Physico-Chemical Properties and Phase Behavior of the Ionic Liquid-β-Cyclodextrin Complexes
Abstract
:1. Introduction
2. Results and Discussion
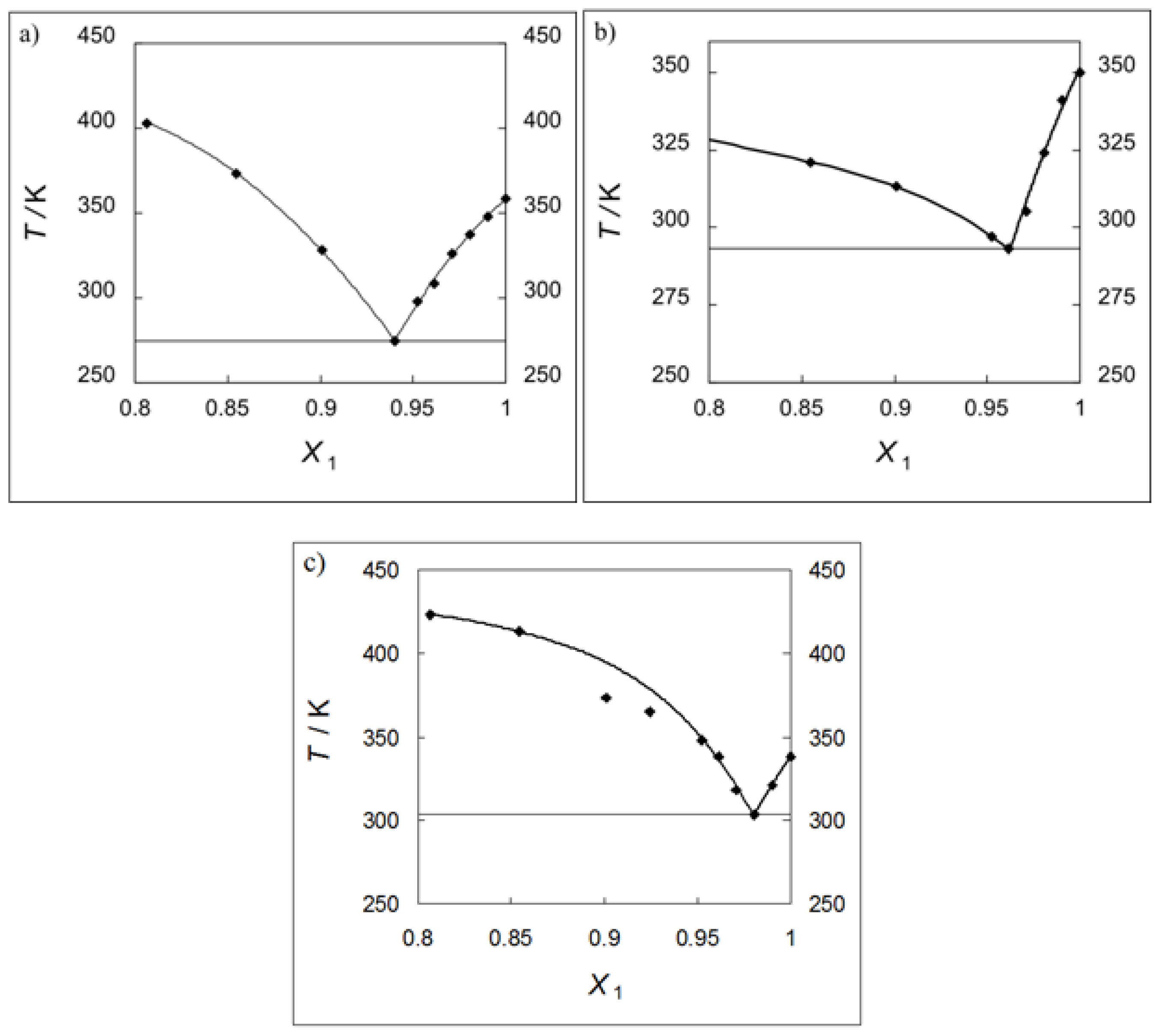
2.1. Solubility Measurements
2.2. IR Spectroscopy
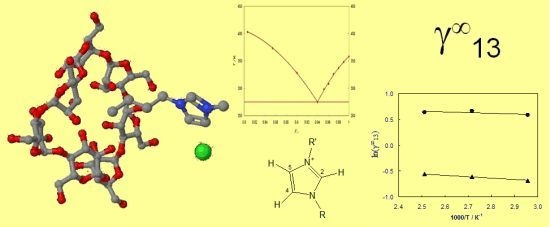
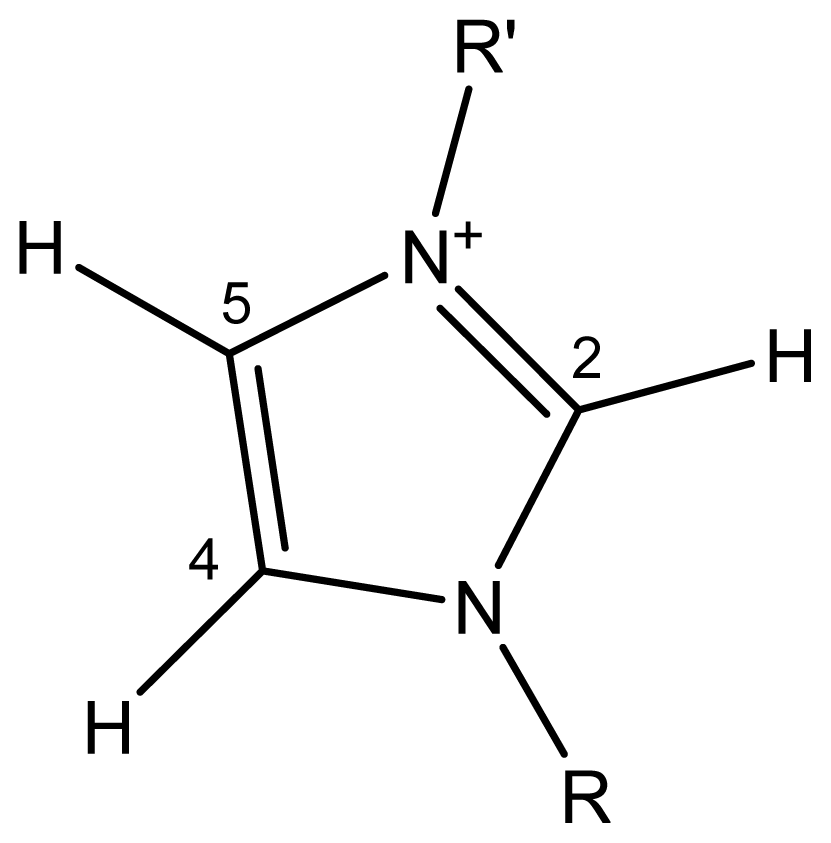
2.3. Activity Coefficients at Infinite Dilution–Theoretical Basis
2.4. Activity Coefficients at Infinite Dilution
3. Experimental Section
3.1. Materials and Chemicals
3.2. Water Content
3.3. Solubility Measurements
3.4. IR Spectroscopy
3.5. Apparatus and Experimental Procedure
4. Conclusions
Supplementary Information
ijms-14-16638-s001.pdfAcknowledgments
Conflicts of Interest
References
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev 1997, 97, 1325–1358. [Google Scholar]
- Kaneto, U.; Fumitoshi, H.; Tetsumi, I. Cyclodextrin drug carrier systems. Chem. Rev 1998, 98, 2045–2076. [Google Scholar]
- Akira, H. Cyclodextrin-based molecular machines. Acc. Chem. Res 2001, 34, 456–464. [Google Scholar]
- Santra, S.; Zhang, P.; Tan, W. Novel interaction between glutamate and the Cu2+/DMABN/β-CD complex. J. Phys. Chem. A 2000, 104, 12021–12028. [Google Scholar]
- Funasaki, N.; Isikawa, S.; Neya, S. Proton NMR study of α-cyclodextrin inclusion of short-chain surfactants. J. Phys. Chem. B 2003, 107, 10094–10099. [Google Scholar]
- Garcia-Rio, L.; Godoy, A. Use of spectra resolution methodology to investigate surfactant/β-cyclodextrin mixed systems. J. Phys. Chem. B 2007, 111, 6400–6409. [Google Scholar]
- Chao, L.; Chen, X.; Jing, B.; Zhao, Y.; Ma, F. Thermo-sensitive amphiphilic supramolecular assembly based on cyclodextrin inclusion. J. Colloid Interface Sci 2010, 351, 63–68. [Google Scholar]
- Liu, Y.; Liu, Y.; Guo, R. Insights into cyclodextrin-modulated interactions between protein and surfactant at specific and nonspecific binding stages. J. Colloid Interface Sci 2010, 351, 180–189. [Google Scholar]
- Del Valle, E.M.M. Cyclodextrin and their uses: A review. Process Biochem 2004, 39, 1033–1046. [Google Scholar]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrin in foods. J. Food Hydrocolloid 2009, 23, 1631–1640. [Google Scholar]
- Flasiński, M.; Broniatowski, M.; Majewski, J.; Dynarowicz-Łątka, P. X-ray grazing incidence diffraction and Langmuir monolayer studies of the interaction of β-cyclodextrin with model lipid membranes. J. Colloid Interface Sci 2010, 348, 511–521. [Google Scholar]
- Bender, M.L.; Komiyama, M. Cyclodextrin Chemistry; Springer-Verlag: Berlin, Germany; p. 1978.
- Rossi, S.; Bonini, M.; Karlsson, G.; Lo Nostro, P.; Baglioni, P. Self-assembly of β-cyclodextrin in water. 2. Electron spin resonance. Langmuir 2007, 23, 10959–10967. [Google Scholar]
- Holbrey, J.D.; López-Martin, I.; Rottenberg, G.; Seddon, K.S.; Silvero, G.; Zheng, X. Hydrogenation of nitrile in supercritical carbon dioxide: A tunable approach to amine selectivity. Green Chem 2008, 10, 87–92. [Google Scholar]
- Domańska, U. General Reviews of Ionic Liquids and their Properties. In Ionic Liquids in Chemical Analysis; CRC Press, Taylor & Francis Group: Abingdon, UK, 2008; Volume Chapter 1. [Google Scholar]
- Marciniak, A. Influence of cation and anion structure of the ionic liquid on extraction processes based on activity coefficients at infinite dilution. A review. Fluid Phase Equilib 2010, 294, 213–233. [Google Scholar]
- Mutelet, F.; Butet, V.; Jaubert, J.-N. Application of inverse gas chromatography and regular solution theory for characterization of ionic liquids. Ing. Eng. Chem. Res 2005, 44, 4120–4127. [Google Scholar]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.N.V.K.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data 2004, 49, 954–964. [Google Scholar]
- Mutelet, F.; Jaubert, J.-N.; Rogalski, M.; Harmand, J.; Sindt, M.; Mieloszynski, J.-L. Activity coefficients at infinite dilution of organic compounds in 1-(meth)acryloyloxyalkyl-3-methylimidazolium bromide using inverse gas chromatography. J. Phys. Chem. B 2008, 112, 3773–3785. [Google Scholar]
- Bahlmann, M.; Nebig, S.; Gmehling, J. Activity coefficients at infinite dilution of alkanes and alkenes in 1-alkyl-3-methylimidazolium tetrafluoroborate. Fluid Phase Equilib 2009, 282, 113–116. [Google Scholar]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev 2008, 37, 123–150. [Google Scholar]
- Hayes, R.; Warr, G.G.; Atkin, R. At the interface: Solvation and designing ionic liquids. Phys. Chem. Chem. Phys 2010, 12, 1709–1723. [Google Scholar]
- Tran, C.H.D.; de Paoli Lacerada, S.H. Determination of binding constants of cyclodextrins in room-temperature ionic liquids by near-infrared spectrometry. Anal. Chem 2002, 74, 5337–5341. [Google Scholar]
- Gao, Y.-A.; Li, Z.-H.; Du, J.-M.; Han, B.-X.; Li, G.-Z.; Hou, W.-G.; Shen, D.; Zhen, L.-Q.; Zhang, G.-Y. Preparation and characterization of inclusion complexes of β-cyclodextrin with ionic liquid. Chem. Eur. J 2005, 11, 5875–5880. [Google Scholar]
- Gao, Y.-A.; Zhao, X.; Dong, B.; Zheng, L.; Li, N.; Zhang, S. Inclusion complexes of β-cyclodextrin with ionic liquid surfactants. J. Phys. Chem. B 2006, 110, 8576–8581. [Google Scholar]
- Zheng, Y.; Xuan, X.; Wang, J.; Fan, M. The enhanced dissolution of β-cyclodextrin in some hydrophilic ionic liquids. J. Phys. Chem. A 2010, 114, 3926–3931. [Google Scholar]
- Ondo, D.; Tkadlecová, M.; Dohnal, V.; Rak, J.; Kvíčala, J.; Lehmann, J.K.; Heintz, A.; Ignatiev, N. Interaction of ionic liquids ions with natural cyclodextrins. J. Phys. Chem. B 2011, 115, 10285–10297. [Google Scholar]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar]
- Lubomska, M.; Gierycz, P.; Rogalski, M. Enhancement of the anthracene aqueous solubility by a synergistic effect of alcohols and β-cyclodextrin. Fluid Phase Equilib 2005, 238, 39–44. [Google Scholar]
- Ngo, M.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal properties of imidazolium ionic liquids. Thermochim. Acta 2000, 357–358, 97–102. [Google Scholar]
- Domańska, U.; Bogel-Łukasik, E.; Bogel-Łukasik, R. 1-Octanol/water partition coefficients of 1-alkyl-3-methylimidazolium chloride. Chem. Eur. J 2003, 9, 3033–3049. [Google Scholar]
- Francois, Y.; Varenne, A.; Sirieix-Plenet, J.; Gareil, P. Determination of aqueous inclusion complexation constants and stoichiometry of alkyl(methyl)-methylimidazolium-based ionic liquid cations and neutral cyclodextrins by affinity capillary electrophoresis. J. Sep. Sci 2007, 30, 751–760. [Google Scholar]
- He, Y.; Chen, Q.; Xu, Ch.; Zhang, J.; Shen, X. Interaction between ionic liquids and β-cyclodextrin: A discussion of association pattern. J. Phys. Chem. B 2009, 113, 231–238. [Google Scholar]
- Buvari, A.; Barcza, L. β-Cyclodextrin complexes of different type with inorganic compounds. Inorg. Chim. Acta 1979, 33, L179–L183. [Google Scholar]
- Larsen, A.S.; Holbrey, J.D.; Tham, F.S.; Reed, C.A. Designing ionic liquids: Imidazolium melts with inert carborane anions. J. Am. Chem. Soc 2000, 122, 7264–7272. [Google Scholar]
- Van den Broeke, J.; Stam, M.; Lutz, M.; Kooijman, H.; Spek, A.L.; Deelman, B.J.; van Koten, G. Designing ionic liquids: 1-butyl-3-methylimidazolium cations with substituted tetraphenylborate counterions. Eur. J. Inorg. Chem 2003, 2798–2811. [Google Scholar]
- Elaiwi, A.; Hitchcock, B.P.; Seddon, K.R.; Srinivasan, N.; Tan, Y.M.; Welton, T.; Zora, J.A. Hydrogen bonding in imidazolium salts and its implications for ambient-temperature halogenoaluminate(III) ionic liquids. J. Chem. Soc. Dalton Trans 1995, 3467–3472. [Google Scholar]
- Suarez, P.A.Z.; Einloft, S.; Dullius, J.E.L.; de Souza, R.F.; Dupont, J. Synthesis and physical–chemical properties of ionic liquids based on 1-n-butyl-3-methylimidazolium cation. J. Chim. Phys 1998, 95, 1626–1639. [Google Scholar]
- Everett, D.H. Effect of gas imperfection on GLC measurements: A refined method for determining activity coefficients and second virial coefficients. Trans. Faraday Soc 1965, 61, 1637–1639. [Google Scholar]
- Cruickshank, A.J.B.; Gainey, B.W.; Hicks, C.P.; Letcher, T.M.; Moody, R.W.; Young, C.L. Gas-liquid chromatographic determination of cross-term second virial coefficients using glycerol. Benzene + nitrogen and benzene + carbon dioxide at 50 °C. Trans. Faraday Soc 1969, 65, 1014–1031. [Google Scholar]
- McGlashan, M.L.; Potter, D.J.B. An apparatus for the measurement of the second virial coefficients of vapors; the second virial coefficients of some n-alkanes and of some mixtures of n-alkanes. Proc. R. Soc 1951, 267, 478–500. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M. Properties of Gases and Liquids, 5th ed; McGraw-Hill Publishing: New York, NY, USA, 2001. [Google Scholar]
- Hudson, G.H.; McCoubrey, J.C. Intermolecular forces between unlike molecules. A more complete form of the combining rules. Trans. Faraday Soc 1960, 56, 761–771. [Google Scholar]
- Cruickshank, A.J.B.; Windsor, M.L.; Young, C.L. Prediction of second virial coefficients of mixtures from the principle of corresponding states. Trans. Faraday Soc 1966, 62, 2341–2347. [Google Scholar]
- Design Institute for Physical Properties, Sponsored by AIChE, DIPPR Project 801–Full Version: Design Institute for Physical Property Data/AIChE. 2005. Available online: http://www.knovel.com/knovel2/Toc.jsp?BookID=1187&VerticalID=0 (accessed on 20 November 2011).
- Yaws, C.L. Chemical Properties Handbook; McGraw-Hill: New York, Columbus, OH, USA, 1999. [Google Scholar]
- Yaws, C.L.; Narasimhan, P.K.; Gabbula, C. Yaws’ Handbook of Antoine Coefficients for Vapor Pressure (Electronic Edition); Knovel: New York, NY, USA, 2005. [Google Scholar]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: New York, NY, USA, 2003. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]
- Domańska, U.; Redhi, G.G.; Marciniak, A. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-1-methylpyrrolidinium trifluoromethanesulfonate using GLC. Fluid Phase Equilib 2009, 278, 97–102. [Google Scholar]
- Domańska, U.; Marciniak, A. Measurements of activity coefficients at infinite dilution of aromatic and aliphatic hydrocarbons, alcohols, and water in the new ionic liquid [EMIM][SCN] using GLC. J. Chem. Thermodyn 2008, 40, 860–866. [Google Scholar]
- Domańska, U.; Laskowska, M. Measurements of activity coefficients at infinite dilution of aliphatic and aromatic hydrocarbons, alcohols, thiophene, tetrahydrofuran, MTBE, and water in ionic liquid [BMIM][SCN] using GLC. J. Chem. Thermodyn 2009, 41, 645–650. [Google Scholar]
- Domańska, U.; Marciniak, A. Activity coefficients at infinite dilution measurements for organic solutes and water in the ionic liquid 1-butyl-3-methyl-pyridinium trifluoromethanesulfonate. J. Chem. Eng. Data 2010, 55, 3208–3211. [Google Scholar]
- Möllmann, C.; Gmehling, J. Measurement of activity coefficients at infinite dilution using gas-liquid chromatography. 5. Results for N-methylacetamide, N,N-dimethylacetamide, N,N-dibutylformamide, and sulfolane as stationary phases. J. Chem. Eng. Data 1997, 42, 35–40. [Google Scholar]
- Krummen, M.; Gruber, D.; Gmehling, J. Measurement of activity coefficients at infinite dilution in solvent mixtures using the dilutor technique. Ind. Eng. Chem. Res 2000, 39, 2114–2123. [Google Scholar]
- Kato, R.; Gmehling, J. Systems with ionic liquids: Measurement of VLE and γ∞ data and prediction of their thermodynamic behavior using original UNIFAC, mod. UNIFAC(Do) and COSMO-RS(OI). J. Chem. Thermodyn 2005, 37, 603–619. [Google Scholar]
- Domańska, U. Vapour-liquid-solid equilibrium of eicosanoic acid in one- and two-component solvents. Fluid Phase Equilib 1986, 26, 201–220. [Google Scholar]
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 338.15 | 338.15 a | 368.15 | 368.15 a | 398.15 | 398.15 a | |
| n-decane | 93.7 | |||||
| hept-1-yne | 14.7 | |||||
| oct-1-yne | 23.9 | 26.4 | ||||
| benzene | 2.61 | 1.79 | 2.16 | |||
| toluene | 5.21 | 7.39 | 5.15 | 8.31 | ||
| ethylbenzene | 10.8 | 17.5 | 8.15 | 15.4 | ||
| o-xylene | 9.07 | 16.3 | 8.23 | 14.5 | ||
| m-xylene | 12,0 | 13.6 | 9.28 | 18.4 | ||
| p-xylene | 10.3 | 18.4 | 10.7 | 14.5 | ||
| methanol | 0.212 | 0.378 | 0.167 | 0.304 | 0.229 | 0.232 |
| ethanol | 0.284 | 0.965 | 0.289 | 0.888 | 0.613 | 0.532 |
| propan-1-ol | 0.457 | 1.42 | 0.429 | 1.2 | 0.954 | 0.92 |
| butan-1-ol | 1.03 | 1.99 | 0.767 | 1.66 | 1.75 | 1.28 |
| THF | 3.01 | 3.07 | ||||
| tiophene | 1.34 | 1.73 | 1.35 | 0.637 | ||
| acetone | 1.18 | 3.43 | ||||
| pentan-2-one | 5.22 | 7.93 | 4.53 | |||
| water | 0.336 | 0.096 | 0.416 | 0.257 | 0.196 | |
| propionic acid | 0.244 | 0.209 | 0.193 | |||
| tetrahydronaphthalene | 41.1 | 34.8 | ||||
| butylamine | 0.796 | 0.835 | ||||
| butyric acid | 0.33 | 0.346 | ||||
| chinolinium | 6.36 | 2.8 | ||||
| pyridinium | 2.77 | 1.94 | ||||
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 338.15 | 338.15 a | 368.15 | 368.15 a | 398.15 | 398.15 a | |
| n-decane | 259 | |||||
| hept-1-yne | 13.4 | |||||
| oct-1-yne | 18.2 | 13.2 | ||||
| benzene | 4.08 | 3.11 | ||||
| toluene | 7.51 | 5.30 | ||||
| ethylbenzene | 13.3 | 71.2 | 8.99 | |||
| o-xylene | 8.90 | 73.9 | 7.86 | 30.9 | ||
| m-xylene | 13.6 | 9.78 | ||||
| p-xylene | 11.9 | 9.19 | ||||
| methanol | 0.098 | 0.619 | 0.116 | 0.590 | 0.112 | 0.594 |
| ethanol | 0.200 | 1.44 | 0.228 | 1.22 | 0.191 | 1.18 |
| propan-1-ol | 0.254 | 2.18 | 0.260 | 1.71 | 0.248 | 1.68 |
| butan-1-ol | 0.328 | 2.66 | 0.331 | 2.33 | 0.343 | 2.43 |
| THF | 4.47 | |||||
| tiophene | 1.63 | 5.19 | 1.55 | 3.12 | ||
| acetone | 2.72 | |||||
| pentan-2-one | 6.37 | 5.05 | ||||
| water | 0.107 | 0.354 | 0.089 | 0.429 | 0.086 | 0.455 |
| propionic acid | 1.32 | 0.210 | 1.20 | |||
| tetrahydronaphthalene | 15.2 | 30.1 | 14.4 | 28.2 | ||
| butylamine | 0.380 | 0.691 | ||||
| butyric acid | 0.381 | |||||
| chinolinium | 1.26 | 3.54 | ||||
| pyridinium | 0.994 | 1.90 | ||||
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 338.15 | 338.15 a | 368.15 | 368.15 a | 398.15 | 398.15 a | |
| n-nonane | 167 | |||||
| n-decane | 115 | 213 | ||||
| ethylbenzene | 35.5 | |||||
| o-xylene | 49.0 | 26.3 | ||||
| m-xylene | 50.0 | |||||
| p-xylene | 48.2 | |||||
| methanol | 0.68 | 0.594 | 0.548 | |||
| ethanol | 2.01 | 1.58 | 1.25 | |||
| propan-1-ol | 3.41 | 2.43 | 1.70 | |||
| butan-1-ol | 4.48 | 3.37 | 2.44 | |||
| tiophene | 7.1 | |||||
| water | 0.836 | 0.396 | 0.899 | 0.479 | 1.52 | 0.085 |
| propionic acid | 0.515 | 1.27 | 0.632 | 0.412 | ||
| tetrahydronaphthalene | 29.4 | 21.2 | ||||
| butylamine | 0.648 | |||||
| butyric acid | 0.685 | 0.663 | ||||
| chinolinium | 7.97 | 2.39 | ||||
| pyridinium | 0.18 | 2.25 | ||||
| Solute | T/K | ||||||
|---|---|---|---|---|---|---|---|
| 338.15 | 338.15 a | 338.15 b | 368.15 | 368.15 a | 398.15 | 398.15 a | |
| n-hexane | |||||||
| n-heptane | 4.28 | 9.16 | 9.44 | ||||
| n-octane | 4.41 | 12.0 | 13.6 | 5.37 | 9.99 | ||
| n-nonane | 5.99 | 14.4 | 20.6 | 4.94 | 13.0 | ||
| n-decane | 2.36 | 17.9 | 29.6 | 7.27 | 16.4 | ||
| cyclohexane | 2.06 | 5.06 | 8.22 | ||||
| hex-1-ene | 1.91 | 5.14 | |||||
| hept-1-yne | 1.22 | 3.44 | 7.26 | 1.41 | 3.29 | ||
| oct-1-yne | 1.54 | 4.22 | 9.61 | 1.96 | 4.21 | ||
| benzene | 0.404 | 1.15 | 6.58 | 0.375 | 1.17 | ||
| toluene | 0.539 | 1.51 | 9.34 | 1.10 | 1.58 | ||
| ethylbenzene | 0.750 | 2.02 | 16.2 | 0.742 | 2.12 | ||
| o-xylene | 0.506 | 1.79 | 21.5 | 0.544 | 1.94 | 0.572 | 1.89 |
| m-xylene | 0.685 | 1.91 | 17.4 | 0.724 | 2.13 | ||
| p-xylene | 0.693 | 1.94 | 16.5 | 0.709 | 2.18 | ||
| methanol | 0.384 | 1.21 | 0.703 | 0.288 | 1.10 | 0.161 | 0.724 |
| ethanol | 0.733 | 1.78 | 4.36 | 0.721 | 1.31 | ||
| propan-1-ol | 0.803 | 1.94 | 6.98 | 0.514 | 1.76 | ||
| butan-1-ol | 0.808 | 2.20 | 5.9 | 0.669 | 1.85 | ||
| THF | 0.300 | 0.819 | 2.52 | 0.244 | 0.841 | ||
| dipropyl ether | 1.38 | 3.89 | 3.77 | ||||
| tiophene | 0.364 | 1.09 | 4.03 | 0.323 | 1.11 | ||
| acetone | 0.215 | 0.642 | 0.192 | 0.654 | |||
| pentan-2-one | 0.288 | 0.858 | 5.02 | 0.293 | 0.932 | ||
| water | 1.21 | 1.04 | 0.860 | 0.551 | |||
| propionic acid | 70.1 | 0.433 | 1.17 | 0.290 | 0.687 | ||
| tetrahydronaphthalene | 8.7 | 2.75 | |||||
| butylamine | 4.38 | 0.229 | 0.344 | ||||
| butyric acid | 4.63 | 1.40 | |||||
| chinolinium | 0.217 | 0.936 | |||||
| pyridinium | 0.265 | 0.750 | |||||
| Solvent | |||||
|---|---|---|---|---|---|
| n-heptane/benzene | cyclohexane/benzene | benzene | n-decane/thiophene | thiophene | |
| [EMIM][Br] | 69.9 | 0.75 | |||
| [BMPYR][Cl] | 158.9 | 0.61 | |||
| [BMPYR][Br] + β-CD | 30.0 | 0.14 | |||
| [OMIM][NTf2] | 10.6 | 5.1 | 2.48 | 21.3 | 2.75 |
| [OMIM][NTf2] + β-CD | 8.0 | 4.4 | 0.87 | 16.4 | 0.92 |
| β-CD | 1.4 | 0.15 | 7.3 | 0.25 | |
| [BMPYR][CF3SO3] a | 33.6 | 12.1 | 0.68 | 118.2 | 0.91 |
| [EMIM][SCN] b | 96.0 | 22.3 | 0.28 | ||
| [BMIM][SCN] c | 76.6 | 16.7 | 0.45 | 329.2 | 0.77 |
| [1,3-BMPY][CF3SO3] d | 22.8 | 8.7 | 0.79 | 85.6 | 1.07 |
| Sulfolane e | 20.5 f | 6.9 f | 0.43 f | ||
| NMP g | 5.9 f | 0.94 f | |||
| NMP + 6% (w/w) water g | 7.3 f | 0.50 f | |||
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rogalski, M.; Modaressi, A.; Magri, P.; Mutelet, F.; Grydziuszko, A.; Wlazło, M.; Domańska, U. Physico-Chemical Properties and Phase Behavior of the Ionic Liquid-β-Cyclodextrin Complexes. Int. J. Mol. Sci. 2013, 14, 16638-16655. https://doi.org/10.3390/ijms140816638
Rogalski M, Modaressi A, Magri P, Mutelet F, Grydziuszko A, Wlazło M, Domańska U. Physico-Chemical Properties and Phase Behavior of the Ionic Liquid-β-Cyclodextrin Complexes. International Journal of Molecular Sciences. 2013; 14(8):16638-16655. https://doi.org/10.3390/ijms140816638
Chicago/Turabian StyleRogalski, Marek, Ali Modaressi, Pierre Magri, Fabrice Mutelet, Aleksandra Grydziuszko, Michał Wlazło, and Urszula Domańska. 2013. "Physico-Chemical Properties and Phase Behavior of the Ionic Liquid-β-Cyclodextrin Complexes" International Journal of Molecular Sciences 14, no. 8: 16638-16655. https://doi.org/10.3390/ijms140816638