Rab27 GTPases Distribute Extracellular Nanomaps for Invasive Growth and Metastasis: Implications for Prognosis and Treatment
Abstract
:1. Introduction
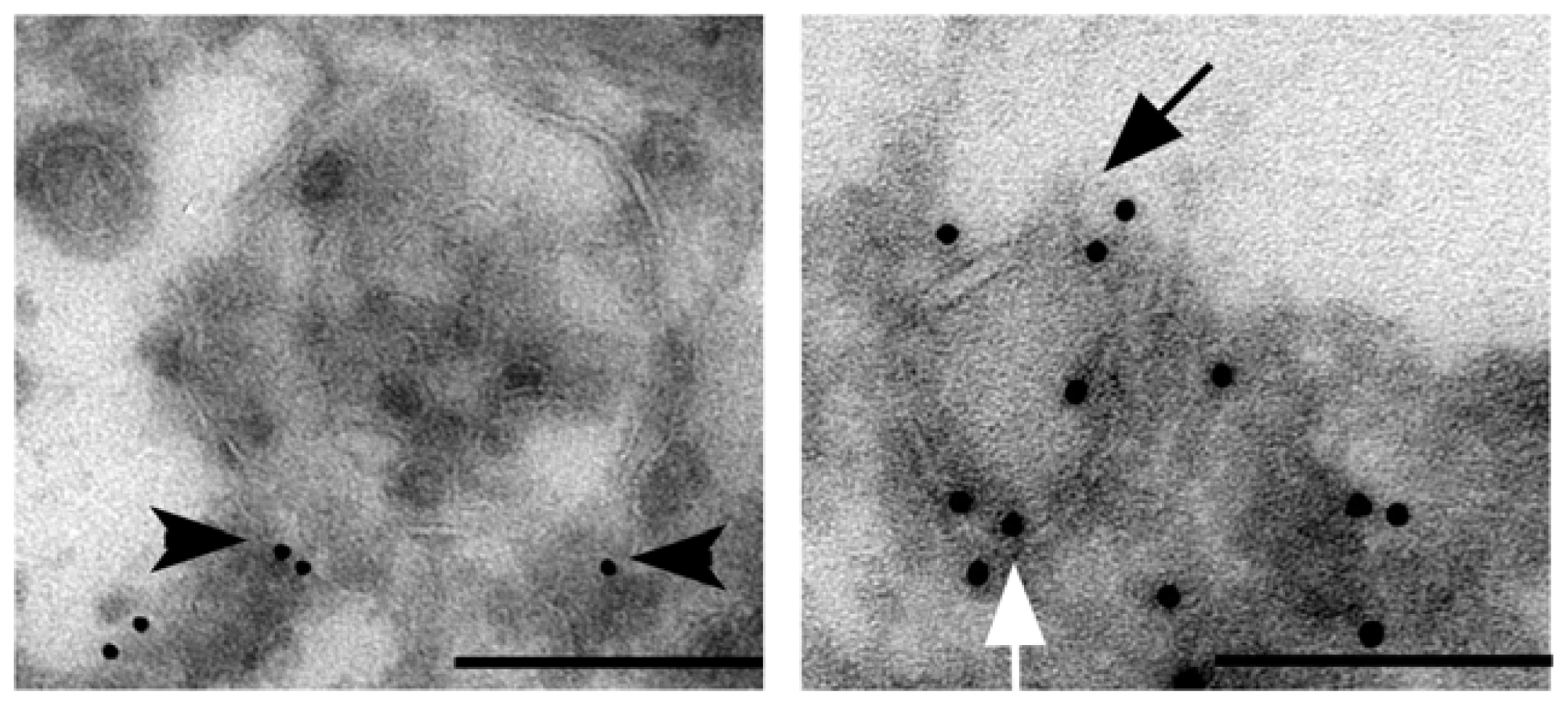
2. Rab27 GTPases Guide Vesicle Exocytosis
3. Rab27 GTPases Drive Invasive Growth and Metastasis
4. Clinical Assessment of Experimentally Validated Pro-Invasive Rab27 GTPases
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Hendrix, A.; Gespach, C.; Bracke, M.; de Wever, O. The tumor ecosystem regulates the roads for invasion and metastasis. Clin. Res. Hepatol. Gastroenterol 2011, 35, 714–719. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar]
- Peinado, H.; Lavotshkin, S.; Lyden, D. The secreted factors responsible for pre-metastatic niche formation: Old sayings and new thoughts. Semin. Cancer Biol 2011, 21, 139–146. [Google Scholar]
- Malanchi, I.; Santamaria-Martinez, A.; Susanto, E.; Peng, H.; Lehr, H.A.; Delaloye, J.F.; Huelsken, J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 2012, 481, 85–89. [Google Scholar]
- McAllister, S.S.; Weinberg, R.A. Tumor-host interactions: A far-reaching relationship. J. Clin. Oncol 2010, 28, 4022–4028. [Google Scholar]
- Hendrix, A.; Westbroek, W.; Bracke, M.; de Wever, O. An ex(o)citing machinery for invasive tumor growth. Cancer Res 2010, 70, 9533–9537. [Google Scholar]
- De Boeck, A.; Pauwels, P.; Hensen, K.; Rummens, J.L.; Westbroek, W.; Hendrix, A.; Maynard, D.; Denys, H.; Lambein, K.; Braems, G.; et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut 2013, 62, 550–560. [Google Scholar]
- Hendrix, A.; Hume, A.N. Exosome signaling in mammary gland development and cancer. Int. J. Dev. Biol 2011, 55, 879–887. [Google Scholar]
- Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol 2009, 10, 513–525. [Google Scholar]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol 2010, 12, 19–30. [Google Scholar]
- Hendrix, A.; Maynard, D.; Pauwels, P.; Braems, G.; Denys, H.; van den Broecke, R.; Lambert, J.; van Belle, S.; Cocquyt, V.; Gespach, C.; et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J. Natl. Cancer Inst 2010, 102, 866–880. [Google Scholar]
- Hendrix, A.; Braems, G.; Bracke, M.; Seabra, M.; Gahl, W.; de Wever, O.; Westbroek, W. The secretory small GTPase Rab27B as a marker for breast cancer progression. Oncotarget 2010, 1, 304–308. [Google Scholar]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Thery, C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012, 72, 4920–4930. [Google Scholar]
- Fukuda, M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell. Mol. Life Sci 2008, 65, 2801–2813. [Google Scholar]
- Pfeffer, S.R. Rab GTPase localization and Rab cascades in Golgi transport. Biochem. Soc. Trans 2012, 40, 1373–1377. [Google Scholar]
- Fukuda, M. Versatile role of Rab27 in membrane trafficking: Focus on the Rab27 effector families. J. Biochem 2005, 137, 9–16. [Google Scholar]
- Hendrix, A.; Lambein, K.; Westbroek, W.; Seabra, M.C.; Cocquyt, V.; Pauwels, P.; Bracke, M.; Gespach, C.; de Wever, O. An immunohistochemical analysis of Rab27B distribution in fetal and adult tissue. Int. J. Dev. Biol 2012, 56, 363–368. [Google Scholar]
- Catz, S.D. Regulation of vesicular trafficking and leukocyte function by Rab27 GTPases and their effectors. J. Leukoc. Biol. 2013. [Google Scholar] [CrossRef]
- Zheng, Y.; Campbell, E.C.; Lucocq, J.; Riches, A.; Powis, S.J. Monitoring the Rab27 associated exosome pathway using nanoparticle tracking analysis. Exp. Cell Res. 2012. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol 2012, 10, e1001450. [Google Scholar]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 2007, 9, 654–659. [Google Scholar]
- Hendrix, A.; Ciccone, C.; Gespach, C.; Bracke, M.; de Wever, O.; Westbroek, W. Rab27B-mediated metabolic programming induces secretome acidification and chemoresistance in breast cancer cells. Exosomes Microvesicles 2013, 3, 15–20. [Google Scholar]
- Wang, X.; Song, X.; Zhuo, W.; Fu, Y.; Shi, H.; Liang, Y.; Tong, M.; Chang, G.; Luo, Y. The regulatory mechanism of Hsp90alpha secretion and its function in tumor malignancy. Proc. Natl. Acad. Sci. USA 2009, 106, 21288–21293. [Google Scholar]
- Epple, L.M.; Griffiths, S.G.; Dechkovskaia, A.M.; Dusto, N.L.; White, J.; Ouellette, R.J.; Anchordoquy, T.J.; Bemis, L.T.; Graner, M.W. Medulloblastoma exosome proteomics yield functional roles for extracellular vesicles. PLoS One 2012, 7, e42064. [Google Scholar]
- Taraboletti, G.; D’Ascenzo, S.; Giusti, I.; Marchetti, D.; Borsotti, P.; Millimaggi, D.; Giavazzi, R.; Pavan, A.; Dolo, V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia 2006, 8, 96–103. [Google Scholar]
- Eustace, B.K.; Sakurai, T.; Stewart, J.K.; Yimlamai, D.; Unger, C.; Zehetmeier, C.; Lain, B.; Torella, C.; Henning, S.W.; Beste, G.; et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat. Cell Biol 2004, 6, 507–514. [Google Scholar]
- Sims, J.D.; McCready, J.; Jay, D.G. Extracellular heat shock protein (Hsp)70 and Hsp90alpha assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One 2011, 6, e18848. [Google Scholar]
- Akavia, U.D.; Litvin, O.; Kim, J.; Sanchez-Garcia, F.; Kotliar, D.; Causton, H.C.; Pochanard, P.; Mozes, E.; Garraway, L.A.; Pe’er, D. An integrated approach to uncover drivers of cancer. Cell 2010, 143, 1005–1017. [Google Scholar]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med 2012, 18, 883–891. [Google Scholar] [Green Version]
- Ho, J.R.; Chapeaublanc, E.; Kirkwood, L.; Nicolle, R.; Benhamou, S.; Lebret, T.; Allory, Y.; Southgate, J.; Radvanyi, F.; Goud, B. Deregulation of Rab and Rab effector genes in bladder cancer. PLoS One 2012, 7, e39469. [Google Scholar]
- Hendrix, A.; Sormunen, R.; Westbroek, W.; Lambein, K.; Denys, H.; Sys, G.; Braems, G.; van den Broecke, R.; Cocquyt, V.; Gespach, C.; et al. Vacuolar H+ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer. Int. J. Cancer 2013. [Google Scholar] [CrossRef]
- Liegeois, S.; Benedetto, A.; Garnier, J.M.; Schwab, Y.; Labouesse, M. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol 2006, 173, 949–961. [Google Scholar]
- Kuznetsov, H.S.; Marsh, T.; Markens, B.A.; Castano, Z.; Greene-Colozzi, A.; Hay, S.A.; Brown, V.E.; Richardson, A.L.; Signoretti, S.; Battinelli, E.M.; et al. Identification of luminal breast cancers that establish a tumor-supportive macroenvironment defined by proangiogenic platelets and bone marrow-derived cells. Cancer Discov 2012, 2, 1150–1165. [Google Scholar]
- Zhang, J.X.; Huang, X.X.; Cai, M.B.; Tong, Z.T.; Chen, J.W.; Qian, D.; Liao, Y.J.; Deng, H.X.; Liao, D.Z.; Huang, M.Y.; et al. Overexpression of the secretory small GTPase Rab27B in human breast cancer correlates closely with lymph node metastasis and predicts poor prognosis. J. Transl. Med. 2012, 10. [Google Scholar] [CrossRef]
- Dong, W.W.; Mou, Q.; Chen, J.; Cui, J.T.; Li, W.M.; Xiao, W.H. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J. Gastroenterol 2012, 18, 1806–1813. [Google Scholar]
- Lovis, P.; Gattesco, S.; Regazzi, R. Regulation of the expression of components of the exocytotic machinery of insulin-secreting cells by microRNAs. Biol. Chem 2008, 389, 305–312. [Google Scholar]
- Lacroix, R.; Judicone, C.; Poncelet, P.; Robert, S.; Arnaud, L.; Sampol, J.; Dignat-George, F. Impact of pre-analytical parameters on the measurement of circulating microparticles: Towards standardization of protocol. J. Thromb. Haemost 2012, 10, 437–446. [Google Scholar]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med 2012, 18, 1835–1840. [Google Scholar]
- Deschout, H.; Raemdonck, K.; Stremersch, S.; Maoddi, P.; Mernier, G.; Renaud, P.; Jiguet, S.; Hendrix, A.; Bracke, M.; van den Broecke, R.; et al. Disposible microfluidic chip with integrated light sheet illumination enables size and concentration measurements of submicron membrane vesicles in biofluids for diagnostics. 2013. submitted for publication. [Google Scholar]
| Protein identity | Matched peptides (#) | Sequence coverage (%) |
|---|---|---|
| Tetraspanins | ||
| CD9 | 3 | 29.6 |
| CD63 | 1 | 14.2 |
| CD81 | 3 | 21.5 |
| Heat shock proteins | ||
| HSP70 | 21 | 44.3 |
| HSP90 alpha | 13 | 32.9 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hendrix, A.; De Wever, O. Rab27 GTPases Distribute Extracellular Nanomaps for Invasive Growth and Metastasis: Implications for Prognosis and Treatment. Int. J. Mol. Sci. 2013, 14, 9883-9892. https://doi.org/10.3390/ijms14059883
Hendrix A, De Wever O. Rab27 GTPases Distribute Extracellular Nanomaps for Invasive Growth and Metastasis: Implications for Prognosis and Treatment. International Journal of Molecular Sciences. 2013; 14(5):9883-9892. https://doi.org/10.3390/ijms14059883
Chicago/Turabian StyleHendrix, An, and Olivier De Wever. 2013. "Rab27 GTPases Distribute Extracellular Nanomaps for Invasive Growth and Metastasis: Implications for Prognosis and Treatment" International Journal of Molecular Sciences 14, no. 5: 9883-9892. https://doi.org/10.3390/ijms14059883




