Mirk/dyrk1B Kinase in Ovarian Cancer
Abstract
:1. Introduction
2. Results and Discussion
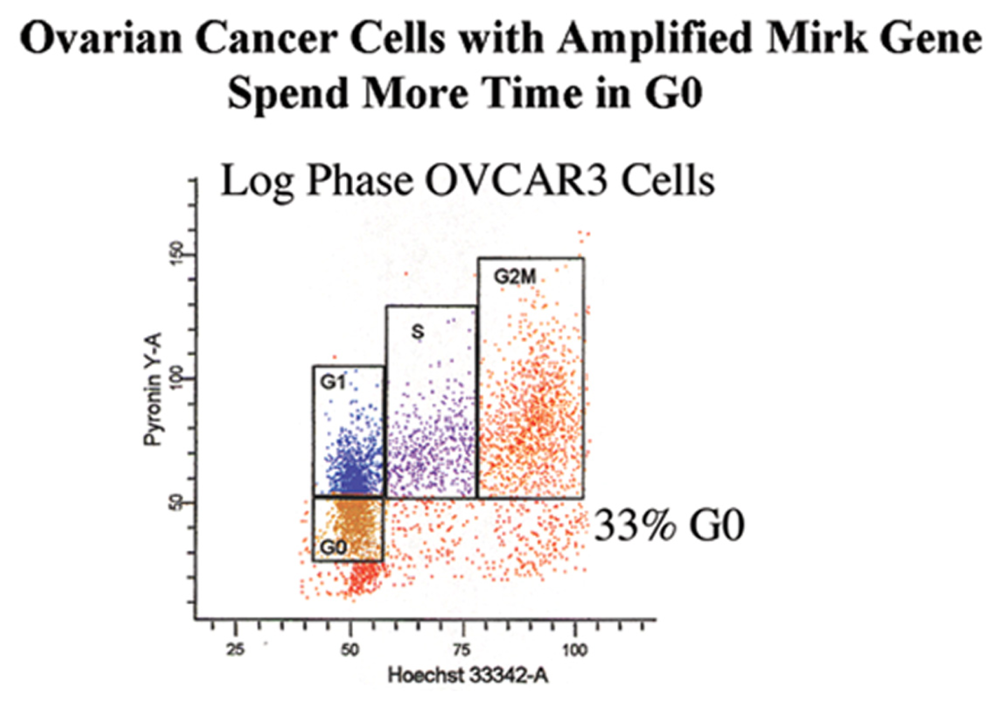
2.1. Mirk Gene Amplification
2.2. Mirk Kinase
2.3. Mirk Kinase Function
2.4. Mirk Kinase Function
2.5. Mirk Kinase Activation
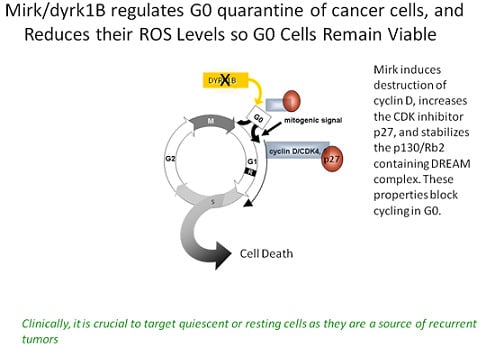
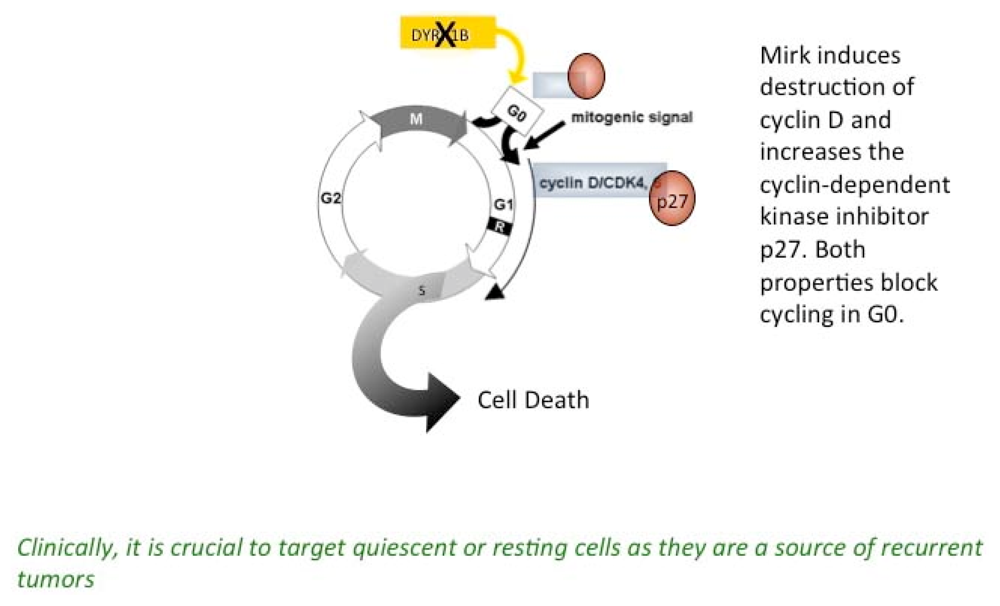
2.6. Mirk Has Two Major Functions in vivo, Growth Arrest in a Quiescent G0/G1 State and Maintenance of Viabilit
2.7. Both Normal Diploid Cells and Ovarian Cancer Cells Can Enter a Quiescent G0 State, Although the Presence of G0 Tumor Cells Has Been Controversial
2.8. G0 Is not a Permanent State
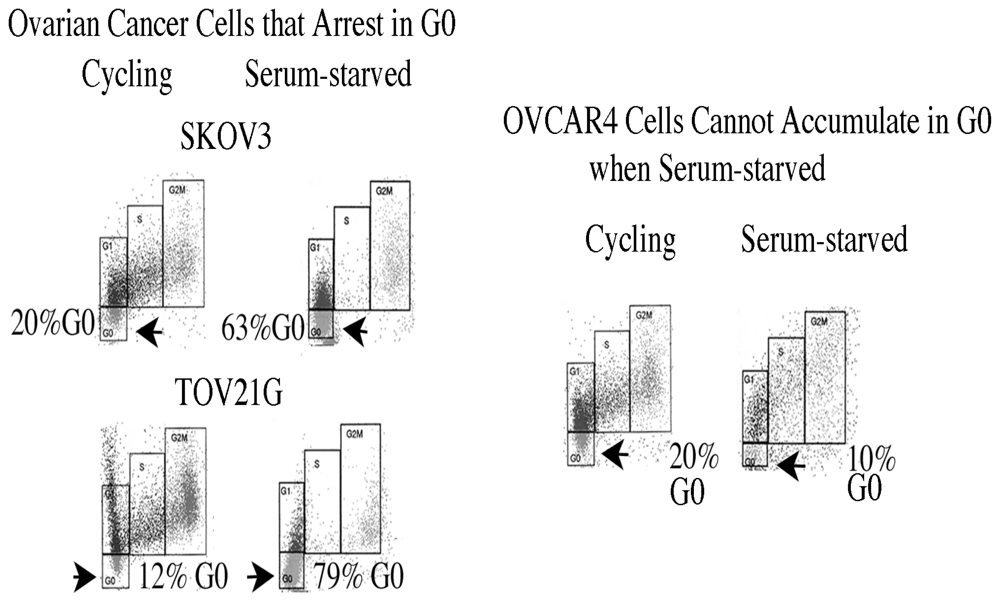
2.9. G0 Is Induced by Various Suboptimal Culture Conditions, and not All Ovarian Cancers Can Enter a Reversible Quiescent State
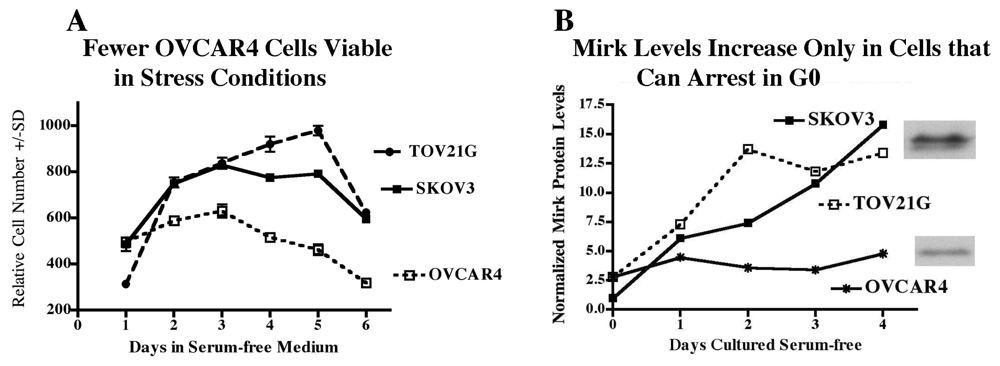
2.10. Why didn’t OVCAR4 (Figure 5) or OV90 Cells Arrest Reversibly in G0, and did this Lack of Arrest Affect Their Viability?
2.11. Accumulation in G0 by Mirk Action Allows Ovarian Cancer Cells to Survive Suboptimal Culture Conditions
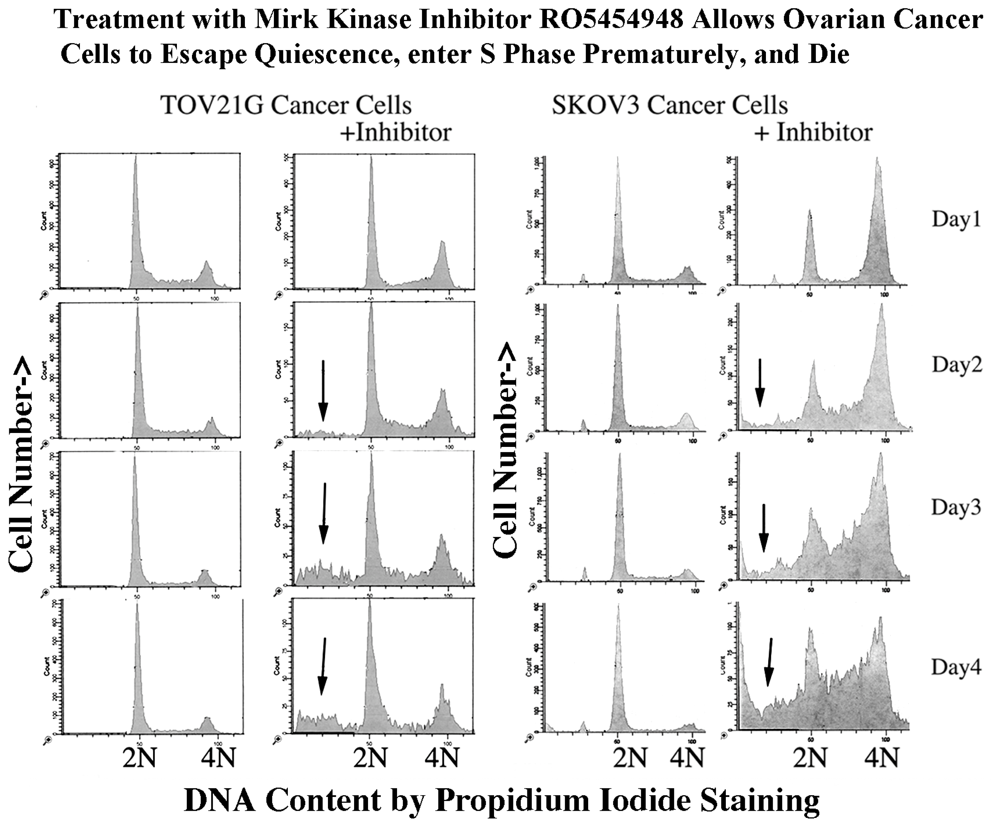
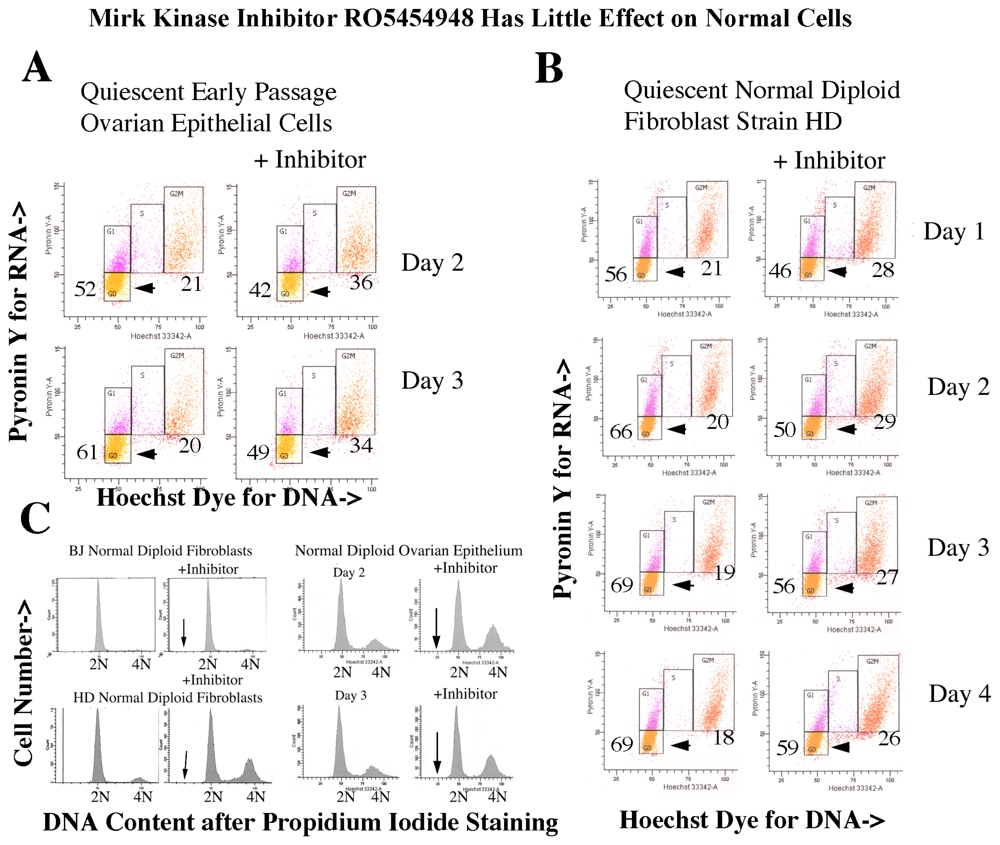
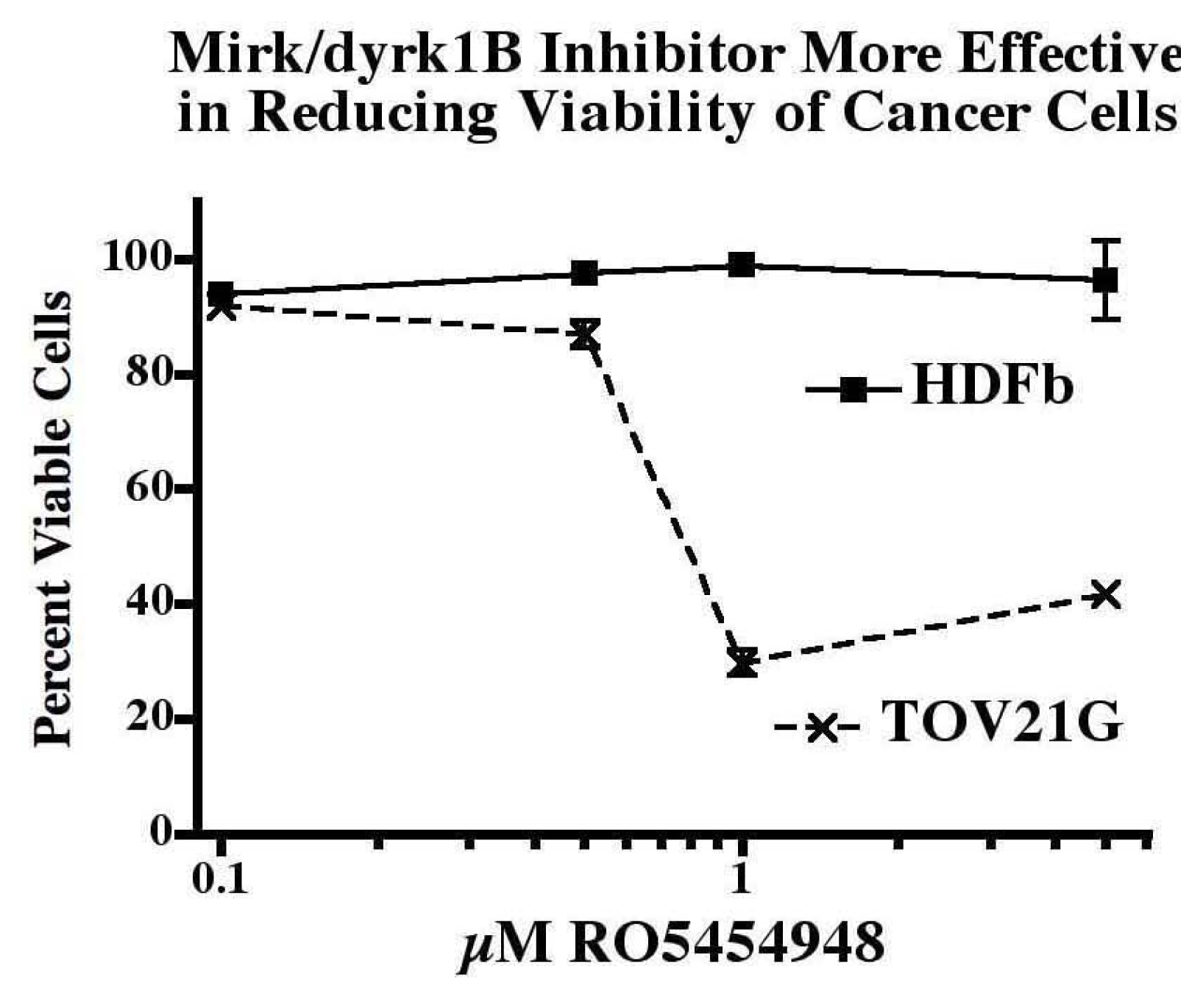
2.12. Ovarian Cancer Cells, not Normal Diploid Cells, Are Damaged by Mirk/dyrk1B Kinase Inhibition
2.13. Mirk Kinase Activity Is Higher in Cancer Cells than Normal Cells
2.14. Effect of Mirk Kinase Inhibitors on CDK4/cyclin D Complexes, as a Measure of the Ability of Mirk Kinase Inhibition to Push Cells into Cycle from Quiescence
2.15. Many Normal Cells in the Body Are Quiescent, so We Questioned Whether Targeting Mirk might Allow Them to Escape Quiescence
2.16. Test of Mirk Kinase Inhibitor on Patient Ascites
3. Conclusions
Conflict of Interest
References
- Barnes, D.; Melo, J. Primitive, quiescent and difficult to kill: The role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle 2006, 5, 2862–2866. [Google Scholar]
- Hu, J.; Friedman, E. Depleting mirk kinase increases cisplatin toxicity in ovarian cancer cells. Genes Cancer 2010, 1, 803–811. [Google Scholar]
- Janumyan, Y.; Cui, Q.; Yan, L.; Sansam, C.G.; Vanlentin, M.; Yang, E. G0 function of BCL2 and BCL-xL requires BAX, BAK, and p27 phosphorylation by Mirk, revealing a novel role of BAX and BAK in quiescence regulation. J. Biol. Chem 2008, 283, 34108–34120. [Google Scholar]
- Lu, Z.; Luo, R.Z.; Lu, Y.; Zhang, X.; Yu, Q.; Khare, S.; Kondo, S.; Kondo, Y.; Yu, Y.; Mills, G.B.; et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J. Clin. Invest 2008, 118, 3917–3929. [Google Scholar]
- Amaravadi, R.K. Autophagy-induced tumor dormancy in ovarian cancer. J. Clin. Invest 2008, 118, 3837–40. [Google Scholar]
- Adam, A.P.; George, A.; Schewe, D.; Bragado, P.; Iglesias, B.V.; Ranganathan, A.C.; Kourtidis, A.; Conklin, D.S.; Aguirre-Ghiso, J.A. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res 2009, 69, 5664–5672. [Google Scholar]
- Chen, C.; Liu, Y.; Liu, R.; Ikenoue, T.; Guan, K.L.; Liu, Y.; Zheng, P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J. Exp. Med 2008, 205, 2397–2408. [Google Scholar]
- Deng, X.; Ewton, D.Z.; Friedman, E. Mirk/Dyrk1B maintains the viability of quiescent pancreatic cancer cells by decreasing ROS levels. Cancer Res 2009, 69, 3317–3324. [Google Scholar]
- Jin, K.; Park, S.-J.; Ewton, D.; Friedman, E. The survival kinase Mirk/dyrk1B is a downstream effector of oncogenic K-ras. Cancer Res 2007, 67, 7247–7255. [Google Scholar]
- Collins, C.S.; Hong, J.; Sapinoso, L.; Zhou, Y.; Liu, Z.; Micklash, K.; Schultz, P.G.; Hampton, G.M. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. PNAS 2006, 103, 3775–3780. [Google Scholar]
- Thompson, F.H.; Nelson, M.A.; Trent, J.M.; Guan, X.Y.; Liu, Y.; Yang, J.M.; Emerson, J.; Adair, L.; Wymer, J.; Balfour, C.; et al. Amplification of 19q13.1-q13.2 sequences in ovarian cancer: G-band, FISH, and molecular studies. Cancer Gene. Cytogenet 1996, 87, 55. [Google Scholar]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615.[Green Version]
- Kuuselo, R.; Savinainen, K.; Azorsa, D.O.; Basu, G.D.; Karhu, R.; Tuzmen, S.; Mousses, S.; Kallioniemi, A. Intersex-like (IXL) is a cell survival regulator in pancreatic cancer with 19q13 amplification. Cancer Res 2007, 67, 1943–1949. [Google Scholar]
- Cheng, J.Q.; Godwin, A.K.; Bellacosa, A.; Taguchi, T.; Franke, T.F.; Hamilton, T.C.; Tsichlis, P.N.; Testa, J.R. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. PNAS 1992, 89, 9267–9271. [Google Scholar]
- Hu, J.; Nakhla, H.; Friedman, E. Transient arrest in a quiescent state allows ovarian cancer cells to survive suboptimal growth conditions and is mediated by Mirk/dyrk1B and p130/Rb2. Int. J. Cancer 2011, 129, 307–318. [Google Scholar]
- Lee, K.; Deng, X.; Friedman, E. Mirk protein kinase is a mitogen-activated protein kinase substrate that mediates survival of colon cancer cells. Cancer Res 2000, 60, 3631–3637. [Google Scholar]
- Gao, J.; Yang, X.; Yin, P.; Hu, W.; Liao, H.; Miao, Z.; Pan, C.; Li, N. The involvement of FoxO in cell survival and chemosensitivity mediated by Mirk/Dyrk1B in ovarian cancer. Int. J. Oncol 2012, 40, 1203–1209. [Google Scholar]
- Deng, X.; Mercer, S.E.; Shah, S.; Ewton, D.Z.; Friedman, E. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G0 by mirk/dyrk1B kinase. J. Biol. Chem 2004, 279, 22498–22504. [Google Scholar]
- Zou, Y.; Ewton, D.; Deng, D.; Mercer, S.; Friedman, E. Mirk/dyrk1B kinase destabilizes cyclin D1 by phosphorylation at threonine 288. J. Biol. Chem 2004, 279, 27790–27798. [Google Scholar]
- Becker, W. Emerging role of DYRK family protein kinases as regulators of protein stability in cell cycle control. Cell Cycle 2012, 11, 3389–3394. [Google Scholar]
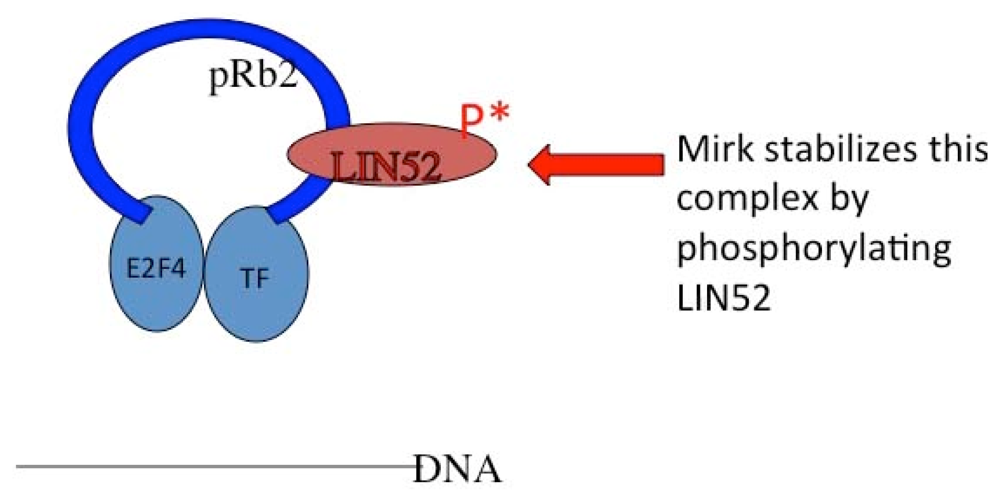
- Litovchick, L.; Florens, L.A.; Swanson, S.K.; Washburn, M.P.; DeCaprio, J.A. DYRK1A protein kinase promotes quiescence and senescence through DREAM complex assembly. Genes Dev 2011, 25, 801–813. [Google Scholar]
- Deng, X.; Ewton, D.Z.; Pawlikowski, B.; Maimone, M.; Friedman, E. Mirk/dyrk1B is a Rho-induced kinase active in skeletal muscle differentiation. J. Biol. Chem 2003, 278, 41347–41354. [Google Scholar]
- Mercer, S.E.; Ewton, D.Z.; Deng, X.; Lim, S.; Mazur, T.R.; Friedman, E. Mirk/dyrk1B mediates survival during the differentiation of C2C12 myoblasts. J. Biol. Chem 2005, 280, 25788–257801. [Google Scholar]
- Gao, J.; Zhao, Y.; Lv, Y.; Chen, Y.; Wei, B.; Tian, J.; Yan, Z.; Kong, F.; Pang, J.; Liu, J.; et al. Mirk/Dyrk1B mediates G0/G1 to S phase cell cycle progression and cell survival involving MAPK/ERK signaling in human cancer cells. Cancer Cell Int 2013, 13, 2. [Google Scholar]
- Coller, H.; Sang, L.; Roberts, J.M. A new description of cellular quiescence. PLoS Biol 2006, 4, 329–349. [Google Scholar]
- Smith, E.; Leone, G.; Nevins, J. Distinct mechanisms control the accumulation of the Rb-related p107 and p130 proteins during cell growth. Cell Growth Diff 1998, 9, 297–303. [Google Scholar]
- D’Andrilli, G.; Masciullo, V.; Bagella, L.; Tonini, T.; Minimo, C.; Zannoni, G.F.; Giuntoli, R.L., II; Carlson, J.A., Jr.; Soprano, D.R.; Soprano, K.J.; et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin. Cancer Res. 2004, 10, 3098–3103. [Google Scholar]
- Hu, J.; Deng, H.; Friedman, E. Ovarian cancer cells, not normal cells, are damaged by mirk/dyrk1B kinase inhibition. Int. J. Cancer 2013. [Google Scholar] [CrossRef]
- Correa, R.J.M.; Peart, T.; Valdes, Y.R.; DiMattia, G.E.; Shepherd, T.G. Modulation of AKT activity is associated with reversible dormancy in ascites-derived epithelial ovarian cancer spheroids. Carcinogenesis 2012, 33, 49–58. [Google Scholar]
- Leder, S.; Czajkowska, H.; Maenz, B.; de Graaf, K.; Barthel, A.; Joost, H.-G.; Becker, W. Alternative splicing variants of dual specificity tyrosine phosphorylated and regulated kinase 1B exhibit distinct patterns of expression and functional properties. Biochem. J 2003, 372, 881–888. [Google Scholar]


© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Friedman, E. Mirk/dyrk1B Kinase in Ovarian Cancer. Int. J. Mol. Sci. 2013, 14, 5560-5575. https://doi.org/10.3390/ijms14035560
Friedman E. Mirk/dyrk1B Kinase in Ovarian Cancer. International Journal of Molecular Sciences. 2013; 14(3):5560-5575. https://doi.org/10.3390/ijms14035560
Chicago/Turabian StyleFriedman, Eileen. 2013. "Mirk/dyrk1B Kinase in Ovarian Cancer" International Journal of Molecular Sciences 14, no. 3: 5560-5575. https://doi.org/10.3390/ijms14035560
APA StyleFriedman, E. (2013). Mirk/dyrk1B Kinase in Ovarian Cancer. International Journal of Molecular Sciences, 14(3), 5560-5575. https://doi.org/10.3390/ijms14035560