Epidermal Development in Mammals: Key Regulators, Signals from Beneath, and Stem Cells
Abstract
:1. Introduction
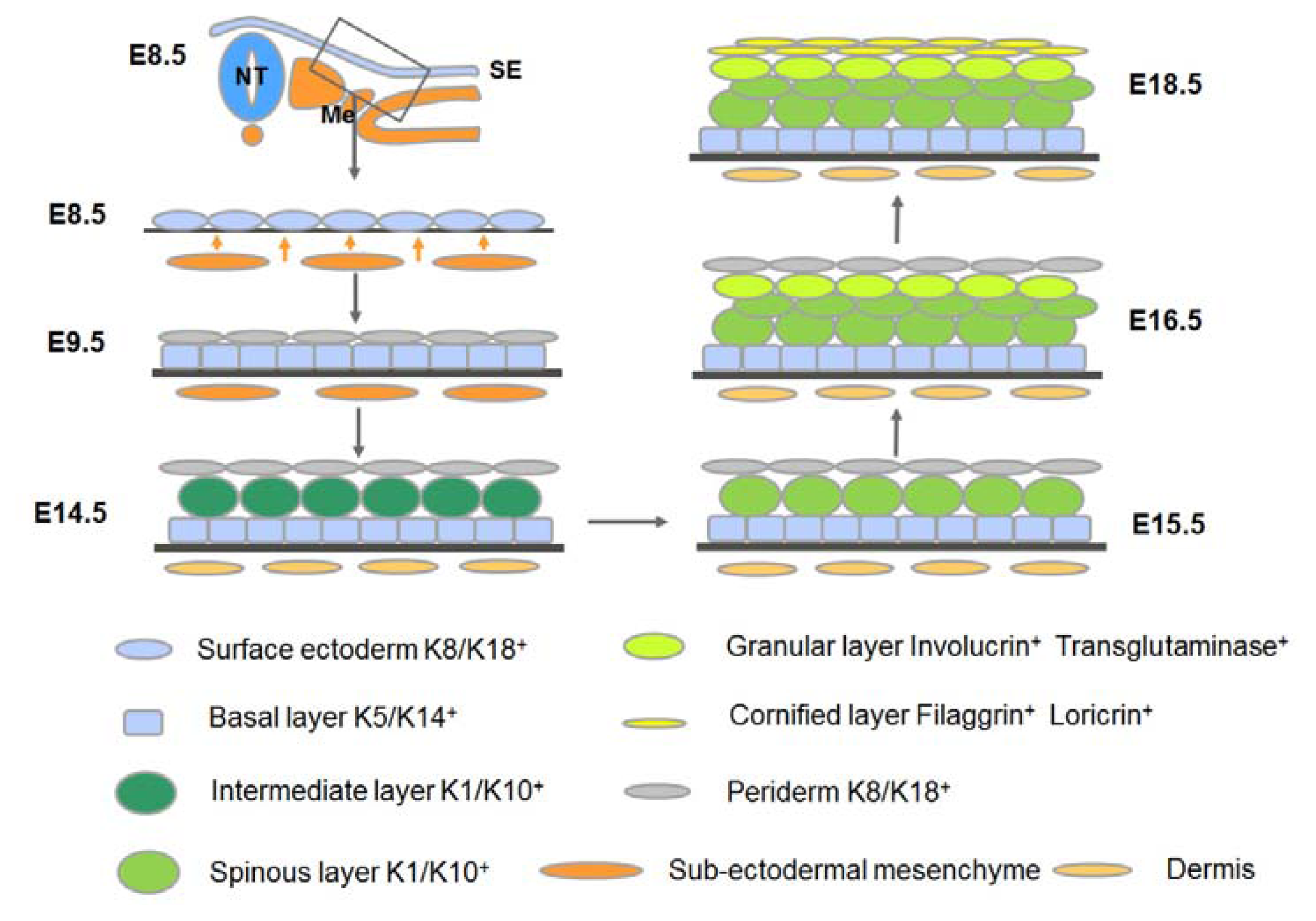
2. An Overview of the Development of Mammalian Epidermis and Its Appendages
3. Key Signaling Events and Mesenchymal-Epithelial Interactions during the Development of the Epidermis and its Appendages
3.1. Adoption of the Epidermal Fate
3.2. Commitment of the Surface Ectoderm to Stratification: Formation of the Embryonic Basal Layer
3.3. Stratification of the Epidermis
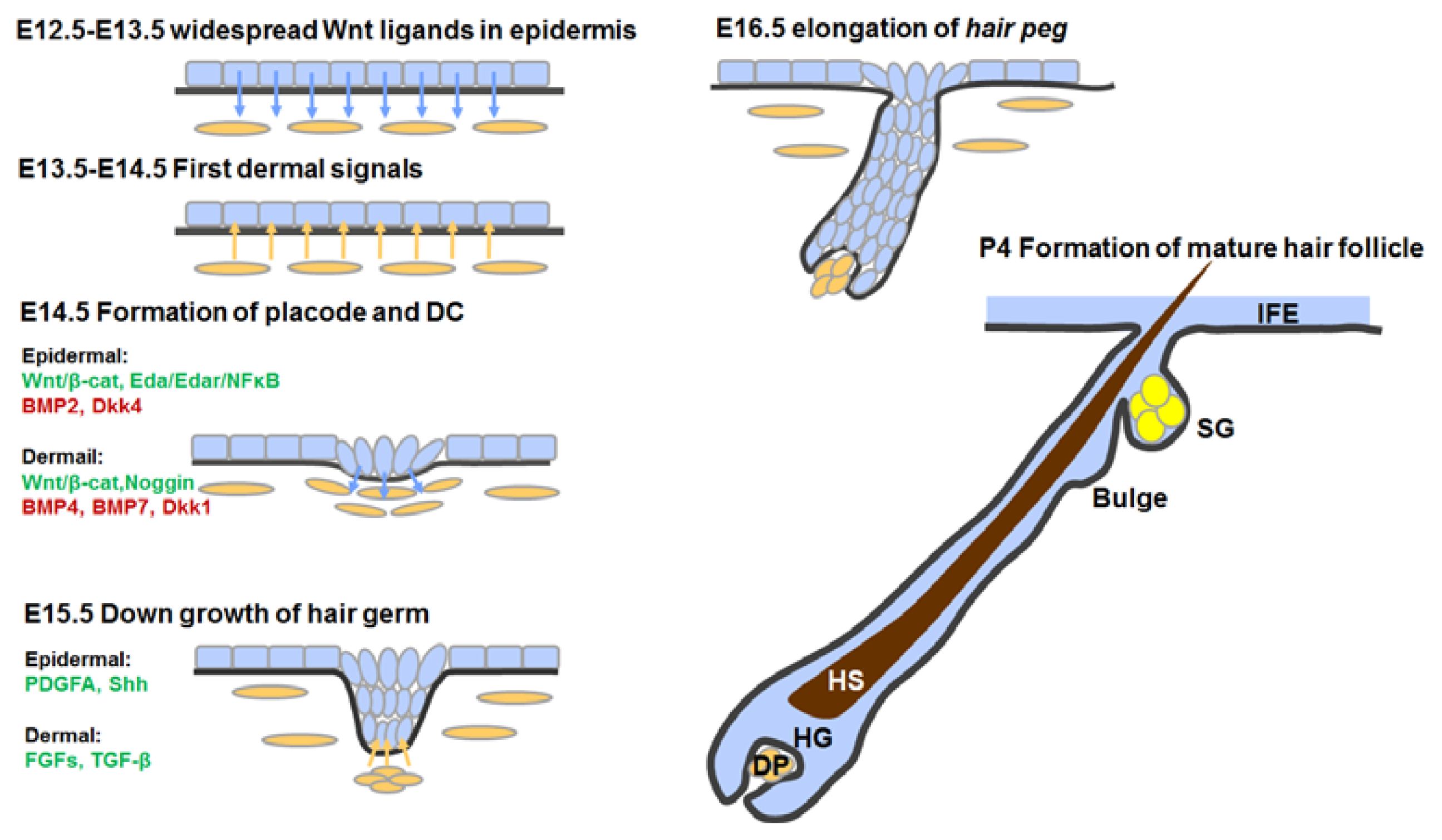
3.4. Hair Follicle Morphogenesis
3.4.1. First Dermal Signal(s)
3.4.2. Formation of Hair Placodes and Dermal Condensates
3.4.3. Hair Follicle Down-Growth
3.4.4. Hair Follicle Maturation
3.5. Development of Sweat Glands
4. Stem Cells in Epidermal Development and Homeostasis
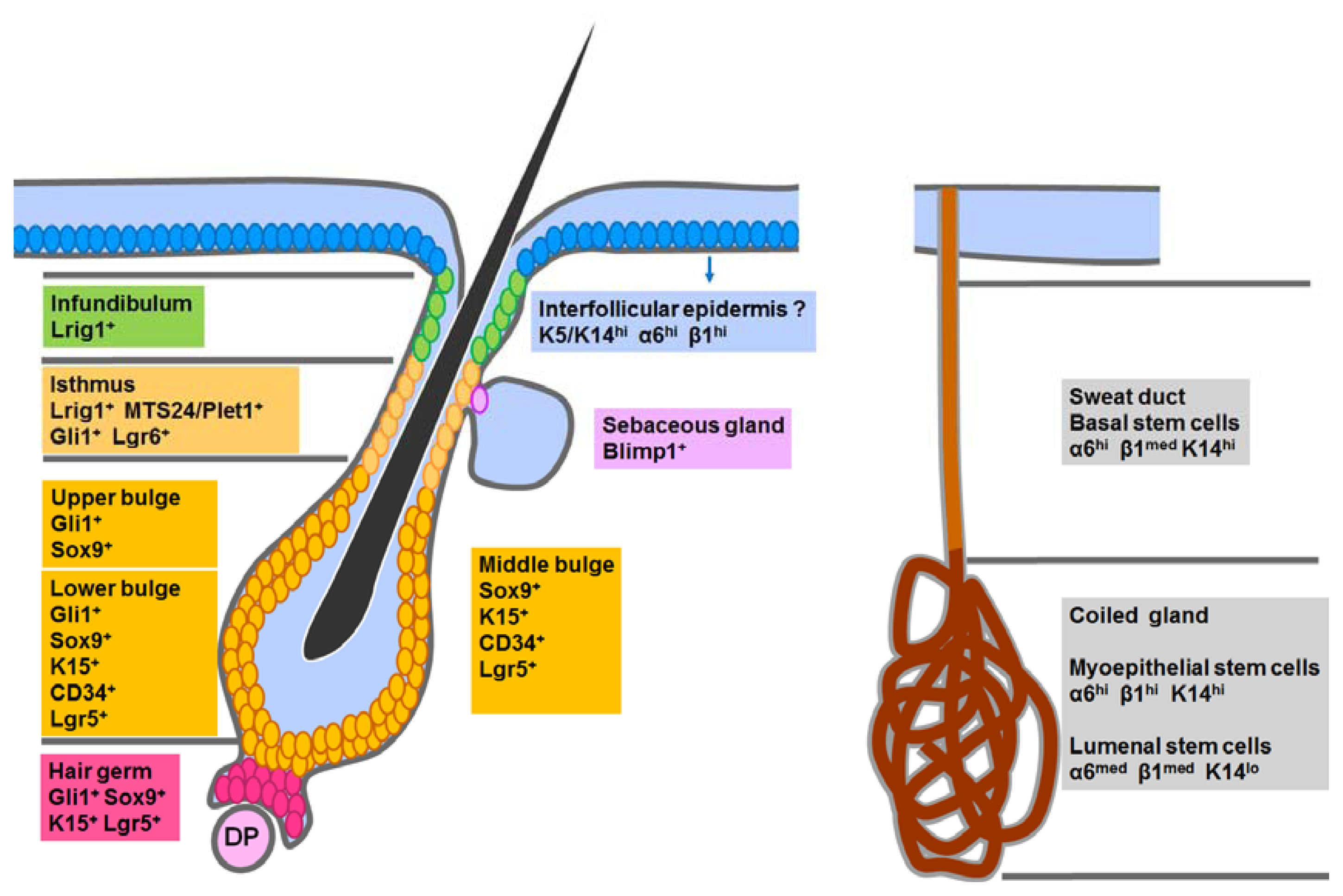
4.1. Epidermal Stem Cells in the Interfollicular Epidermis (IFE)
4.2. Hair Follicle Stem Cells in the Classic Bulge Niche and the Hair Germ
4.3. Stem Cells in the Isthmus, Sebaceous Gland and Infundibulum
4.4. Sweat Gland Stem Cells
5. Derivation of Epidermal Lineages from Pluripotent Stem Cells In Vitro
5.1. From ES Cells to Epidermal Lineages
5.1.1. BMP4
5.1.2. RA
5.1.3. ECM Components
5.2. From iPS Cells to Epidermal Lineages
5.3. Comments on These In Vitro Studies
6. Concluding Remarks
Acknowledgments
Conflict of Interest
References
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.; Barnabe-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol 2001, 3, 778–784. [Google Scholar]
- Liu, S.; Liu, S.; Wang, X.; Zhou, J.; Cao, Y.; Wang, F.; Duan, E. The PI3K-Akt pathway inhibits senescence and promotes self-renewal of human skin-derived precursors in vitro. Aging Cell 2011, 10, 661–674. [Google Scholar]
- Jackson, B.W.; Grund, C.; Winter, S.; Franke, W.W.; Illmensee, K. Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation 1981, 20, 203–216. [Google Scholar]
- Moll, R.; Moll, I.; Wiest, W. Changes in the pattern of cytokeratin polypeptides in epidermis and hair follicles during skin development in human fetuses. Differentiation 1982, 23, 170–178. [Google Scholar]
- Byrne, C.; Tainsky, M.; Fuchs, E. Programming gene expression in developing epidermis. Development 1994, 120, 2369–2383. [Google Scholar]
- Smart, I.H. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br. J. Dermatol 1970, 82, 276–282. [Google Scholar]
- Koster, M.I.; Roop, D.R. Mechanisms regulating epithelial stratification. Annu. Rev. Cell. Dev. Biol 2007, 23, 93–113. [Google Scholar]
- Paus, R.; Muller-Rover, S.; van der Veen, C.; Maurer, M.; Eichmuller, S.; Ling, G.; Hofmann, U.; Foitzik, K.; Mecklenburg, L.; Handjiski, B. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Investig. Dermatol 1999, 113, 523–532. [Google Scholar]
- Niemann, C.; Horsley, V. Development and homeostasis of the sebaceous gland. Semin. Cell Dev. Biol 2012, 23, 928–936. [Google Scholar]
- Sato, K. Biology of the eccrine sweat gland. Dermatol. Gen. Med. N. Y. McGraw-Hill 1993, 1, 221–241. [Google Scholar]
- Rinn, J.L.; Wang, J.K.; Allen, N.; Brugmann, S.A.; Mikels, A.J.; Liu, H.; Ridky, T.W.; Stadler, H.S.; Nusse, R.; Helms, J.A.; et al. A dermal HOX transcriptional program regulates site-specific epidermal fate. Genes Dev 2008, 22, 303–307. [Google Scholar]
- Stern, C.D. Neural induction: Old problem, new findings, yet more questions. Development 2005, 132, 2007–2021. [Google Scholar]
- Fuchs, E. Scratching the surface of skin development. Nature 2007, 445, 834–842. [Google Scholar]
- Yang, A.; Schweitzer, R.; Sun, D.; Kaghad, M.; Walker, N.; Bronson, R.T.; Tabin, C.; Sharpe, A.; Caput, D.; Crum, C. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 1999, 398, 714–717. [Google Scholar]
- Mills, A.A.; Zheng, B.; Wang, X.-J.; Vogel, H.; Roop, D.R.; Bradley, A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 1999, 398, 708–713. [Google Scholar]
- Senoo, M.; Pinto, F.; Crum, C.P.; McKeon, F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 2007, 129, 523–536. [Google Scholar]
- Koster, M.I.; Kim, S.; Huang, J.; Williams, T.; Roop, D.R. TAp63alpha induces AP-2gamma as an early event in epidermal morphogenesis. Dev. Biol 2006, 289, 253–261. [Google Scholar]
- Koster, M.I.; Kim, S.; Mills, A.A.; DeMayo, F.J.; Roop, D.R. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev 2004, 18, 126–131. [Google Scholar]
- McKeon, F. p63 and the epithelial stem cell: More than status quo? Genes Dev 2004, 18, 465–469. [Google Scholar]
- Romano, R.A.; Birkaya, B.; Sinha, S. A functional enhancer of keratin14 is a direct transcriptional target of deltaNp63. J. Investig. Dermatol 2007, 127, 1175–1186. [Google Scholar]
- Candi, E.; Rufini, A.; Terrinoni, A.; Dinsdale, D.; Ranalli, M.; Paradisi, A.; de Laurenzi, V.; Spagnoli, L.G.; Catani, M.V.; Ramadan, S.; et al. Differential roles of p63 isoforms in epidermal development: Selective genetic complementation in p63 null mice. Cell Death Differ 2006, 13, 1037–1047. [Google Scholar]
- Cheng, X.; Koch, P.J. In vivo function of desmosomes. J. Dermatol 2004, 31, 171–187. [Google Scholar]
- Ihrie, R.A.; Marques, M.R.; Nguyen, B.T.; Horner, J.S.; Papazoglu, C.; Bronson, R.T.; Mills, A.A.; Attardi, L.D. Perp is a p63-regulated gene essential for epithelial integrity. Cell 2005, 120, 843–856. [Google Scholar]
- Jones, P.H.; Watt, F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 1993, 73, 713–724. [Google Scholar]
- Watt, F.M.; Green, H. Stratification and terminal differentiation of cultured epidermal cells. Nature 1982, 295, 434–436. [Google Scholar]
- Lechler, T.; Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 2005, 437, 275–280. [Google Scholar]
- Koster, M.I.; Dai, D.; Marinari, B.; Sano, Y.; Costanzo, A.; Karin, M.; Roop, D.R. p63 induces key target genes required for epidermal morphogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 3255–3260. [Google Scholar]
- Vooijs, M.; Ong, C.T.; Hadland, B.; Huppert, S.; Liu, Z.; Korving, J.; van den Born, M.; Stappenbeck, T.; Wu, Y.; Clevers, H.; et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 2007, 134, 535–544. [Google Scholar]
- Okuyama, R.; Nguyen, B.C.; Talora, C.; Ogawa, E.; Tommasi di Vignano, A.; Lioumi, M.; Chiorino, G.; Tagami, H.; Woo, M.; et al. High commitment of embryonic keratinocytes to terminal differentiation through a Notch1-caspase 3 regulatory mechanism. Dev. Cell 2004, 6, 551–562. [Google Scholar]
- Rangarajan, A.; Talora, C.; Okuyama, R.; Nicolas, M.; Mammucari, C.; Oh, H.; Aster, J.C.; Krishna, S.; Metzger, D.; Chambon, P.; et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J 2001, 20, 3427–3436. [Google Scholar]
- Uyttendaele, H.; Panteleyev, A.A.; de Berker, D.; Tobin, D.T.; Christiano, A.M. Activation of Notch1 in the hair follicle leads to cell-fate switch and Mohawk alopecia. Differentiation 2004, 72, 396–409. [Google Scholar]
- Nair, M.; Teng, A.; Bilanchone, V.; Agrawal, A.; Li, B.; Dai, X. Ovol1 regulates the growth arrest of embryonic epidermal progenitor cells and represses c-myc transcription. J. Cell Biol 2006, 173, 253–264. [Google Scholar]
- Ingraham, C.R.; Kinoshita, A.; Kondo, S.; Yang, B.; Sajan, S.; Trout, K.J.; Malik, M.I.; Dunnwald, M.; Goudy, S.L.; Lovett, M.; et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6). Nat. Genet 2006, 38, 1335–1340. [Google Scholar]
- Richardson, R.J.; Dixon, J.; Malhotra, S.; Hardman, M.J.; Knowles, L.; Boot-Handford, R.P.; Shore, P.; Whitmarsh, A.; Dixon, M.J. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat. Genet 2006, 38, 1329–1334. [Google Scholar]
- Li, Q.; Lu, Q.; Estepa, G.; Verma, I.M. Identification of 14–3-3sigma mutation causing cutaneous abnormality in repeated-epilation mutant mouse. Proc. Natl. Acad. Sci. USA 2005, 102, 15977–15982. [Google Scholar]
- Herron, B.J.; Liddell, R.A.; Parker, A.; Grant, S.; Kinne, J.; Fisher, J.K.; Siracusa, L.D. A mutation in stratifin is responsible for the repeated epilation (Er) phenotype in mice. Nat. Genet 2005, 37, 1210–1212. [Google Scholar]
- Blanpain, C.; Lowry, W.E.; Pasolli, H.A.; Fuchs, E. Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev 2006, 20, 3022–3035. [Google Scholar]
- Segre, J.A.; Bauer, C.; Fuchs, E. Klf4 is a transcription factor required for establishing the barrier function of the skin. Nat. Genet 1999, 22, 356–360. [Google Scholar]
- Yu, Z.; Lin, K.K.; Bhandari, A.; Spencer, J.A.; Xu, X.; Wang, N.; Lu, Z.; Gill, G.N.; Roop, D.R.; Wertz, P.; Andersen, B. The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev. Biol 2006, 299, 122–136. [Google Scholar]
- Ting, S.B.; Caddy, J.; Wilanowski, T.; Auden, A.; Cunningham, J.M.; Elias, P.M.; Holleran, W.M.; Jane, S.M. The epidermis of grhl3-null mice displays altered lipid processing and cellular hyperproliferation. Organogenesis 2005, 2, 33–35. [Google Scholar]
- Hardy, M.H. The secret life of the hair follicle. Trends Genet 1992, 8, 55–61. [Google Scholar]
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar]
- Millar, S.E. Molecular mechanisms regulating hair follicle development. J. Investig. Dermatol 2002, 118, 216–225. [Google Scholar]
- Sennett, R.; Rendl, M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol 2012, 23, 917–927. [Google Scholar]
- Sick, S.; Reinker, S.; Timmer, J.; Schlake, T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 2006, 314, 1447–1450. [Google Scholar]
- Zhang, Y.; Tomann, P.; Andl, T.; Gallant, N.M.; Huelsken, J.; Jerchow, B.; Birchmeier, W.; Paus, R.; Piccolo, S.; Mikkola, M.L.; et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 2009, 17, 49–61. [Google Scholar]
- DasGupta, R.; Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 1999, 126, 4557–4568. [Google Scholar]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar]
- Monaghan, A.P.; Kioschis, P.; Wu, W.; Zuniga, A.; Bock, D.; Poustka, A.; Delius, H.; Niehrs, C. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech. Dev 1999, 87, 45–56. [Google Scholar]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev 2001, 107, 69–82. [Google Scholar]
- Bazzi, H.; Fantauzzo, K.A.; Richardson, G.D.; Jahoda, C.A.; Christiano, A.M. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Dev. Biol 2007, 305, 498–507. [Google Scholar]
- Cui, C.Y.; Schlessinger, D. EDA signaling and skin appendage development. Cell Cycle 2006, 5, 2477–2483. [Google Scholar]
- Koppinen, P.; Pispa, J.; Laurikkala, J.; Thesleff, I.; Mikkola, M.L. Signaling and subcellular localization of the TNF receptor Edar. Exp. Cell Res 2001, 269, 180–192. [Google Scholar]
- Kumar, A.; Eby, M.T.; Sinha, S.; Jasmin, A.; Chaudhary, P.M. The ectodermal dysplasia receptor activates the nuclear factor-kappaB, JNK, and cell death pathways and binds to ectodysplasin A. J. Biol. Chem 2001, 276, 2668–2677. [Google Scholar]
- Yan, M.; Wang, L.C.; Hymowitz, S.G.; Schilbach, S.; Lee, J.; Goddard, A.; de Vos, A.M.; Gao, W.Q.; Dixit, V.M. Two-amino acid molecular switch in an epithelial morphogen that regulates binding to two distinct receptors. Science 2000, 290, 523–527. [Google Scholar]
- Fliniaux, I.; Mikkola, M.L.; Lefebvre, S.; Thesleff, I. Identification of dkk4 as a target of Eda-A1/Edar pathway reveals an unexpected role of ectodysplasin as inhibitor of Wnt signalling in ectodermal placodes. Dev. Biol 2008, 320, 60–71. [Google Scholar]
- Pummila, M.; Fliniaux, I.; Jaatinen, R.; James, M.J.; Laurikkala, J.; Schneider, P.; Thesleff, I.; Mikkola, M.L. Ectodysplasin has a dual role in ectodermal organogenesis: Inhibition of Bmp activity and induction of Shh expression. Development 2007, 134, 117–125. [Google Scholar]
- Schmidt-Ullrich, R.; Aebischer, T.; Hulsken, J.; Birchmeier, W.; Klemm, U.; Scheidereit, C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development 2001, 128, 3843–3853. [Google Scholar]
- Mou, C.; Jackson, B.; Schneider, P.; Overbeek, P.A.; Headon, D.J. Generation of the primary hair follicle pattern. Proc. Natl. Acad. Sci. USA 2006, 103, 9075–9080. [Google Scholar]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev. Biol 1995, 172, 126–138. [Google Scholar]
- Noramly, S.; Morgan, B.A. BMPs mediate lateral inhibition at successive stages in feather tract development. Development 1998, 125, 3775–3787. [Google Scholar]
- Botchkarev, V.A.; Botchkareva, N.V.; Roth, W.; Nakamura, M.; Chen, L.H.; Herzog, W.; Lindner, G.; McMahon, J.A.; Peters, C.; Lauster, R.; et al. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell. Biol 1999, 1, 158–164. [Google Scholar]
- Feijen, A.; Goumans, M.J.; van den Eijnden-van Raaij, A.J. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development 1994, 120, 3621–3637. [Google Scholar]
- Ohyama, A.; Saito, F.; Ohuchi, H.; Noji, S. Differential expression of two BMP antagonists, gremlin and Follistatin, during development of the chick feather bud. Mech. Dev 2001, 100, 331–333. [Google Scholar]
- Karlsson, L.; Bondjers, C.; Betsholtz, C. Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 1999, 126, 2611–2621. [Google Scholar]
- Woo, W.M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev 2012, 26, 1235–1246. [Google Scholar]
- Jamora, C.; Lee, P.; Kocieniewski, P.; Azhar, M.; Hosokawa, R.; Chai, Y.; Fuchs, E. A Signaling pathway involving TGF-beta 2 and snail in hair follicle morphogenesis. PLoS Biol 2004, 3, e11. [Google Scholar]
- Foitzik, K.; Paus, R.; Doetschman, T.; Paolo Dotto, G. The TGF-beta 2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev. Biol 1999, 212, 278–289. [Google Scholar]
- Inoue, K.; Aoi, N.; Yamauchi, Y.; Sato, T.; Suga, H.; Eto, H.; Kato, H.; Tabata, Y.; Yoshimura, K. TGF-β2 is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J. Cell. Mol. Med 2009, 13, 4643–4656. [Google Scholar]
- Merrill, B.J.; Gat, U.; DasGupta, R.; Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 2001, 15, 1688–1705. [Google Scholar]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev 2000, 14, 1181–1185. [Google Scholar]
- Kobielak, K.; Pasolli, H.A.; Alonso, L.; Polak, L.; Fuchs, E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell. Biol 2003, 163, 609–623. [Google Scholar]
- Kulessa, H.; Turk, G.; Hogan, B.L. Inhibition of Bmp signaling affects growth and differentiation in the anagen hair follicle. EMBO J 2000, 19, 6664–6674. [Google Scholar]
- Yuhki, M.; Yamada, M.; Kawano, M.; Iwasato, T.; Itohara, S.; Yoshida, H.; Ogawa, M.; Mishina, Y. BMPR1A signaling is necessary for hair follicle cycling and hair shaft differentiation in mice. Development 2004, 131, 1825–1833. [Google Scholar]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev 2008, 22, 543–557. [Google Scholar]
- Collins, C.A.; Kretzschmar, K.; Watt, F.M. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development 2011, 138, 5189–5199. [Google Scholar]
- Fu, X.; Li, J.; Sun, X.; Sun, T.; Sheng, Z. Regeneration science: Epidermal stem cells are the source of sweat glands in human fetal skin: Evidence of synergetic development of stem cells, sweat glands, growth factors, and matrix metalloproteinases. Wound Repair Regen 2005, 13, 102–108. [Google Scholar]
- Kunisada, M.; Cui, C.Y.; Piao, Y.; Ko, M.S.; Schlessinger, D. Requirement for Shh and Fox family genes at different stages in sweat gland development. Hum. Mol. Genet 2009, 18, 1769–1778. [Google Scholar]
- Van Genderen, C.; Okamura, R.M.; Farinas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev 1994, 8, 2691–2703. [Google Scholar]
- Potten, C.S. The epidermal proliferative unit: The possible role of the central basal cell. Cell Tissue Kinet 1974, 7, 77–88. [Google Scholar]
- Mackenzie, I.C. Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Investig. Dermatol 1997, 109, 377–383. [Google Scholar]
- Barrandon, Y.; Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 1987, 84, 2302–2306. [Google Scholar]
- Clayton, E.; Doupe, D.P.; Klein, A.M.; Winton, D.J.; Simons, B.D.; Jones, P.H. A single type of progenitor cell maintains normal epidermis. Nature 2007, 446, 185–189. [Google Scholar]
- Doupe, D.P.; Klein, A.M.; Simons, B.D.; Jones, P.H. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 2012, 18, 317–323. [Google Scholar]
- Fuchs, E. Skin stem cells: Rising to the surface. J. Cell. Biol 2008, 180, 273–284. [Google Scholar]
- Mascre, G.; Dekoninck, S.; Drogat, B.; Youssef, K.K.; Brohee, S.; Sotiropoulou, P.A.; Simons, B.D.; Blanpain, C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 2012, 489, 257–262. [Google Scholar]
- Truong, A.B.; Kretz, M.; Ridky, T.W.; Kimmel, R.; Khavari, P.A. p63 regulates proliferation and differentiation of developmentally mature keratinocytes. Genes Dev 2006, 20, 3185–3197. [Google Scholar]
- Zhou, J.X.; Chen, S.Y.; Liu, W.M.; Cao, Y.J.; Duan, E.K. Enrichment and identification of human “fetal” epidermal stem cells. Hum. Reprod 2004, 19, 968–974. [Google Scholar]
- Li, J.; Miao, C.; Guo, W.; Jia, L.; Zhou, J.; Ma, B.; Peng, S.; Liu, S.; Cao, Y.; Duan, E. Enrichment of putative human epidermal stem cells based on cell size and collagen type IV adhesiveness. Cell Res 2008, 18, 360–371. [Google Scholar]
- Zhou, J.X.; Jia, L.W.; Liu, W.M.; Miao, C.L.; Liu, S.; Cao, Y.J.; Duan, E.K. Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis. Hum. Reprod 2006, 21, 1698–1704. [Google Scholar]
- Jia, L.; Zhou, J.; Peng, S.; Li, J.; Cao, Y.; Duan, E. Effects of Wnt3a on proliferation and differentiation of human epidermal stem cells. Biochem. Biophys. Res. Commun 2008, 368, 483–488. [Google Scholar]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol 2004, 22, 411–417. [Google Scholar]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar]
- Nowak, J.A.; Polak, L.; Pasolli, H.A.; Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008, 3, 33–43. [Google Scholar]
- Troy, T.C.; Arabzadeh, A.; Turksen, K. Re-assessing K15 as an epidermal stem cell marker. Stem Cell Rev 2012, 7, 927–934. [Google Scholar]
- Youssef, K.K.; Van Keymeulen, A.; Lapouge, G.; Beck, B.; Michaux, C.; Achouri, Y.; Sotiropoulou, P.A.; Blanpain, C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell. Biol 2010, 12, 299–305. [Google Scholar]
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgard, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet 2008, 40, 1291–1299. [Google Scholar]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar]
- Zhang, S.; Hu, H.; Zhang, H.; Liu, S.; Liu, S.; Zhang, Y.; Lei, X.; Ning, L.; Cao, Y.; Duan, E. Hair follicle stem cells derived from single rat vibrissa via organ culture reconstitute hair follicles in vivo. Cell Transplant 2012, 21, 1075–1085. [Google Scholar]
- Jaks, V.; Kasper, M.; Toftgard, R. The hair follicle-a stem cell zoo. Exp. Cell Res 2010, 316, 1422–1428. [Google Scholar]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; Dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar]
- Botchkarev, V.A.; Sharov, A.A. BMP signaling in the control of skin development and hair follicle growth. Differentiation 2004, 72, 512–526. [Google Scholar]
- Haegebarth, A.; Clevers, H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am. J. Pathol 2009, 174, 715–721. [Google Scholar]
- Kandyba, E.; Leung, Y.; Chen, Y.B.; Widelitz, R.; Chuong, C.M.; Kobielak, K. Competitive balance of intrabulge BMP/Wnt signaling reveals a robust gene network ruling stem cell homeostasis and cyclic activation. Proc. Natl. Acad. Sci. USA 2012, 110, 1351–1356. [Google Scholar]
- Oshimori, N.; Fuchs, E. Paracrine TGF-beta signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2011, 10, 63–75. [Google Scholar]
- Niimori, D.; Kawano, R.; Felemban, A.; Niimori-Kita, K.; Tanaka, H.; Ihn, H.; Ohta, K. Tsukushi controls the hair cycle by regulating TGF-beta 1 signaling. 2012, 372, 81–87. [Google Scholar]
- Yang, L.; Wang, L.; Yang, X. Disruption of Smad4 in mouse epidermis leads to depletion of follicle stem cells. Mol. Biol. Cell 2009, 20, 882–890. [Google Scholar]
- Hu, H.M.; Zhang, S.B.; Lei, X.H.; Deng, Z.L.; Guo, W.X.; Qiu, Z.F.; Liu, S.; Wang, X.Y.; Zhang, H.; Duan, E.K. Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS One 2011, 7, e40124. [Google Scholar]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med 2005, 11, 1351–1354. [Google Scholar]
- Levy, V.; Lindon, C.; Zheng, Y.; Harfe, B.D.; Morgan, B.A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J 2007, 21, 1358–1366. [Google Scholar]
- Levy, V.; Lindon, C.; Harfe, B.D.; Morgan, B.A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 2005, 9, 855–861. [Google Scholar]
- Vidal, V.P.; Chaboissier, M.C.; Lutzkendorf, S.; Cotsarelis, G.; Mill, P.; Hui, C.C.; Ortonne, N.; Ortonne, J.P.; Schedl, A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol 2005, 15, 1340–1351. [Google Scholar]
- Snippert, H.J.; Haegebarth, A.; Kasper, M.; Jaks, V.; van Es, J.H.; Barker, N.; van de Wetering, M.; van den Born, M.; Begthel, H.; Vries, R.G. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 2010, 327, 1385–1389. [Google Scholar]
- Nijhof, J.G.; Braun, K.M.; Giangreco, A.; van Pelt, C.; Kawamoto, H.; Boyd, R.L.; Willemze, R.; Mullenders, L.H.; Watt, F.M.; de Gruijl, F.R.; et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 2006, 133, 3027–3037. [Google Scholar]
- Jensen, K.B.; Collins, C.A.; Nascimento, E.; Tan, D.W.; Frye, M.; Itami, S.; Watt, F.M. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 2009, 4, 427–439. [Google Scholar]
- Horsley, V.; O’Carroll, D.; Tooze, R.; Ohinata, Y.; Saitou, M.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; Fuchs, E. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 2006, 126, 597–609. [Google Scholar]
- Frances, D.; Niemann, C. Stem cell dynamics in sebaceous gland morphogenesis in mouse skin. Dev. Biol 2012, 363, 138–146. [Google Scholar]
- Lu, C.; Polak, L.; Rocha, A.; Pasolli, H.M.; Chen, S.-C.; Sharma, N.; Blanpain, C.; Fuchs, E. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 2012, 150, 136–150. [Google Scholar]
- Gat, U.; DasGupta, R.; Degenstein, L.; Fuchs, E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 1998, 95, 605–614. [Google Scholar]
- Bagutti, C.; Hutter, C.; Chiquet-Ehrismann, R.; Fassler, R.; Watt, F.M. Dermal fibroblast-derived growth factors restore the ability of beta(1) integrin-deficient embryonal stem cells to differentiate into keratinocytes. Dev. Biol 2001, 231, 321–333. [Google Scholar]
- Coraux, C.; Hilmi, C.; Rouleau, M.; Spadafora, A.; Hinnrasky, J.; Ortonne, J.P.; Dani, C.; Aberdam, D. Reconstituted skin from murine embryonic stem cells. Curr. Biol 2003, 13, 849–853. [Google Scholar]
- Troy, T.C.; Turksen, K. Commitment of embryonic stem cells to an epidermal cell fate and differentiation in vitro. Dev. Dyn 2005, 232, 293–300. [Google Scholar]
- Haase, I.; Knaup, R.; Wartenberg, M.; Sauer, H.; Hescheler, J.; Mahrle, G. In vitro differentiation of murine embryonic stem cells into keratinocyte-like cells. Eur. J. Cell Biol 2007, 86, 801–805. [Google Scholar]
- Guenou, H.; Nissan, X.; Larcher, F.; Feteira, J.; Lemaitre, G.; Saidani, M.; del Rio, M.; Barrault, C.C.; Bernard, F.X.; Peschanski, M.; et al. Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: A preclinical study. Lancet 2009, 374, 1745–1753. [Google Scholar]
- Kawasaki, H.; Mizuseki, K.; Nishikawa, S.; Kaneko, S.; Kuwana, Y.; Nakanishi, S.; Nishikawa, S.I.; Sasai, Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron 2000, 28, 31–40. [Google Scholar]
- Zhang, K.; Li, L.; Huang, C.; Shen, C.; Tan, F.; Xia, C.; Liu, P.; Rossant, J.; Jing, N. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development 2012, 137, 2095–2105. [Google Scholar]
- Gambaro, K.; Aberdam, E.; Virolle, T.; Aberdam, D.; Rouleau, M. BMP-4 induces a Smad-dependent apoptotic cell death of mouse embryonic stem cell-derived neural precursors. Cell Death Differ 2006, 13, 1075–1087. [Google Scholar]
- Qiao, Y.; Zhu, Y.; Sheng, N.; Chen, J.; Tao, R.; Zhu, Q.; Zhang, T.; Qian, C.; Jing, N. AP2gamma regulates neural and epidermal development downstream of the BMP pathway at early stages of ectodermal patterning. Cell Res 2012, 22, 1546–1561. [Google Scholar]
- Zhao, G.; Skeath, J.B. The Sox-domain containing gene Dichaete/fish-hook acts in concert with vnd and ind to regulate cell fate in the Drosophila neuroectoderm. Development 2002, 129, 1165–1174. [Google Scholar]
- Walcher, T.; Xie, Q.; Sun, J.; Irmler, M.; Beckers, J.; Ozturk, T.; Niessing, D.; Stoykova, A.; Cvekl, A.; Ninkovic, J.; Gotz, M. Functional dissection of the paired domain of Pax6 reveals molecular mechanisms of coordinating neurogenesis and proliferation. Development 2013, 140, 1123–1136. [Google Scholar]
- Metallo, C.M.; Ji, L.; de Pablo, J.J.; Palecek, S.P. Retinoic acid and bone morphogenetic protein signaling synergize to efficiently direct epithelial differentiation of human embryonic stem cells. Stem Cells 2008, 26, 372–380. [Google Scholar]
- Morriss-Kay, G.M.; Sokolova, N. Embryonic development and pattern formation. FASEB J 1996, 10, 961–968. [Google Scholar]
- Haselbeck, R.J.; Hoffmann, I.; Duester, G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev. Genet 1999, 25, 353–364. [Google Scholar]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. J. Neurobiol 2006, 66, 606–630. [Google Scholar]
- Inanc, B.; Elcin, A.E.; Elcin, Y.M. Human embryonic stem cell differentiation on tissue engineering scaffolds: Effects of NGF and retinoic acid induction. Tissue Eng. Part A 2008, 14, 955–964. [Google Scholar]
- Shalom-Feuerstein, R.; Lena, A.M.; Zhou, H.; De La Forest Divonne, S.; van Bokhoven, H.; Candi, E.; Melino, G.; Aberdam, D. DeltaNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ 2011, 18, 887–896. [Google Scholar]
- Bamberger, C.; Pollet, D.; Schmale, H. Retinoic acid inhibits downregulation of DeltaNp63alpha expression during terminal differentiation of human primary keratinocytes. J. Investig. Dermatol 2002, 118, 133–138. [Google Scholar]
- Aberdam, E.; Barak, E.; Rouleau, M.; de LaForest, S.; Berrih-Aknin, S.; Suter, D.M.; Krause, K.H.; Amit, M.; Itskovitz-Eldor, J.; Aberdam, D. A pure population of ectodermal cells derived from human embryonic stem cells. Stem Cells 2008, 26, 440–444. [Google Scholar]
- Ji, L.; Allen-Hoffmann, B.L.; de Pablo, J.J.; Palecek, S.P. Generation and differentiation of human embryonic stem cell-derived keratinocyte precursors. Tissue Eng 2006, 12, 665–679. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hamalainen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar]
- Zhou, H.; Wu, S.; Joo, J.Y.; Zhu, S.; Han, D.W.; Lin, T.; Trauger, S.; Bien, G.; Yao, S.; Zhu, Y.; et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009, 4, 381–384. [Google Scholar]
- Miyoshi, N.; Ishii, H.; Nagano, H.; Haraguchi, N.; Dewi, D.L.; Kano, Y.; Nishikawa, S.; Tanemura, M.; Mimori, K.; Tanaka, F.; et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell 2011, 8, 633–638. [Google Scholar]
- Warren, L.; Ni, Y.; Wang, J.; Guo, X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci. Rep 2012, 2, 657. [Google Scholar]
- Yagi, T.; Ito, D.; Okada, Y.; Akamatsu, W.; Nihei, Y.; Yoshizaki, T.; Yamanaka, S.; Okano, H.; Suzuki, N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum. Mol. Genet 2011, 20, 4530–4539. [Google Scholar]
- Kondo, T.; Asai, M.; Tsukita, K.; Kutoku, Y.; Ohsawa, Y.; Sunada, Y.; Imamura, K.; Egawa, N.; Yahata, N.; Okita, K.; et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular abeta and differential drug responsiveness. Cell Stem Cell 2013, 12, 487–496. [Google Scholar]
- Bilousova, G.; Chen, J.; Roop, D.R. Differentiation of mouse induced pluripotent stem cells into a multipotent keratinocyte lineage. J. Investig. Dermatol 2010, 131, 857–864. [Google Scholar]
- Tolar, J.; Xia, L.; Riddle, M.J.; Lees, C.J.; Eide, C.R.; McElmurry, R.T.; Titeux, M.; Osborn, M.J.; Lund, T.C.; Hovnanian, A.; et al. Induced pluripotent stem cells from individuals with recessive dystrophic epidermolysis bullosa. J. Investig. Dermatol 2010, 131, 848–856. [Google Scholar]
- Itoh, M.; Kiuru, M.; Cairo, M.S.; Christiano, A.M. Generation of keratinocytes from normal and recessive dystrophic epidermolysis bullosa-induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8797–8802. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, S.; Zhang, H.; Duan, E. Epidermal Development in Mammals: Key Regulators, Signals from Beneath, and Stem Cells. Int. J. Mol. Sci. 2013, 14, 10869-10895. https://doi.org/10.3390/ijms140610869
Liu S, Zhang H, Duan E. Epidermal Development in Mammals: Key Regulators, Signals from Beneath, and Stem Cells. International Journal of Molecular Sciences. 2013; 14(6):10869-10895. https://doi.org/10.3390/ijms140610869
Chicago/Turabian StyleLiu, Shuang, Huishan Zhang, and Enkui Duan. 2013. "Epidermal Development in Mammals: Key Regulators, Signals from Beneath, and Stem Cells" International Journal of Molecular Sciences 14, no. 6: 10869-10895. https://doi.org/10.3390/ijms140610869



