zhang, X.; Sakamaki, T.; Yeung, Y.G.; Chang, C.; Hopp, T.; Fuqua, S.A.; Jaffray, E.; et al. Androgen receptor acetylation governs trans activation and Mekk1-induced apoptosis without affecting in vitro sumoylation and trans-repression function. Mol. Cell. Biol 2002, 22, 3373–3388. [Google Scholar]
- Gaughan, L.; Logan, I.R.; Cook, S.; Neal, D.E.; Robson, C.N. Tip60 and histone Deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J. Biol. Chem 2002, 277, 25904–25913. [Google Scholar]
- Lin, H.K.; Hu, Y.C.; Yang, L.; Altuwaijri, S.; Chen, Y.T.; Kang, H.Y.; Chang, C. Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-Kinase/Akt pathway in prostate cancer lncap cells with different passage numbers. J. Biol. Chem 2003, 278, 50902–50907. [Google Scholar]
- Mellinghoff, I.K.; Vivanco, I.; Kwon, A.; Tran, C.; Wongvipat, J.; Sawyers, C.L. Her2/Neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 2004, 6, 517–527. [Google Scholar]
- Gordon, V.; Bhadel, S.; Wunderlich, W.; Zhang, J.; Ficarro, S.B.; Mollah, S.A.; Shabanowitz, J.; Hunt, D.F.; Xenarios, I.; Hahn, W.C.; et al. Cdk9 regulates Ar promoter selectivity and cell growth through serine 81 phosphorylation. Mol. Endocrinol 2010, 24, 2267–2280. [Google Scholar]
- Shigemura, K.; Isotani, S.; Wang, R.; Fujisawa, M.; Gotoh, A.; Marshall, F.F.; Zhau, H.E.; Chung, L.W. Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate lncap cells: Roles of Erk/map kinase. Prostate 2009, 69, 949–955. [Google Scholar]
- Rochette-Egly, C. Nuclear receptors: Integration of multiple signalling pathways through phosphorylation. Cell Signal 2003, 15, 355–366. [Google Scholar]
- Mccall, P.; Gemmell, L.K.; Mukherjee, R.; Bartlett, J.M.; Edwards, J. Phosphorylation of the androgen receptor is associated with reduced survival in hormone-refractory prostate cancer patients. Br. J. Cancer 2008, 98, 1094–1101. [Google Scholar]
- Mccall, P.; Witton, C.J.; Grimsley, S.; Nielsen, K.V.; Edwards, J. Is pten loss associated with clinical outcome measures in human prostate cancer? Br. J. Cancer 2008, 99, 1296–1301. [Google Scholar]
- Black, B.E.; Vitto, M.J.; Gioeli, D.; Spencer, A.; Afshar, N.; Conaway, M.R.; Weber, M.J.; Paschal, B.M. Transient, ligand-dependent arrest of the androgen receptor in subnuclear foci alters phosphorylation and coactivator interactions. Mol. Endocrinol 2004, 18, 834–850. [Google Scholar]
- Taneja, S.S.; Ha, S.; Swenson, N.K.; Huang, H.Y.; Lee, P.; Melamed, J.; Shapiro, E.; Garabedian, M.J.; Logan, S.K. Cell-specific regulation of androgen receptor phosphorylation in vivo. J. Biol. Chem 2005, 280, 40916–40924. [Google Scholar]
- Wen, Y.; Hu, M.C.; Makino, K.; Spohn, B.; Bartholomeusz, G.; Yan, D.H.; Hung, M.C. Her-2/Neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res 2000, 60, 6841–6845. [Google Scholar]
- Zhou, Z.X.; Kemppainen, J.A.; Wilson, E.M. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol. Endocrinol 1995, 9, 605–615. [Google Scholar]
- Tam, L.; Mcglynn, L.M.; Traynor, P.; Mukherjee, R.; Bartlett, J.M.; Edwards, J. Expression levels of the Jak/Stat pathway in the transition from hormone-sensitive to hormone-refractory prostate cancer. Br. J. Cancer 2007, 97, 378–383. [Google Scholar]
- Traynor, P.; Mcglynn, L.M.; Mukhergee, R.; Grimsley, S.J.; Bartlett, J.M.; Edwards, J. An increase in N-Ras expression is associated with development of hormone refractory prostate cancer in a subset of patients. Dis. Markers 2008, 24, 157–165. [Google Scholar]
- Chen, S.; Xu, Y.; Yuan, X.; Bubley, G.J.; Balk, S.P. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc. Natl. Acad. Sci. USA 2006, 103, 15969–15974. [Google Scholar]
- Zong, H.; Chi, Y.; Wang, Y.; Yang, Y.; Zhang, L.; Chen, H.; Jiang, J.; Li, Z.; Hong, Y.; Wang, H.; et al. Cyclin D3/Cdk11p58 complex is involved in the repression of androgen receptor. Mol. Cell. Biol 2007, 27, 7125–7142. [Google Scholar]
- Gioeli, D.; Black, B.E.; Gordon, V.; Spencer, A.; Kesler, C.T.; Eblen, S.T.; Paschal, B.M.; Weber, M.J. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol. Endocrinol 2006, 20, 503–515. [Google Scholar]
- Hsu, F.N.; Chen, M.C.; Chiang, M.C.; Lin, E.; Lee, Y.T.; Huang, P.H.; Lee, G.S.; Lin, H. Regulation of androgen receptor and prostate cancer growth by cyclin-dependent kinase 5. J. Biol. Chem 2011, 286, 33141–33149. [Google Scholar]
- Wong, C.I.; Zhou, Z.X.; Sar, M.; Wilson, E.M. Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J. Biol. Chem 1993, 268, 19004–19012. [Google Scholar]
- Willder, J.M.; Heng, S.J.; Mccall, P.; Adams, C.E.; Tannahill, C.; Fyffe, G.; Seywright, M.; Horgan, P.G.; Leung, H.Y.; Underwood, M.A.; et al. Androgen receptor phosphorylation at serine 515 by Cdk1 predicts biochemical relapse in prostate cancer patients. Br. J. Cancer 2013, 108, 139–148. [Google Scholar]
- Kirkegaard, T.; Edwards, J.; Tovey, S.; Mcglynn, L.M.; Krishna, S.N.; Mukherjee, R.; Tam, L.; Munro, A.F.; Dunne, B.; Bartlett, J.M. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology 2006, 48, 787–794. [Google Scholar]
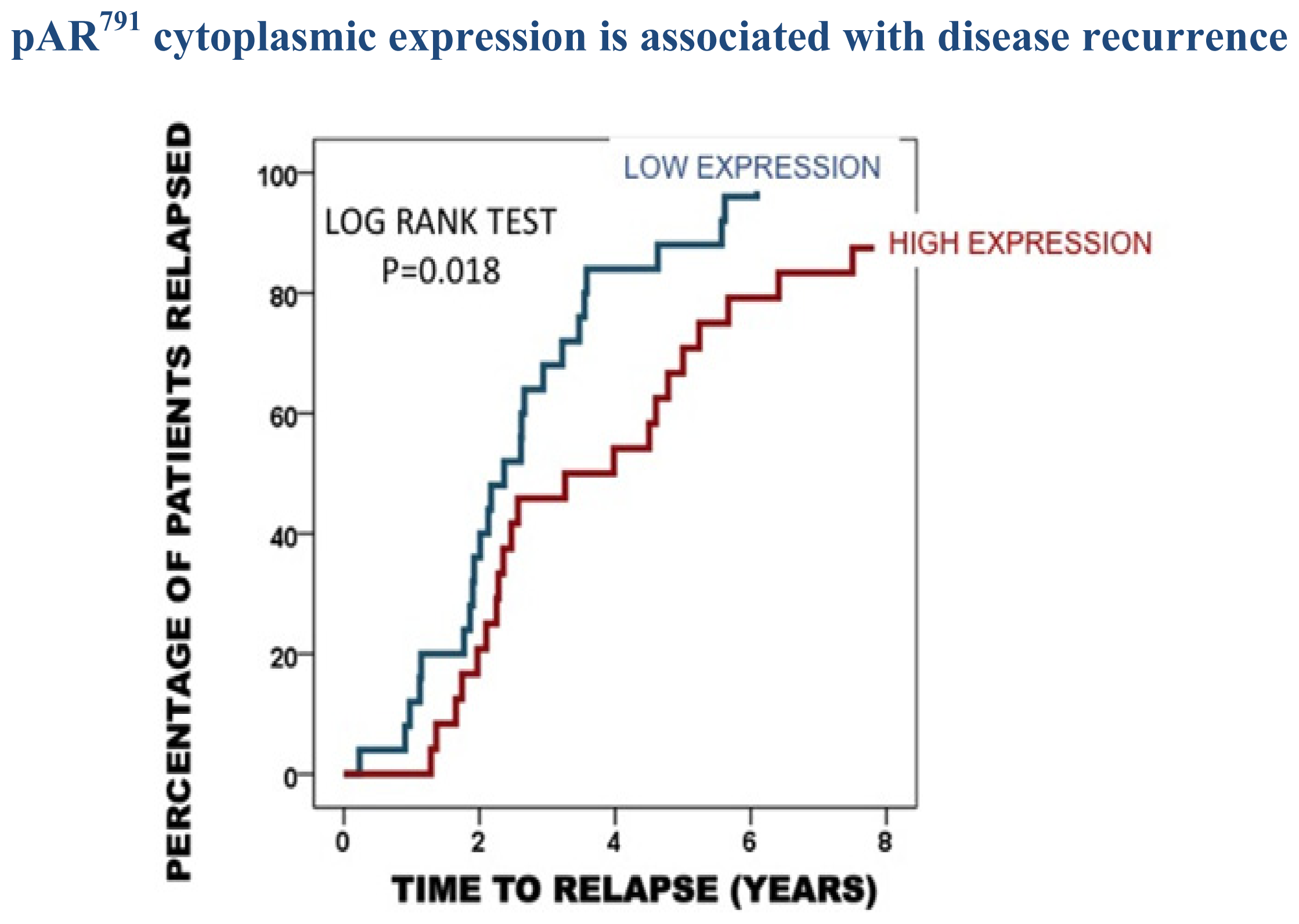
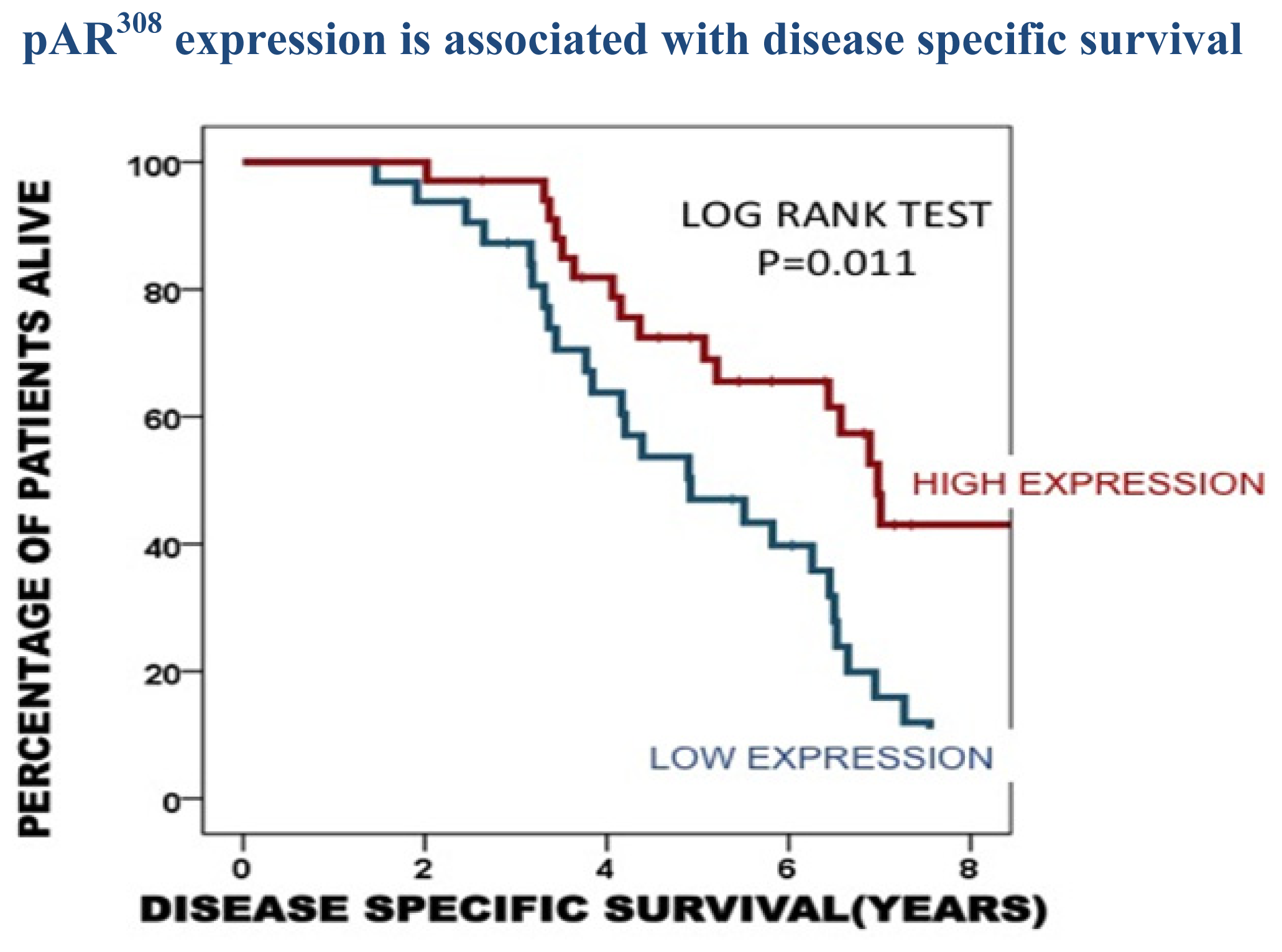
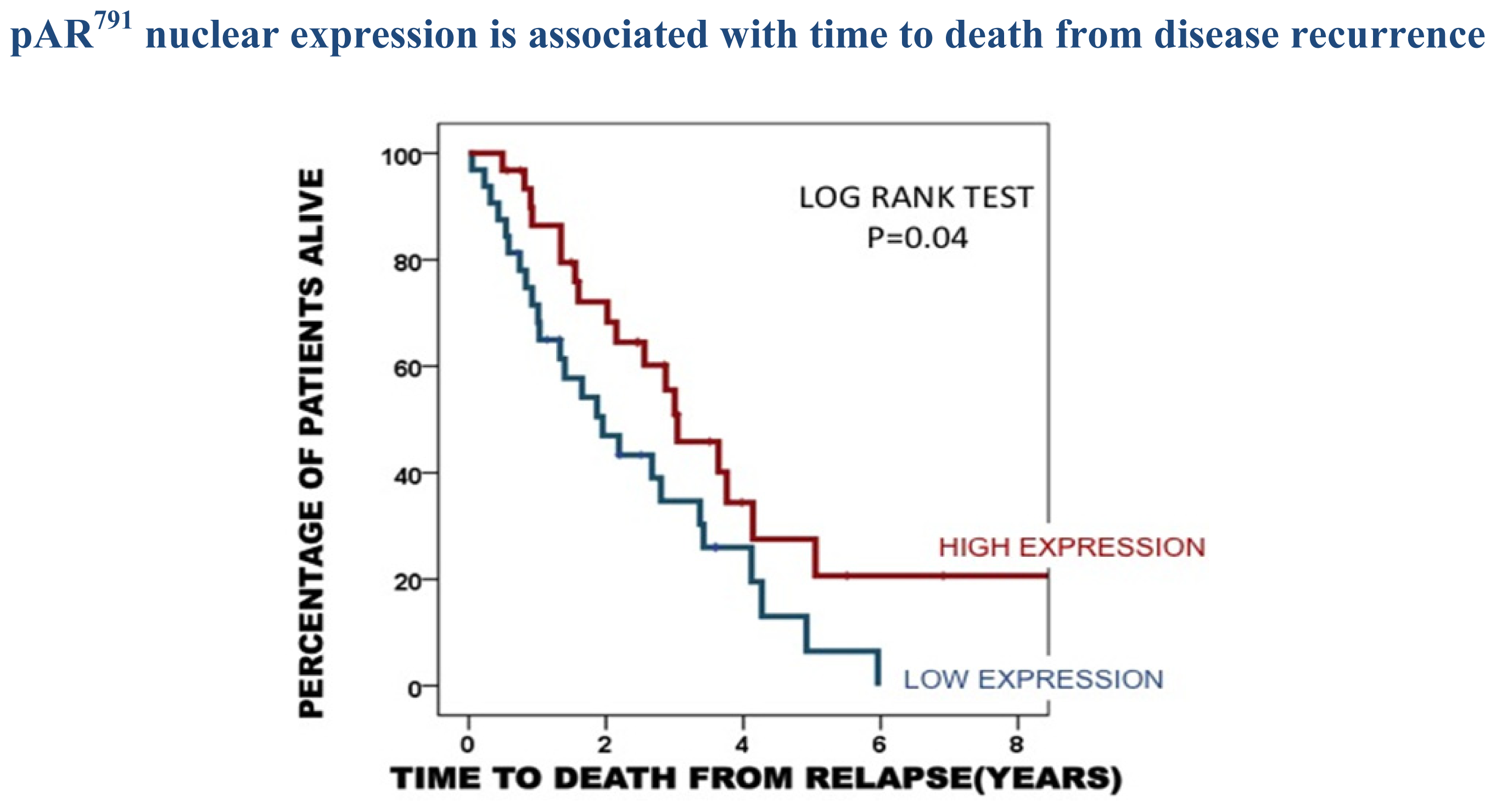
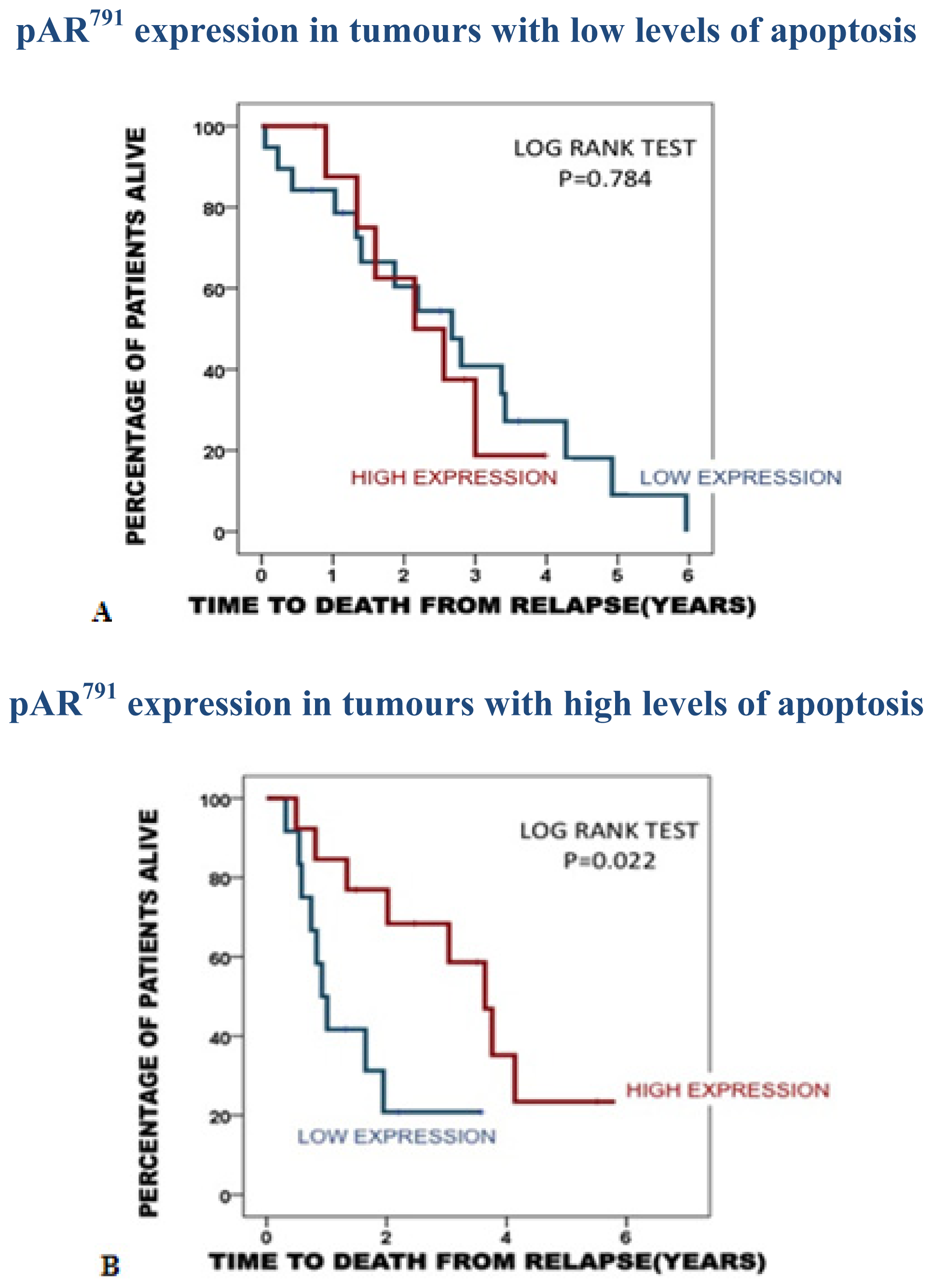


| Time to disease recurrence | Time to death from disease recurrence | Disease specific survival | |
|---|---|---|---|
| Age (<70/>70/unknown) | p = 0.210 (56/28) | p = 0.247 | p = 0.246 |
| Gleason (<7/=7/>7/unknown) | p = 0.020 (33/39/12) | p = 0.165 | p = 0.261 |
| Metastasis at diagnosis (No/Yes/Unknown) | p = 0.007 (66/18) | p = 0.016 | p = 0.0007 |
| PSA at diagnosis (<4/4–10/>10) unknown | p = 0.044 (3/13/68) | p = 0.246 | p = 0.261 |
| Metastasis at recurrence (No/Yes/Unknown) | p = 0.001 (19/54/13) | p = 0.006 | |
| PSA at recurrence (<10/10–20/>20) unknown | p = 0.019 (37/11/36) | p = 0.001 |
| HNPC (IQR) | CRPC (IQR) | p value | ICCC | |
|---|---|---|---|---|
| pAR94, C | 30 (0–50) | 15 (0–36) | p = 0.404 | 0.71 |
| pAR94, N | 25 (10–39) | 20 (9–38) | p = 0.158 | 0.70 |
| pAR308, N | 135 (110–153) | 153 (140–173) | p =0.006 | 0.77 |
| pAR650, C | 0 (0–15) | 5 (0–11) | p = 0.980 | 0.92 |
| pAR650, N | 85 (44–110) | 104 (74–125) | p =0.003 | 0.95 |
| pAR791, C | 20 (0–50) | 10 (0–33) | p = 0.798 | 0.92 |
| pAR791, N | 34 (16–62) | 29 (12–54) | p = 0.291 | 0.95 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McCall, P.; Adams, C.E.; Willder, J.M.; Bennett, L.; Qayyum, T.; Orange, C.; Underwood, M.A.; Edwards, J. Androgen Receptor Phosphorylation at Serine 308 and Serine 791 Predicts Enhanced Survival in Castrate Resistant Prostate Cancer Patients. Int. J. Mol. Sci. 2013, 14, 16656-16671. https://doi.org/10.3390/ijms140816656
McCall P, Adams CE, Willder JM, Bennett L, Qayyum T, Orange C, Underwood MA, Edwards J. Androgen Receptor Phosphorylation at Serine 308 and Serine 791 Predicts Enhanced Survival in Castrate Resistant Prostate Cancer Patients. International Journal of Molecular Sciences. 2013; 14(8):16656-16671. https://doi.org/10.3390/ijms140816656
Chicago/Turabian StyleMcCall, Pamela, Claire E. Adams, Jennifer M. Willder, Lindsay Bennett, Tahir Qayyum, Clare Orange, Mark A. Underwood, and Joanne Edwards. 2013. "Androgen Receptor Phosphorylation at Serine 308 and Serine 791 Predicts Enhanced Survival in Castrate Resistant Prostate Cancer Patients" International Journal of Molecular Sciences 14, no. 8: 16656-16671. https://doi.org/10.3390/ijms140816656




