New Advances in Urea Transporter UT-A1 Membrane Trafficking
Abstract
:1. Introduction
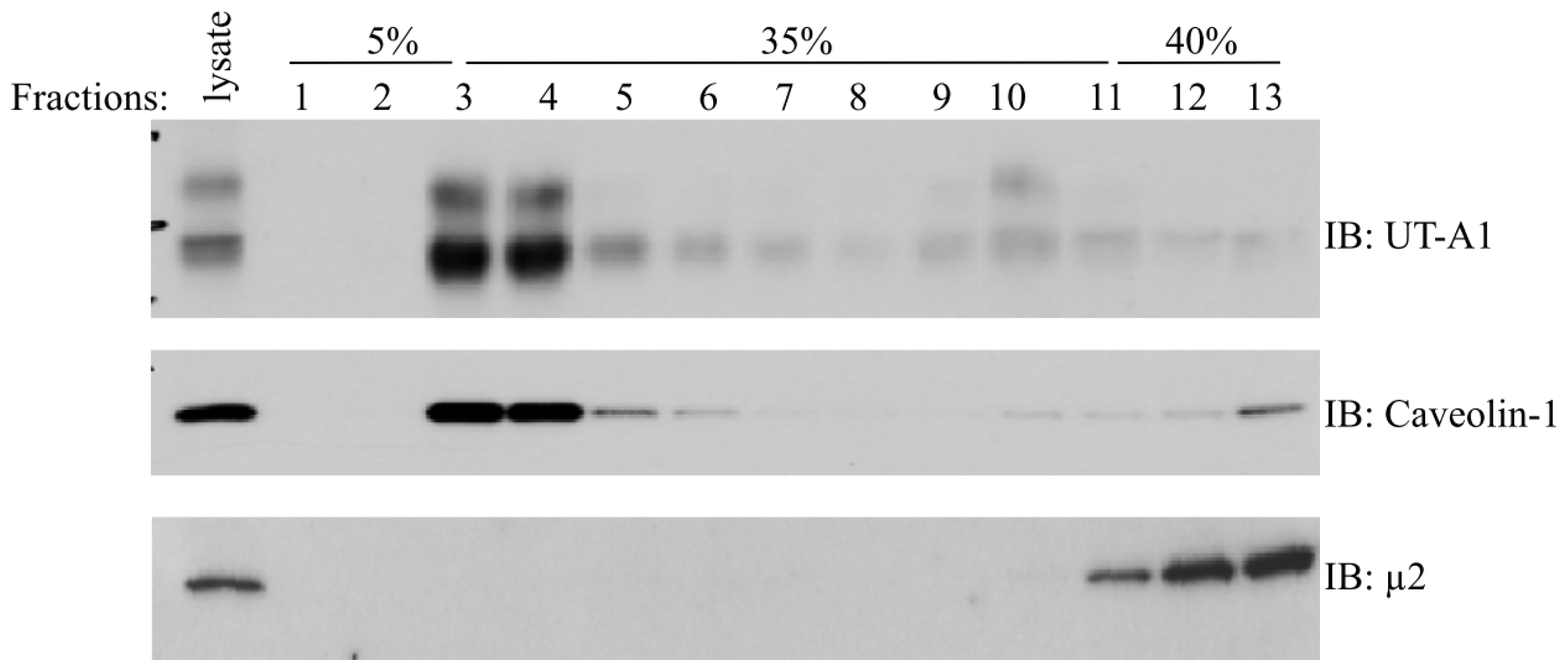
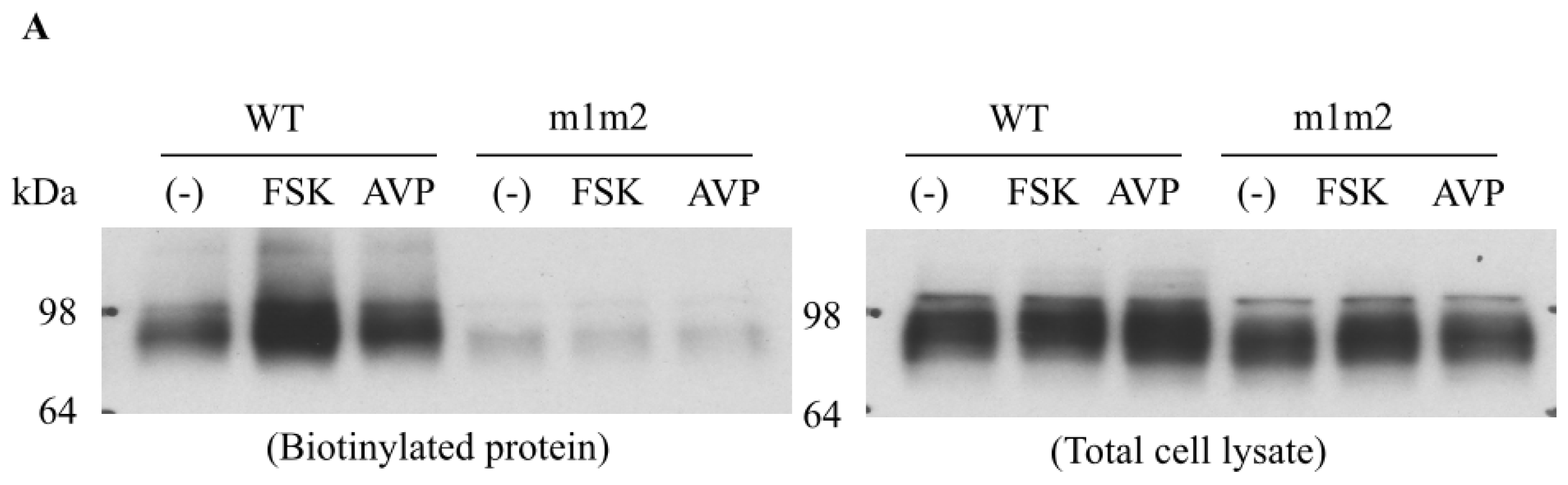
2. Role of Lipid Rafts
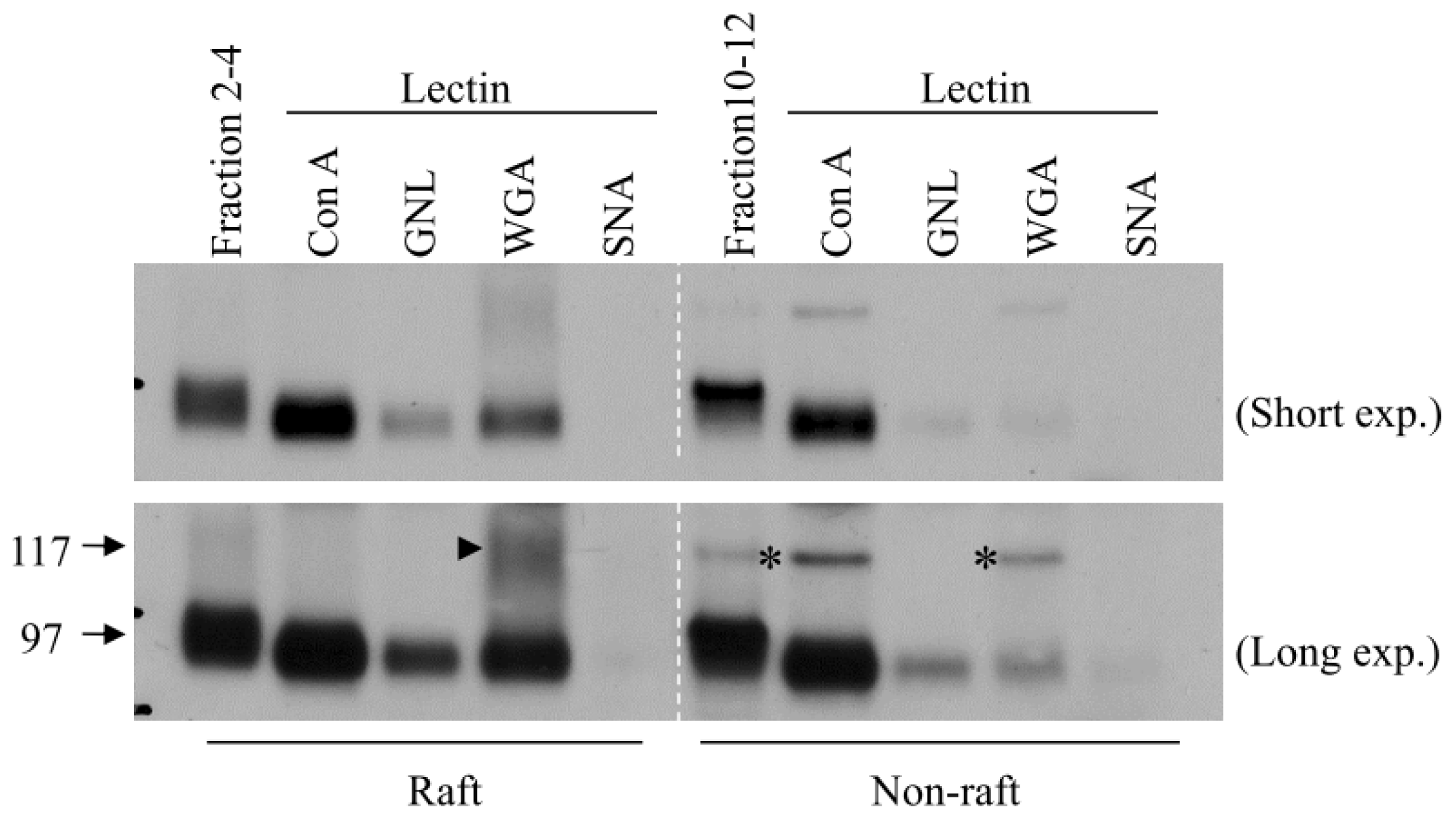
3. Role of N-Glycosylation
4. Role of Accessory Proteins
4.1. Caveolin
4.2. Snapin
4.3. Actin
5. Conclusion
Acknowledgements
Conflicts of Interest
References
- Sands, J.M. Critical role of urea in the urine-concentrating mechanism. J. Am. Soc. Nephrol 2007, 18, 670–671. [Google Scholar]
- Gamble, J.L.; McKhann, C.F.; Butler, A.M.; Tuthill, E. An economy of water in renal function referable to urea. Am. J. Physiol 1934, 109, 139–154. [Google Scholar]
- You, G.; Smith, C.P.; Kanai, Y.; Lee, W.-S.; Stelzner, M.; Hediger, M.A. Cloning and characterization of the vasopressin regulated urea transporter. Nature 1993, 365, 844–847. [Google Scholar]
- Klein, J.D.; Blount, M.A.; Sands, J.M. Molecular mechanisms of urea transport in health and disease. Pflugers. Arch 2012, 464, 561–572. [Google Scholar]
- Nielsen, S.; Terris, J.; Smith, C.P.; Hediger, M.A.; Ecelbarger, C.A.; Knepper, M.A. Cellular and subcellular localization of the vasopressin-regulated urea transporter in rat kidney. Proc. Natl. Acad. Sci. USA 1996, 93, 5495–5500. [Google Scholar]
- Fenton, R.A.; Chou, C.L.; Stewart, G.S.; Smith, C.P.; Knepper, M.A. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc. Natl. Acad. Sci. USA 2004, 101, 7469–7474. [Google Scholar]
- Wall, S.M.; Han, J.S.; Chou, C.L.; Knepper, M.A. Kinetics of urea and water permeability activation by vasopressin in rat terminal IMCD. Am. J. Physiol 1992, 262, F989–F998. [Google Scholar]
- Zhang, C.; Sands, J.M.; Klein, J.D. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am. J. Physiol. Renal Physiol 2002, 282, F85–F90. [Google Scholar]
- Kato, A.; Klein, J.D.; Zhang, C.; Sands, J.M. Angiotensin II increases vasopressin-stimulated facilitated urea permeability in rat terminal IMCDs. Am. J. Physiol. Renal Physiol 2000, 279, F835–F840. [Google Scholar]
- Klein, J.D.; Fröhlich, O.; Blount, M.A.; Martin, C.F.; Smith, T.D.; Sands, J.M. Vasopressin increases plasma membrane accumulation of urea transporter UT-A1 in rat inner medullary collecting ducts. J. Am. Soc. Nephrol 2006, 17, 2680–2686. [Google Scholar]
- Blount, M.A.; Mistry, A.C.; Fröhlich, O.; Price, S.R.; Chen, G.; Sands, J.M.; Klein, J.D. Phosphorylation of UT-A1 urea transporter at serines 486 and 499 is important for vasopressin-regulated activity and membrane accumulation. Am. J. Physiol. Renal Physiol 2008, 295, F295–F299. [Google Scholar]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol 2010, 11, 688–699. [Google Scholar]
- Puri, N.; Roche, P.A. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic 2006, 7, 1482–1494. [Google Scholar]
- Feng, X.; Huang, H.; Yang, Y.; Fröhlich, O.; Klein, J.D.; Sands, J.M.; Chen, G. Caveolin-1 directly interacts with UT-A1 urea transporter: The role of caveolae/lipid rafts in UT-A1 regulation at the cell membrane. Am. J. Physiol. Renal Physiol 2009, 296, F1514–F1520. [Google Scholar]
- Chen, G.; Howe, A.G.; Xu, G.; Fröhlich, O.; Klein, J.D.; Sands, J.M. Mature N-linked glycans facilitate UT-A1 urea transporter lipid raft compartmentalization. FASEB J 2011, 25, 4531–4539. [Google Scholar]
- Huang, H.; Feng, X.; Zhuang, J.; Fröhlich, O.; Janet, D.; Klein, J.D.; Cai, H.; Sands, J.M.; Chen, G. Internalization of UT-A1 urea transporter is dynamin dependent and mediated by both caveolae and clathrin coated pit pathways. Am. J. Physiol. Renal Physiol 2010, 299, F1389–F1395. [Google Scholar]
- Xu, G.; Su, H.; Carter, B.C.; Fröhlich, O.; Chen, G. Depolymerization of cortical actin inhibits UT-A1 urea transporter endocytosis but promotes forskolin stimulated membrane trafficking. Am. J. Physiol. Cell Physiol 2012, 302, C1012–C1018. [Google Scholar]
- Hanzal-Bayer, M.F.; Hancock, J.F. Lipid rafts and membrane traffic. FEBS Lett 2007, 581, 2098–2104. [Google Scholar]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol 2000, 1, 31–39. [Google Scholar]
- Xiong, Y.; Antalffy, G.; Enyedi, A.; Strehler, E.E. Apical localization of PMCA2w/b is lipid raft-dependent. Biochem. Biophys. Res. Commun 2009, 384, 32–36. [Google Scholar]
- Hoover, R.S.; Poch, E.; Monroy, A.; Vázquez, N.; Nishio, T.; Gamba, G.; Hebert, S.C. N-Glycosylation at two sites critically alters thiazide binding and activity of the rat thiazide-sensitive Na+:Cl− cotransporter. J. Am. Soc. Nephrol 2003, 14, 271–282. [Google Scholar]
- Lee, T.K.; Koh, A.S.; Cui, Z.; Pierce, R.H.; Ballatori, N. N-glycosylation controls functional activity of Oatp1, an organic anion transporter. Am. J. Physiol. Gastrointest. Liver Physiol 2003, 285, G371–G381. [Google Scholar]
- Hendriks, G.; Koudijs, M.; van Balkom, B.W.; Oorschot, V.; Klumperman, J.; Deen, P.M.; van der Sluijs, P. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J. Biol. Chem 2004, 279, 2975–2983. [Google Scholar]
- Vagin, O.; Kraut, J.A.; Sachs, G. Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am. J. Physiol. Renal Physiol 2009, 296, F459–F469. [Google Scholar]
- Scheiffele, P.; Peränen, J.; Simons, K. N-glycans as apical sorting signals in epithelial cells. Nature 1995, 378, 96–98. [Google Scholar]
- Chen, G.; Froehlich, O.; Yang, Y.; Klein, J.D.; Sands, J.M. Loss of N-linked glycosylation reduces urea transporter UT-A1 response to vasopressin. J. Biol. Chem 2006, 281, 27436–27442. [Google Scholar]
- Bradford, A.D.; Terris, J.M.; Ecelbarger, C.A.; Klein, J.D.; Sands, J.M.; Chou, C.L.; Knepper, M.A. 97- and 117-kDa forms of collecting duct urea transporter UT-A1 are due to different states of glycosylation. Am. J. Physiol. Renal Physiol 2001, 281, F133–F143. [Google Scholar]
- Pech, V.; Klein, J.D.; Kozlowski, S.D.; Wall, S.M.; Sands, J.M. Vasopressin increases urea permeability in the initial IMCD from diabetic rats. Am. J. Physiol. Renal Physiol 2005, 289, F531–F535. [Google Scholar]
- Parton, R.G.; Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol 2007, 8, 185–194. [Google Scholar]
- Razani, B.; Lisanti, M.P. Caveolins and caveolae: Molecular and functional relationships. Exp. Cell Res 2001, 271, 36–44. [Google Scholar]
- Lajoie, P.; Nabi, I.R. Regulation of raft-dependent endocytosis. J. Cell Mol. Med 2007, 11, 644–653. [Google Scholar]
- Liu, P.; Rudick, M.; Anderson, R.G. Multiple functions of caveolin-1. J. Biol. Chem 2002, 277, 41295–41298. [Google Scholar]
- Lang, T. SNARE proteins and ‘membrane rafts’. J. Physiol 2007, 585, 693–698. [Google Scholar]
- Mistry, A.C.; Mallick, R.; Fröhlich, O.; Klein, J.D.; Rehm, A.; Chen, G.; Sands, J.M. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J. Biol. Chem 2007, 282, 30097–30106. [Google Scholar]
- Chamberlain, L.H.; Burgoyne, R.D.; Gould, G.W. SNARE proteins are highly enriched in lipid rafts in PC12 cells: Implications for the spatial control of exocytosis. Proc. Natl. Acad. Sci. USA 2001, 98, 5619–5624. [Google Scholar]
- Koticha, D.K.; Huddleston, S.J.; Witkin, J.W.; Baldini, G. Role of the cysteine-rich domain of the t-SNARE component, SYNDET, in membrane binding and subcellular localization. J. Biol. Chem 1999, 274, 9053–9060. [Google Scholar]
- Apodaca, G. Endocytic traffic in polarized epithelial cells: Role of the actin and microtubule cytoskeleton. Traffic 2001, 2, 149–159. [Google Scholar]
- Noda, Y.; Horikawa, S.; Katayama, Y.; Sasaki, S. Water channel aquaporin-2 directly binds to actin. Biochem. Biophys. Res. Commun 2004, 322, 740–745. [Google Scholar]
- Mazzochi, C.; Bubien, J.K.; Smith, P.R.; Benos, D.J. The carboxyl terminus of the alpha-subunit of the amiloride-sensitive epithelial sodium channel binds to F-actin. J. Biol. Chem 2006, 281, 6528–6538. [Google Scholar]
- Prat, A.G.; Cunningham, C.C.; Jackson, G.R., Jr; Borkan, S.C.; Wang, Y.; Ausiello, D.A.; Cantiello, H.F. Actin filament organization is required for proper cAMP-dependent activation of CFTR. Am. J. Physiol. Cell Physiol 1999, 277, C1160–C1169. [Google Scholar]
- Liedtke, C.M.; Hubbard, M.; Wang, X. Stability of actin cytoskeleton and PKC-delta binding to actin regulate NKCC1 function in airway epithelial cells. Am. J. Physiol. Cell Physiol 2003, 284, C487–C496. [Google Scholar]
- Sadeghi, A.; Doyle, A.D.; Johnson, B.D. Regulation of the cardiac l-type Ca2+ channel by the actin-binding proteins α-actinin and dystrophin. Am. J. Physiol. Cell Physiol 2002, 282, C1502–C1511. [Google Scholar]
- Kanzaki, M.; Pessin, J.E. Insulin-stimulated GLUT4 translocation in adipocytes is dependent upon cortical actin remodeling. J. Biol. Chem 2001, 276, 42436–42444. [Google Scholar]
- Simon, H.; Gao, Y.; Franki, N.; Hays, R.M. Vasopressin depolymerizes apical F-actin in rat inner medullary collecting duct. Am. J. Physiol. Cell Physiol 1993, 265, C757–C762. [Google Scholar]
- Su, H.; Carter, C.B.; Fröhlich, O.; Cummings, R.D.; Chen, G. Glycoforms of UT-A3 urea transporter with poly-N-acetyllactosamine glycosylation have enhanced transport activity. Am. J. Physiol. Renal Physiol 2012, 303, F201–F208. [Google Scholar]
- Blount, M.A.; Klein, J.D.; Martin, C.F.; Tchapyjnikov, D.; Sands, J.M. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am. J. Physiol. Renal Physiol 2007, 293, F1308–F1313. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, G. New Advances in Urea Transporter UT-A1 Membrane Trafficking. Int. J. Mol. Sci. 2013, 14, 10674-10682. https://doi.org/10.3390/ijms140510674
Chen G. New Advances in Urea Transporter UT-A1 Membrane Trafficking. International Journal of Molecular Sciences. 2013; 14(5):10674-10682. https://doi.org/10.3390/ijms140510674
Chicago/Turabian StyleChen, Guangping. 2013. "New Advances in Urea Transporter UT-A1 Membrane Trafficking" International Journal of Molecular Sciences 14, no. 5: 10674-10682. https://doi.org/10.3390/ijms140510674



