Inhibitory Effect of Baicalin and Baicalein on Ovarian Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Baicalin and Baicalein on Ovarian Cancer Cells Viability
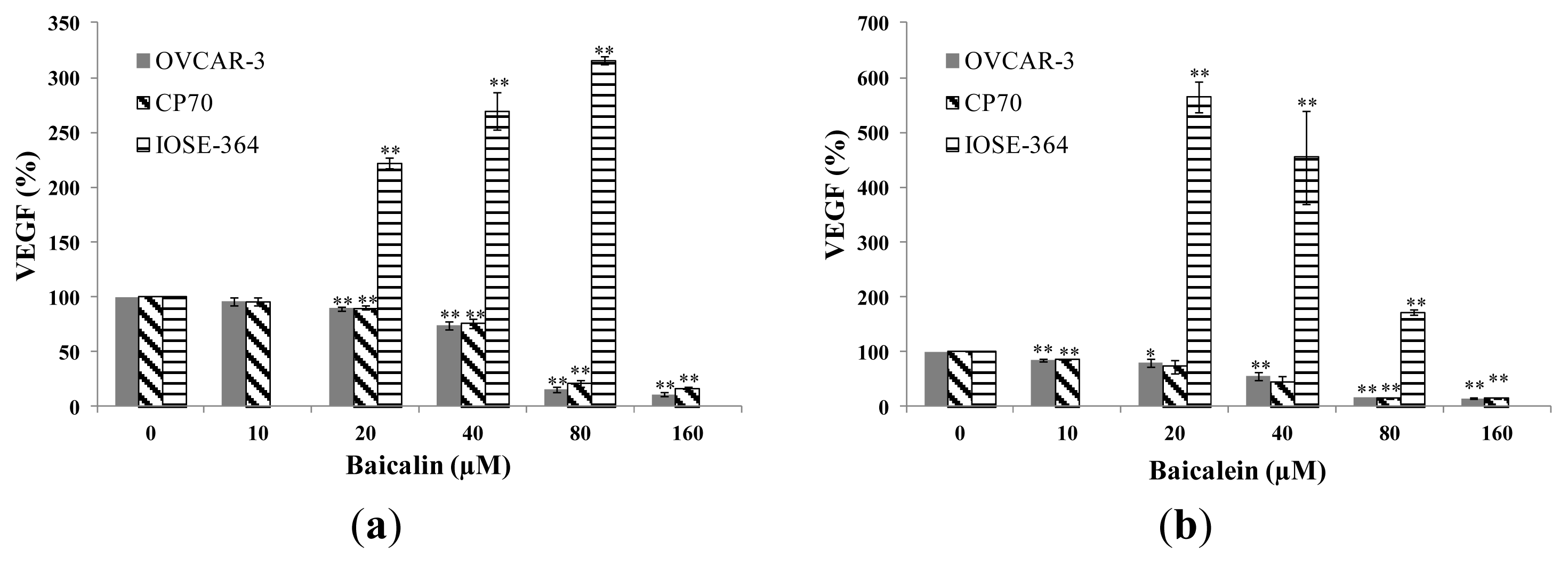
2.2. Effect of Baicalin and Baicalein on VEGF Protein Expression in OVCAR-3, CP70 and IOSE-364 Cells
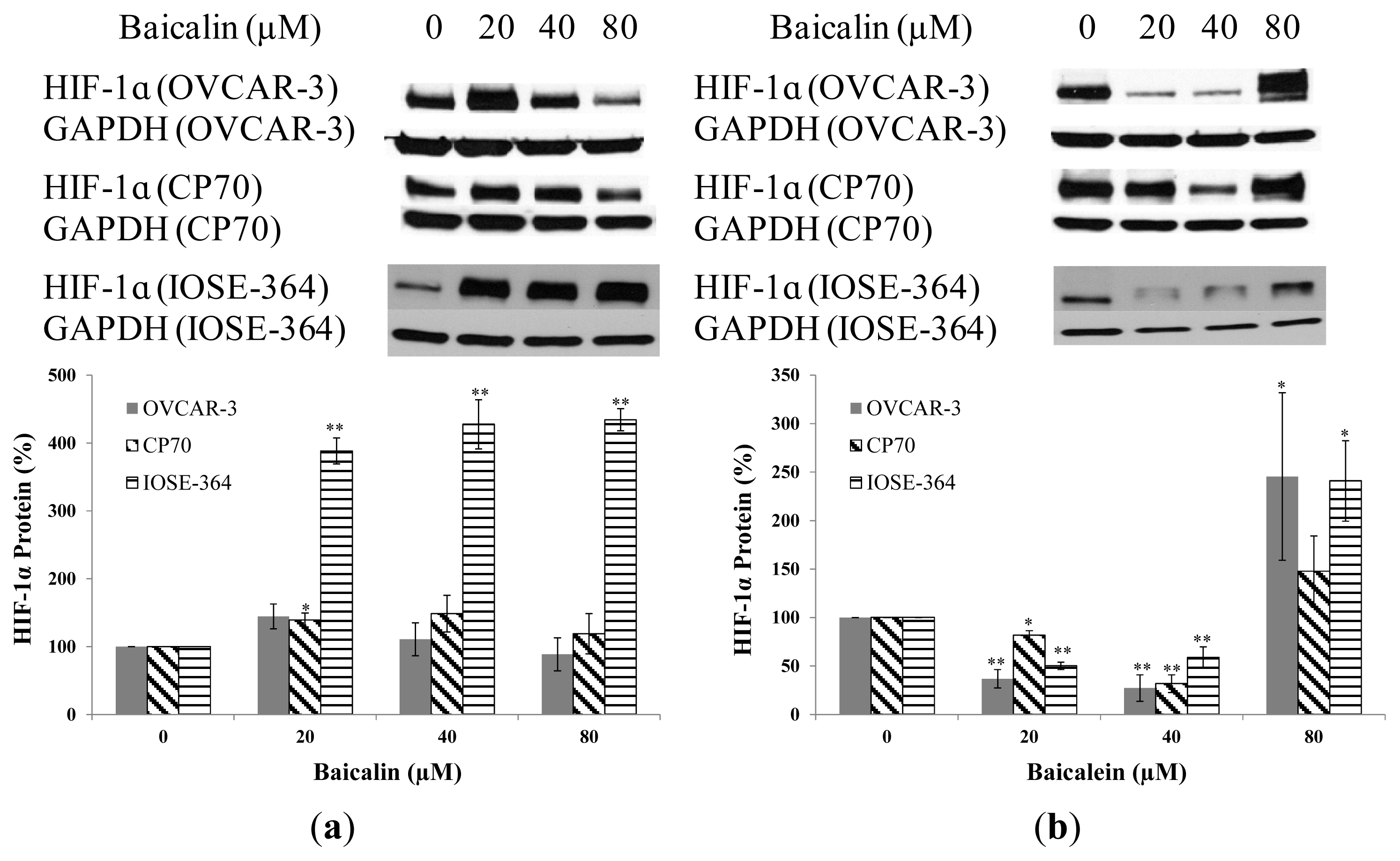
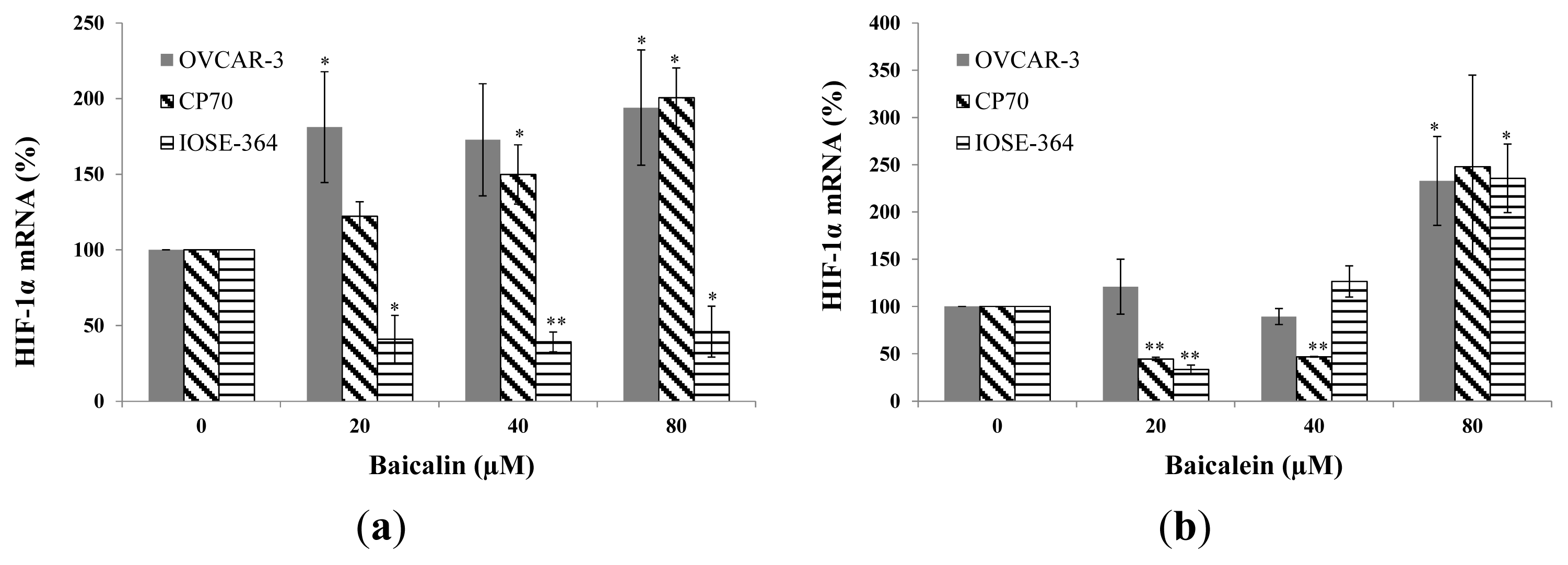
2.3. Effect of Baicalin and Baicalein on HIF-1α Protein and mRNA Expression in OVCAR-3, CP70, and IOSE-364 Cells
2.4. Effect of Baicalin and Baicalein on cMyc Protein Expression in OVCAR-3 and CP70 Cells
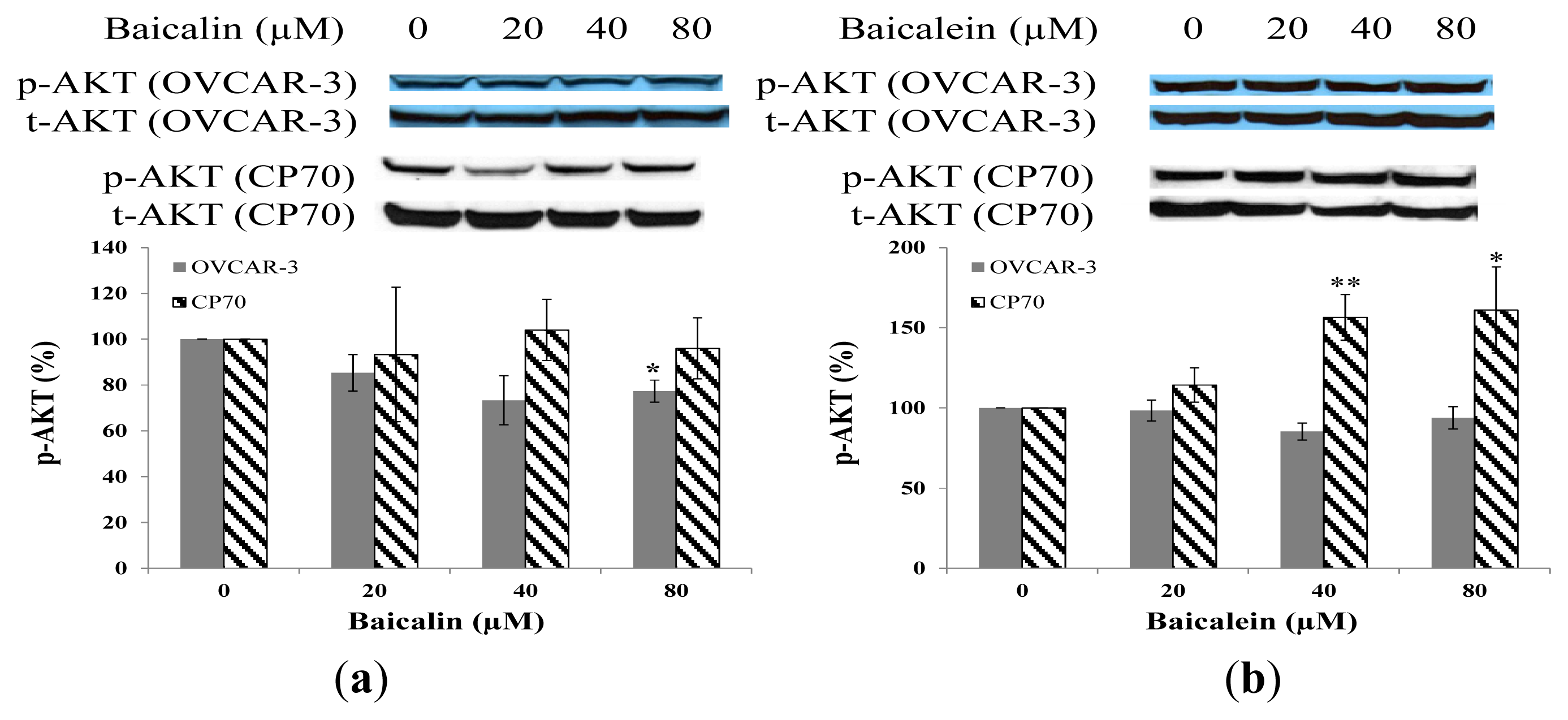
2.5. Effect of Baicalin and Baicalein on AKT Phosphorylation in OVCAR-3 and CP70 Cells
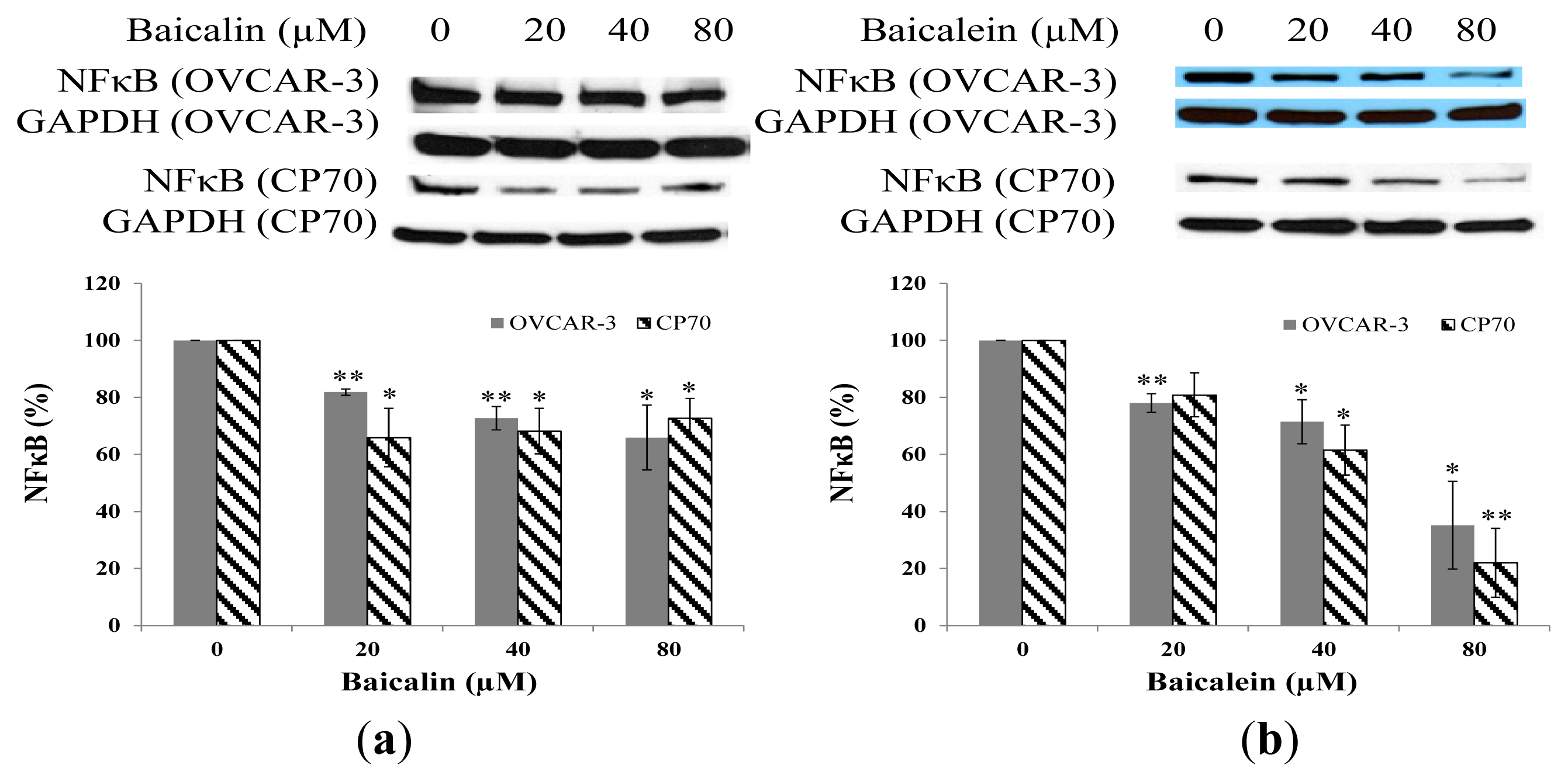
2.6. Effect of Baicalin and Baicalein on NFκB Protein Expression in OVCAR-3 and CP70 Cells
2.7. Effect of Baicalin and Baicalein on PTEN Protein Expression in OVCAR-3 and CP70 Cells
3. Experimental Section
3.1. Cell Culture and Treatment
3.2. Cell Viability
3.3. ELISA for VEGF
3.4. Western Blot
3.5. qRT-PCR
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Jemal, A.; Tiwari, R.C.; Murray, T.; Ghafoor, A.; Samuels, A.; Ward, E.; Feuer, E.J.; Thun, M.J. Cancer statistics, 2004. CA Cancer J. Clin 2004, 54, 8–29. [Google Scholar]
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer statistics. CA Cancer J. Clin 2001, 51, 15–36. [Google Scholar]
- Sarojini, S.; Tamir, A.; Lim, H.; Li, S.; Zhang, S.; Goy, A.; Pecora, A.; Suh, K.S. Early detection biomarkers for ovarian cancer. J. Oncol 2012, 2012, 709049. [Google Scholar]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med 1996, 334, 1–6. [Google Scholar]
- Eckstein, N. Platinum resistance in breast and ovarian cancer cell lines. J. Exp. Clin. Cancer Res 2011, 30, 91. [Google Scholar]
- Bosetti, C.; Rossi, M.; McLaughlin, J.K.; Negri, E.; Talamini, R.; Laqiou, P.; Montella, M.; Ramazzotti, V.; Franceschi, S.; LaVecchia, C. Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol. Biomarkers Prev 2007, 16, 98–101. [Google Scholar]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: where we are, where we are going. Nutr. Cancer 2006, 56, 225–231. [Google Scholar]
- Holick, C.N.; Giovannucci, E.L.; Rosner, B.; Stampfer, M.J.; Michaud, D.S. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am. J. Clin. Nutr 2007, 85, 877–886. [Google Scholar]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar]
- Nishida, N.; Yano, H.; Komai, K.; Nishida, T.; Kamura, T.; Kojiro, M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 2004, 101, 1364–1374. [Google Scholar]
- Bosetti, C.; Bravi, F.; Talamini, R.; Parpinel, M.; Gnagnarella, P.; Negri, E.; Montella, M.; Lagiou, P.; Franceschi, S.; LaVecchia, C. Flavonoids and prostate cancer risk: A study in Italy. Nutr. Cancer 2006, 56, 123–127. [Google Scholar]
- Theodoratou, E.; Kyle, J.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol. Biomarkers Prev 2007, 16, 684–693. [Google Scholar]
- Chao, J.I.; Su, W.C.; Liu, H.F. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and survivin associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol. Cancer Ther 2007, 6, 3039–3048. [Google Scholar]
- Chen, S.S.; Michael, A.; Butler-Manuel, S.A. Advances in the treatment of ovarian cancer: A potential role of antiinflammatory phytochemicals. Discov. Med 2012, 13, 7–17. [Google Scholar]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem 2012, 130, 321–328. [Google Scholar]
- Plochmann, K.; Korte, G.; Koutsilieri, E.; Richling, E.; Riederer, P.; Rethwilm, A.; Schreier, P.; Scheller, C. Structure-Activity relationships of flavonoid-induced cytotoxicity on human leukemia cells. Arch. Biochem. Biophys 2007, 460, 1–9. [Google Scholar]
- Choi, E.J.; Kim, T.; Lee, M.S. Pro-Apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SK-OV-3 cells. Life Sci 2007, 80, 1403–1408. [Google Scholar]
- Franek, K.J.; Zhou, Z.; Zhang, W.D.; Chen, W.Y. In vitro studies of baicalin alone or in combination with Salvia miltiorrhiza extract as a potential anti-cancer agent. Int. J. Oncol 2005, 26, 217–224. [Google Scholar]
- Banks, E. The Epidemiology of Ovarian Cancer. In Ovarian Cancer Methods and Protocols; Bartlett, J.M.S., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 3–6. [Google Scholar]
- Pan, S.Y.; Ugnat, A.M.; Mao, Y.; Wen, S.W.; Johnson, K.C. Canadian Cancer Registries Epidemiology Research Group. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 2004, 13, 1521–1527. [Google Scholar]
- Gates, M.A.; Tworoger, S.S.; Hecht, J.L.; de Vivo, I.; Rosner, B.; Hankinson, S.E. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int. J. Cancer 2007, 121, 2225–2232. [Google Scholar]
- Ikezoe, T.; Chen, S.S.; Heber, D.; Taguchi, H.; Koeffler, H.P. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate 2001, 49, 285–292. [Google Scholar]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev 2009, 35, 57–68. [Google Scholar]
- Li, L.; Zeng, H.; Shan, L.; Yuan, X.; Li, Y.; Liu, R.; Zhang, W. The different inhibitory effects of Huang-Lian-Jie-Du-Tang on cyclooxygenase 2 and 5-lipoxygenase. J. Ethnopharmacol 2012, 143, 732–739. [Google Scholar]
- Cheng, Y.H.; Li, L.A.; Lin, P.; Cheng, L.C.; Hung, C.H.; Chang, N.W.; Lin, C. Baicalein induces G1 arrest in oral cancer cells by enhancing the degradation of cyclin D1 and activating AhR to decrease Rb phosphorylation. Toxicol. Appl. Pharmacol 2012, 263, 360–367. [Google Scholar]
- Ling, Y.; Chen, Y.; Chen, P.; Hui, H.; Song, X.; Lu, Z.; Li, C.; Lu, N.; Guo, Q. Baicalein potently suppresses angiogenesis induced by vascular endothelial growth factor through the p53/Rb signaling pathway leading to G1/S cell cycle arrest. Exp. Biol. Med. (Maywood) 2011, 236, 851–858. [Google Scholar]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin increases VEGF expression and angiogenesis by activating the ERRα/PGC-1α pathway. Cardiovasc. Res 2011, 89, 426–435. [Google Scholar]
- Hwang, K.Y.; Oh, Y.T.; Yoon, H.; Lee, J.; Kim, H.; Choe, W.; Kang, I. Baicalein suppresses hypoxia-induced HIF-1alpha protein accumulation and activation through inhibition of reactive oxygen species and PI 3-kinase/Akt pathway in BV2 murine microglial cells. Neurosci. Lett 2008, 444, 264–269. [Google Scholar]
- Zheng, J.; Hu, J.D.; Huang, Y.; Chen, B.Y. Effects of baicalin on proliferation and apoptosis of adriamycin-resistant human leukemia HL-60/ADR cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009, 17, 1198–1202. [Google Scholar]
- Aggarwal, B.B. Nuclear factor-jB: The enemy within. Cancer Cell 2004, 6, 203–206. [Google Scholar]
- Salmena, L.; Carracedo, A.; Pandolfi, P.P. Tenets of PTEN tumor suppression. Cell 2008, 133, 403–414. [Google Scholar]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem 1998, 273, 13375–13378. [Google Scholar]



© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, J.; Li, Z.; Chen, A.Y.; Ye, X.; Luo, H.; Rankin, G.O.; Chen, Y.C. Inhibitory Effect of Baicalin and Baicalein on Ovarian Cancer Cells. Int. J. Mol. Sci. 2013, 14, 6012-6025. https://doi.org/10.3390/ijms14036012
Chen J, Li Z, Chen AY, Ye X, Luo H, Rankin GO, Chen YC. Inhibitory Effect of Baicalin and Baicalein on Ovarian Cancer Cells. International Journal of Molecular Sciences. 2013; 14(3):6012-6025. https://doi.org/10.3390/ijms14036012
Chicago/Turabian StyleChen, Jianchu, Zhaoliang Li, Allen Y. Chen, Xingqian Ye, Haitao Luo, Gary O. Rankin, and Yi Charlie Chen. 2013. "Inhibitory Effect of Baicalin and Baicalein on Ovarian Cancer Cells" International Journal of Molecular Sciences 14, no. 3: 6012-6025. https://doi.org/10.3390/ijms14036012
APA StyleChen, J., Li, Z., Chen, A. Y., Ye, X., Luo, H., Rankin, G. O., & Chen, Y. C. (2013). Inhibitory Effect of Baicalin and Baicalein on Ovarian Cancer Cells. International Journal of Molecular Sciences, 14(3), 6012-6025. https://doi.org/10.3390/ijms14036012





