Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901
Abstract
:1. Introduction
2. Results and Discussion
2.1. Content of the Poncirin in Four Tissues of Ougan
2.2. Purification of Poncirin from Albedo of Ougan
2.3. Inhibitory Activity of Purified Poncirin on the Growth of Gastric Cancer Cells
3. Experimental Section
3.1. Chemical and Reagents
3.2. Apparatus
3.3. Materials
3.4. Preparation of Sample Solution
3.5. Identification of Poncirin
3.6. Dynamic Adsorption and Desorption Tests
3.7. Selection of the Two-Phase Solvent Systems for HSCCC
3.8. Preparation of Two-Phase-Solvent System and Sample Solution
3.9. HSCCC Separation Procedure
3.10. Cell Culture and Cell Viability Assay
3.11. Statistic Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Diplock, A.; Charleux, J.; Grozier-Willi, G.; Kok, K.; Rice-Evans, C.; Roberfroid, M.; Stahl, W.; Vina-Ribes, J. Functional food sciences and defence against reactive oxidative species. Br. J. Nutr 1998, 80, 77–82. [Google Scholar]
- Soong, Y.Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem 2004, 88, 411–417. [Google Scholar]
- Pérez-Gregorio, M.R.; García-Falcón, M.S.; Simal-Gándara, J. Flavonoids changes in fresh-cut onions during storage in different packaging systems. Food Chem 2011, 124, 652–658. [Google Scholar]
- Figueiredo-González, M.; Cancho-Grande, B.; Boso, S.; Santiago, J.L.; Martínez, M.C.; Simal-Gándara, J. Evolution of flavonoids in Mouratón berries taken from both bunch halves. Food Chem 2013, 138, 1868–1877. [Google Scholar]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; de Luca, D.; Statti, G.A.; Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol 2011, 49, 1549–1555. [Google Scholar]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int 2011, 44, 2088–2099. [Google Scholar]
- Abeysinghe, D.C.; Li, X.; Sun, C.D.; Zhang, W.S.; Zhou, C.H.; Chen, K.S. Bioactive compounds and antioxidant capacity in different edible tissues of citrus fruit of four citrus species. Food Chem 2007, 104, 1338–1344. [Google Scholar]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappa B inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull 2007, 30, 2345–2351. [Google Scholar]
- Lee, J.H.; Lee, S.H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res 2009, 12, 1748–1753. [Google Scholar]
- Kim, D.H.; Jung, E.A.; Sohng, I.S.; Han, J.A.; Kim, T.H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharmacal. Res 1998, 21, 17–23. [Google Scholar]
- Yoon, H.Y.; Yun, S.I.; Kim, B.Y.; Jin, Q.L.; Woo, E.R. Poncirin promotes osteoblast differentiation but inhibits adipocyte differentiation in mesenchymal stem cells. Eur. J. Pharmacol 2011, 664, 54–59. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food. Chem 2008, 56, 6185–6205. [Google Scholar]
- Xu, X.H.; Yan, F.H.; Ye, R.H.; Cheng, X.D. Advances in Citrus reticulate cv. Suavissima research. J. Zhejiang For. Sci. Technol 2008, 28, 75–77. [Google Scholar]
- Fu, B.Q.; Liu, J.; Li, H.; Li, L.; Lee, F.S.C.; Wang, X.R. The application of macroporous resins in the separation of licorice flavonoids and glycyrrhizic acid. J. Chromatogr. A 2005, 1089, 18–24. [Google Scholar]
- Fu, Y.; Zu, Y.; Liu, W.; Hou, C.; Chen, L.; Li, S.; Shi, X.; Tong, M. Preparative separation of vitexin and isovitexin from pigeonpea extracts with macroporous resins. J. Chromatogr. A 2007, 1139, 206–213. [Google Scholar]
- Zhang, B.; Yang, R.Y.; Zhao, Y.; Liu, C.Z. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J. Chromatogr. B 2008, 867, 253–258. [Google Scholar]
- Wan, J.B.; Zhang, Q.W.; Ye, W.C.; Wang, Y.T. Quantification and separation of proto panaxatriol and protopanaxadiol type saponins from Panax notoginseng with macroporous resins. Sep. Purif. Technol 2008, 60, 198–205. [Google Scholar]
- Jiang, Y.; Lu, H.T.; Chen, F. Preparative purification of glycycyrrhirn extracted from the root of liquorice using high-speed countercurrent chromatography. J. Chromatogr. A 2004, 1033, 183–186. [Google Scholar]
- Li, H.B.; Chen, F. Preparative isolation and purification of gastrodin from the Chinese medicinal plant Gastrodia elata by high-speed countercurrent chromatography. J. Chromatogr. A 2004, 1052, 229–232. [Google Scholar]
- Li, H.B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed countercurrent chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar]
- Lee, D.H.; Park, K.I.; Park, H.S.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Hah, Y.S.; Chung, H.J.; et al. Flavonoids isolated from Korea Citrus aurantiumL. induce G2/M phase arrest and apoptosis in human gastric cancer AGS cells. Evid. Based Comple. Alt. 2012. [Google Scholar] [CrossRef]
- Kim, C.Y.; Lee, M.K.; Ahn, M.-J.; Kim, J. One step purification of flavanone glycosides from Poncirus trifoliata by centrifugal partition chromatography. J. Sep. Sci 2007, 30, 2693–2697. [Google Scholar]
- Liu, W.; Zhang, S.; Zu, Y.G.; Fu, Y.J.; Ma, W.; Zhang, D.Y.; Kong, Y.; Li, X.J. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour. Technol 2010, 101, 4667–4675. [Google Scholar]
- Oka, F.; Oka, H.; Ito, Y. Systematic search for suitable two-phase solvent systems for high-speed counter-current chromatography. J. Chromatogr. A 1991, 538, 99–108. [Google Scholar]
- Yoichiro, I. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar]
- Shin, I.S.; Lee, M.Y.; Seo, C.S.; Lim, H.S.; Ha, H.K.; Shin, H.K. Yijin-tang, an oriental herbal formula reduces ethanol-induced acute gastric injury in rats. J. Korean Soc. Appl. Biol 2012, 55, 197–204. [Google Scholar]
- Liu, R.M.; Kong, L.Y.; Li, A.F.; Sun, A.L. Preparative isolation and purification of saponin and flavone glycoside compounds from Clinopodium chinensis (Benth) O. Kuntze by high-speed countercurrent chromatography. J. Liq. Chromatogr. Related Technol 2007, 30, 521–532. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar]
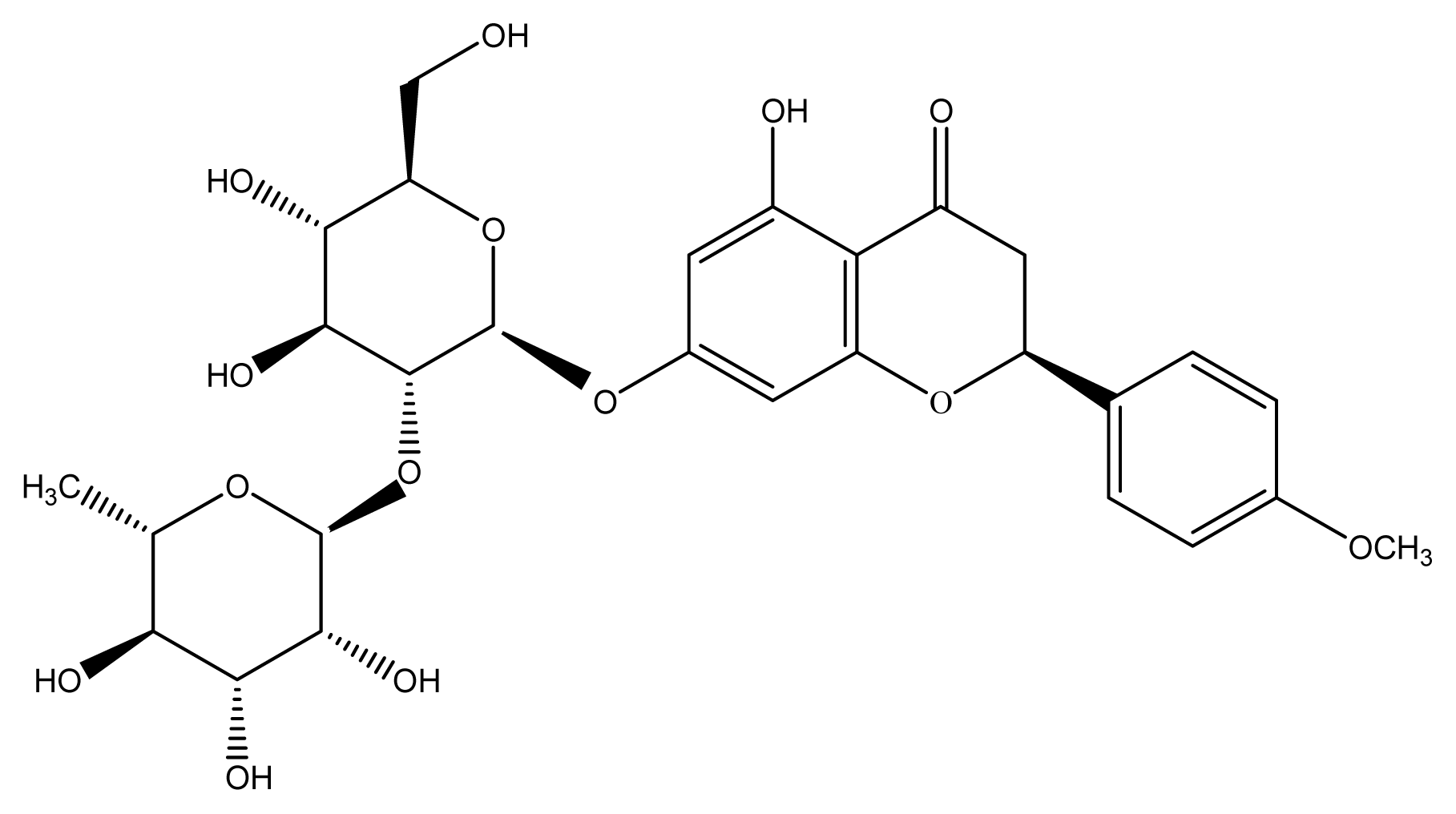

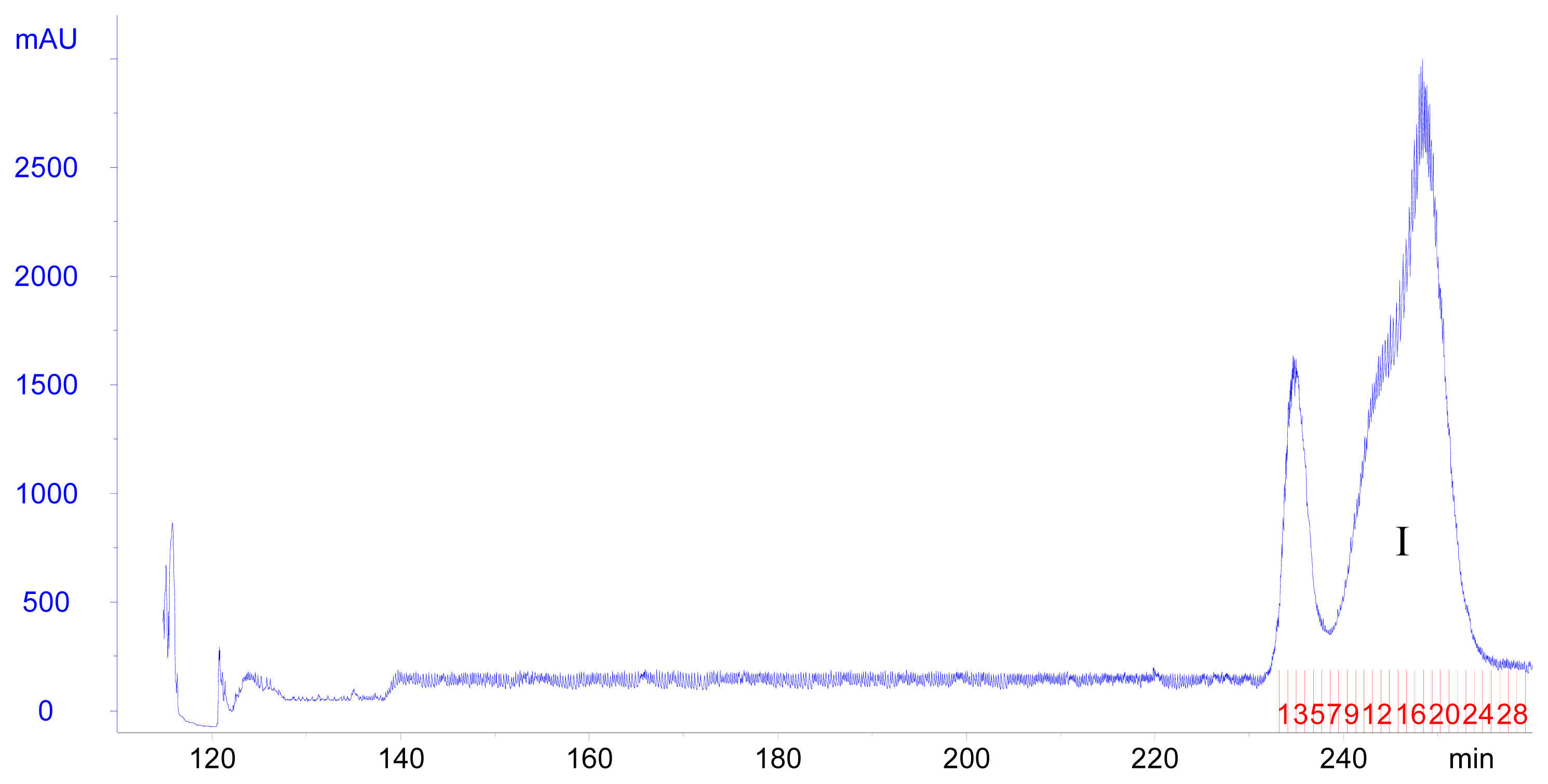

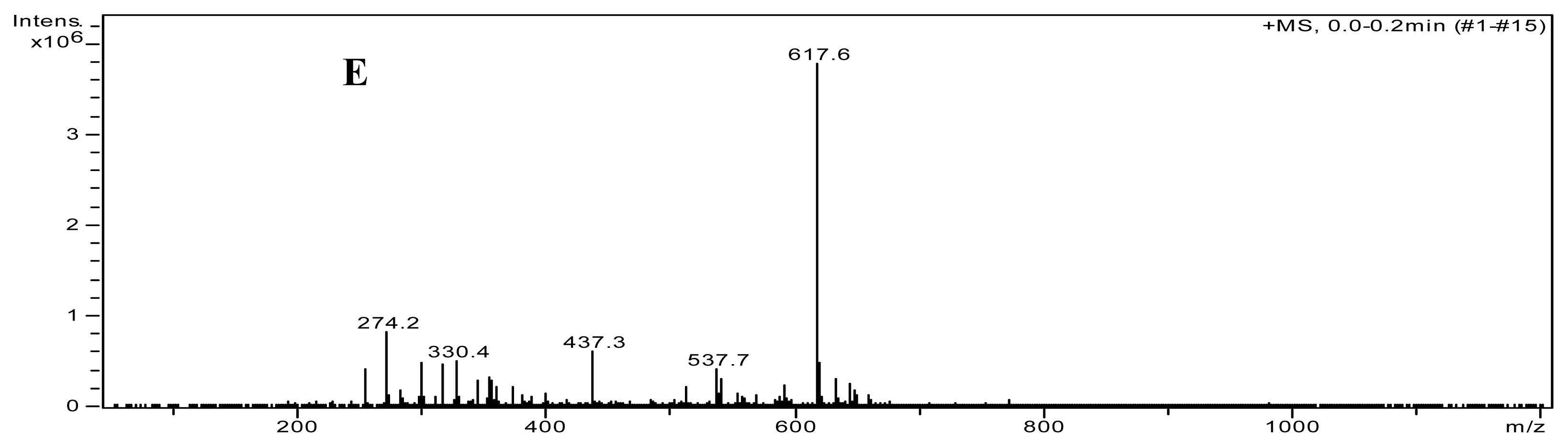
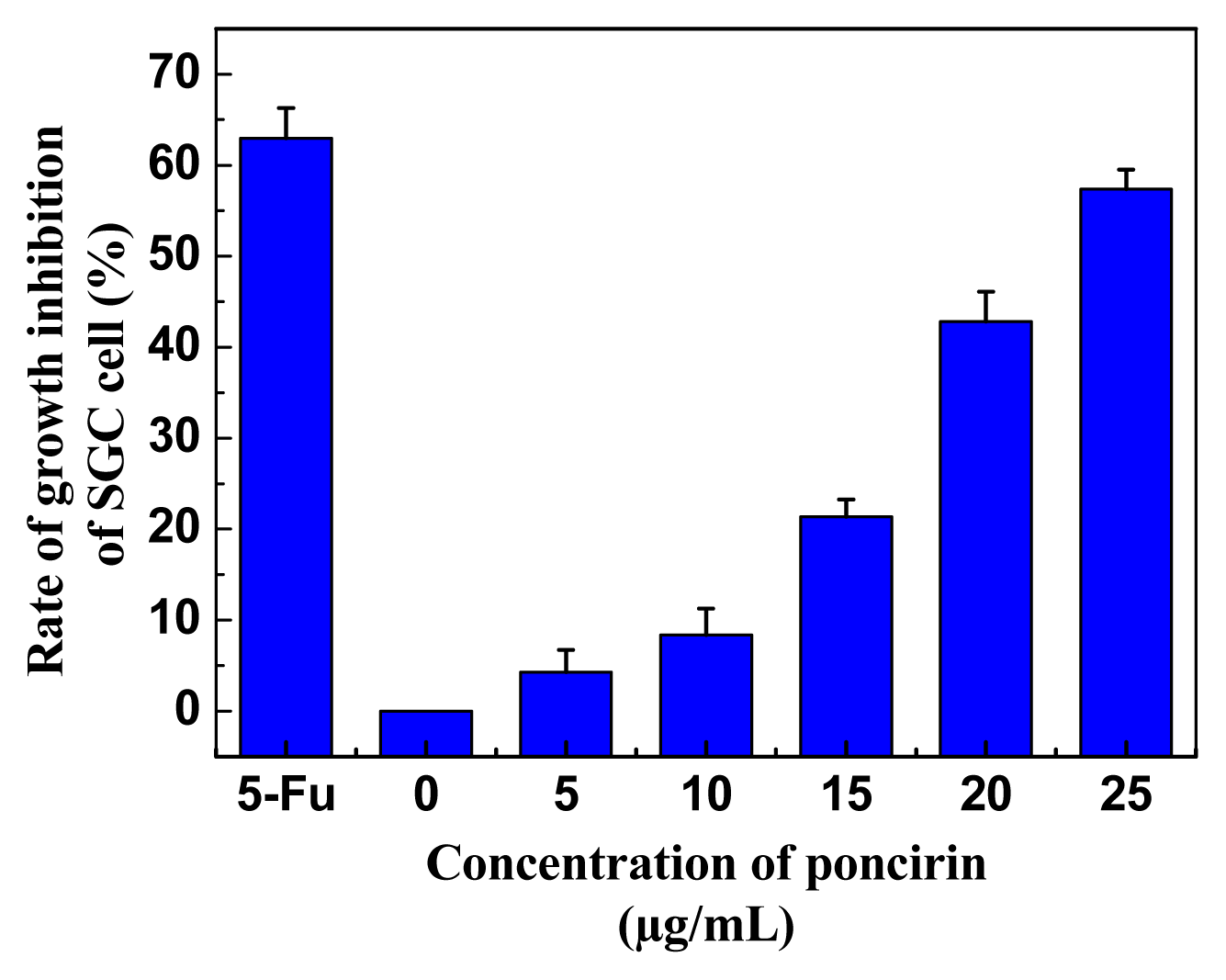
| Tissue | Content of poncirin (mg/g FW) |
|---|---|
| flavedo | 0.15 ± 0.01 |
| albedo | 1.37 ± 0.09 |
| segment membrane | 0.52 ± 0.01 |
| juice sac | 0.07 ± 0.01 |
| Concentration of ethanol (%) | Mass of dried residue (mg) | Mass of the poncirin (mg) | Content of the (poncirin %) |
|---|---|---|---|
| 10 | 19.3 | / | / |
| 20 | 140.5 | 0.78 | 0.56 |
| 30 | 217.4 | 3.91 | 1.80 |
| 40 | 193.5 | 9.48 | 4.90 |
| 50 | 82.9 | 8.42 | 10.16 |
| 60 | 38.3 | 3.65 | 9.52 |
| 70 | 13.7 | 0.24 | 1.76 |
| 80 | 2.3 | / | / |
| 90 | 0.7 | / | / |
| Solvent system | Ratio(v/v/v/v) | K value |
|---|---|---|
| Chloroform–methanol–n-butanol–water | 4:3:0.5:2 | 0.81 |
| Chloroform–methanol–n-butanol–water | 4:3:0.5:3 | 0.36 |
| Ethyl acetate–n-butanol–water | 4:0.5:5 | 3.14 |
| Ethyl acetate–n-butanol–water | 5:1:5 | 2.73 |
| n-Hexane–n-butanol–water | 1:1:2 | 0.55 |
| Purification step | Purity (%) | Recovery (%) | Yield (mg) |
|---|---|---|---|
| Crude extract | 0.14 | / | / |
| D101 Resin | 5.30 | 71.55 | 302.4 a |
| HSCCC | 96.56 | 63.77 | 2.1 b |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhu, X.; Luo, F.; Zheng, Y.; Zhang, J.; Huang, J.; Sun, C.; Li, X.; Chen, K. Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901. Int. J. Mol. Sci. 2013, 14, 8684-8697. https://doi.org/10.3390/ijms14058684
Zhu X, Luo F, Zheng Y, Zhang J, Huang J, Sun C, Li X, Chen K. Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901. International Journal of Molecular Sciences. 2013; 14(5):8684-8697. https://doi.org/10.3390/ijms14058684
Chicago/Turabian StyleZhu, Xiaoyan, Fenglei Luo, Yixiong Zheng, Jiukai Zhang, Jianzhen Huang, Chongde Sun, Xian Li, and Kunsong Chen. 2013. "Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901" International Journal of Molecular Sciences 14, no. 5: 8684-8697. https://doi.org/10.3390/ijms14058684
APA StyleZhu, X., Luo, F., Zheng, Y., Zhang, J., Huang, J., Sun, C., Li, X., & Chen, K. (2013). Characterization, Purification of Poncirin from Edible Citrus Ougan (Citrus reticulate cv. Suavissima) and Its Growth Inhibitory Effect on Human Gastric Cancer Cells SGC-7901. International Journal of Molecular Sciences, 14(5), 8684-8697. https://doi.org/10.3390/ijms14058684






