Sustained Release of Prindopril Erbumine from Its Chitosan-Coated Magnetic Nanoparticles for Biomedical Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Powder X-ray Diffraction
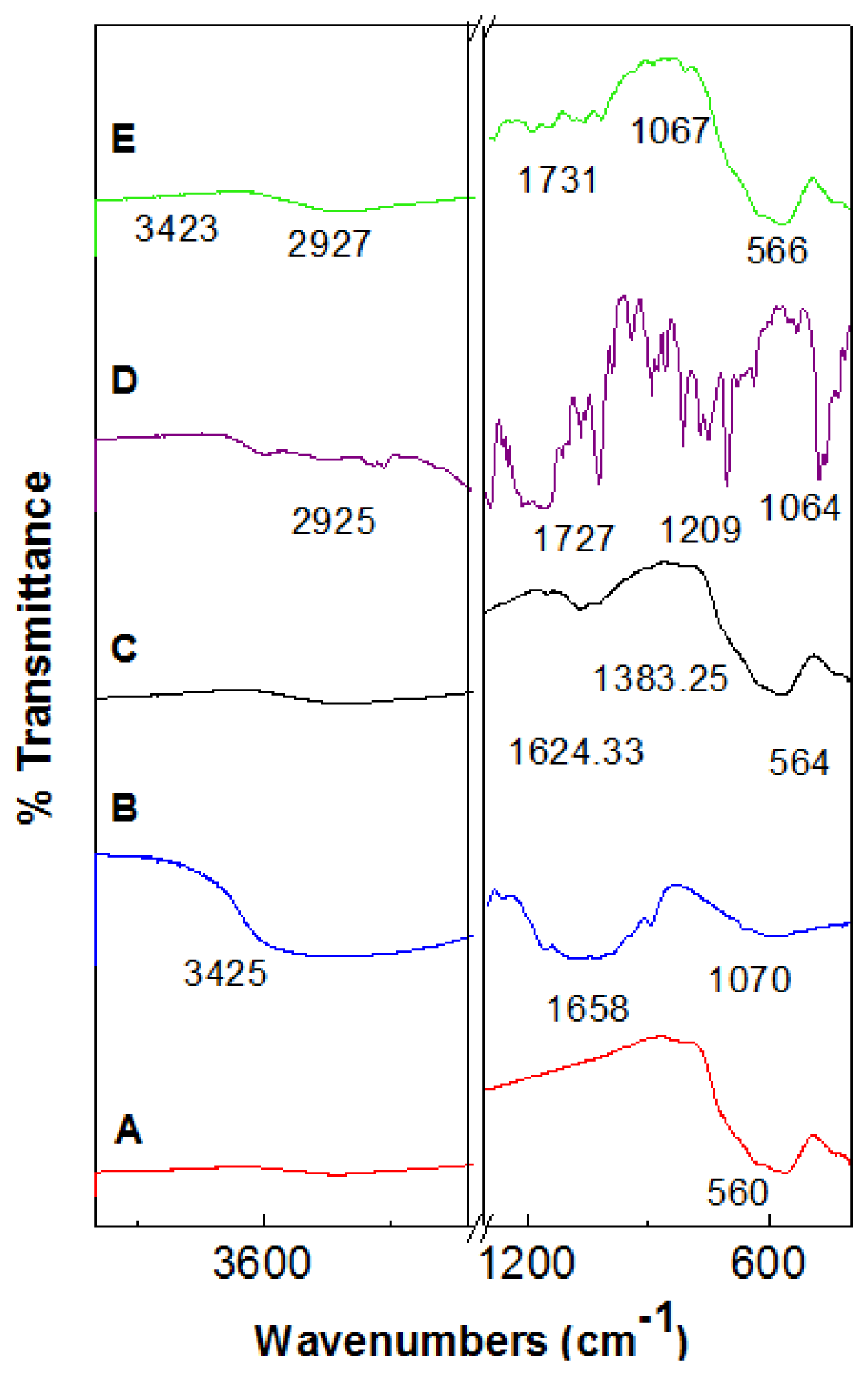
2.2. Fourier Transforms Infrared Spectroscopy
2.3. Magnetic Properties
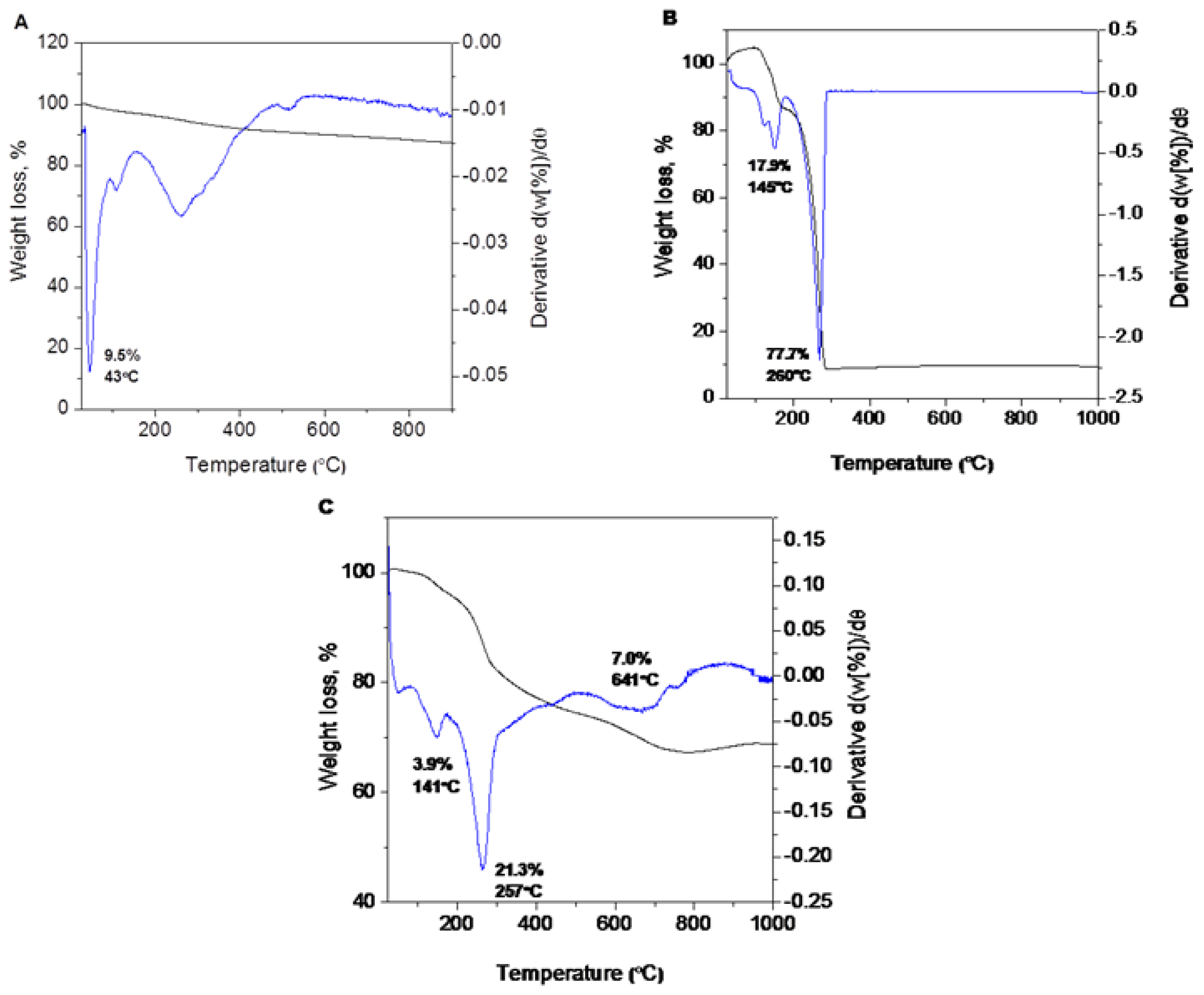
2.4. Thermal Analyses
2.5. Determination of Average Size and Size Distribution Properties
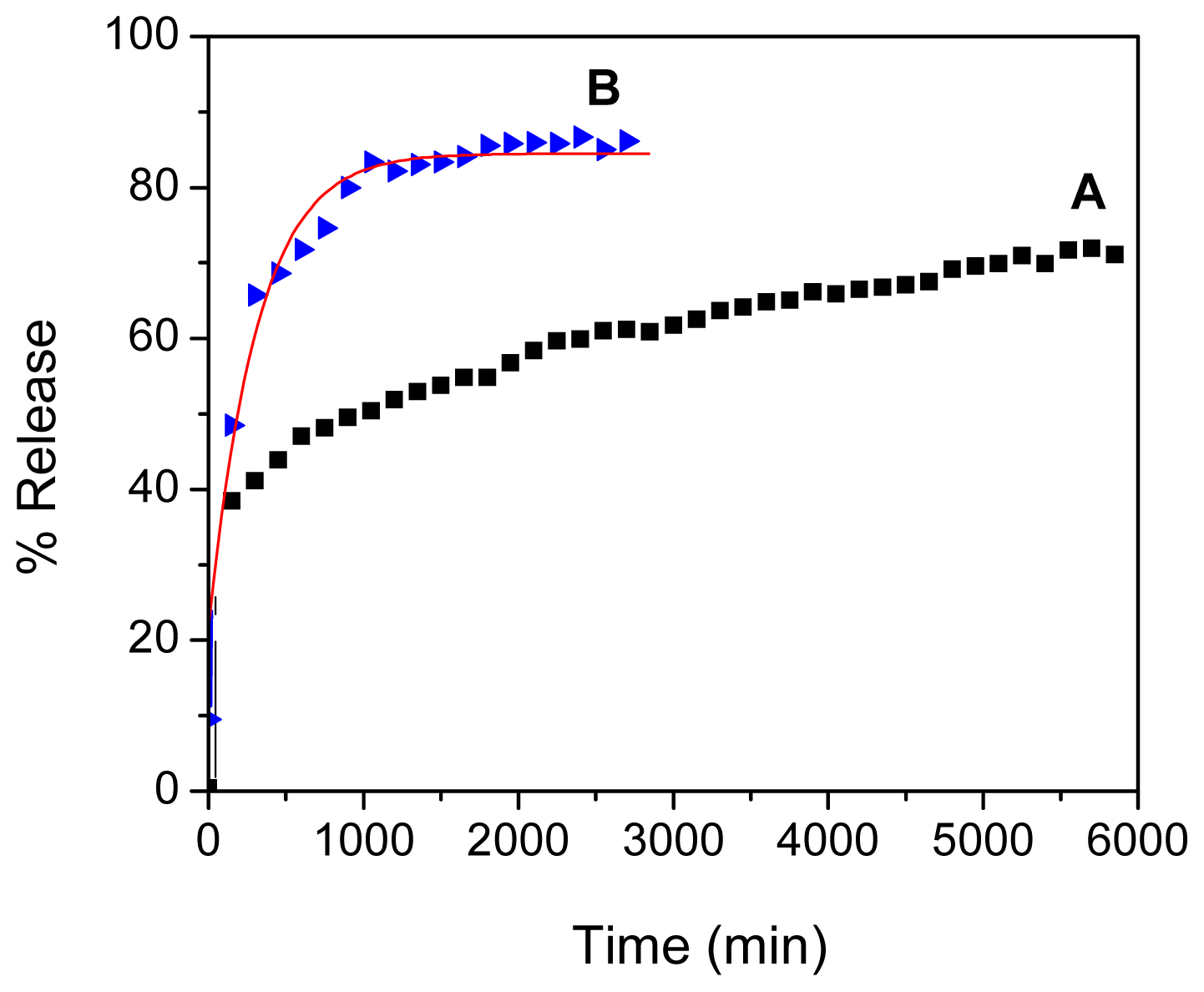
2.6. Loading and Release Behavior of Perindopril Erbumine
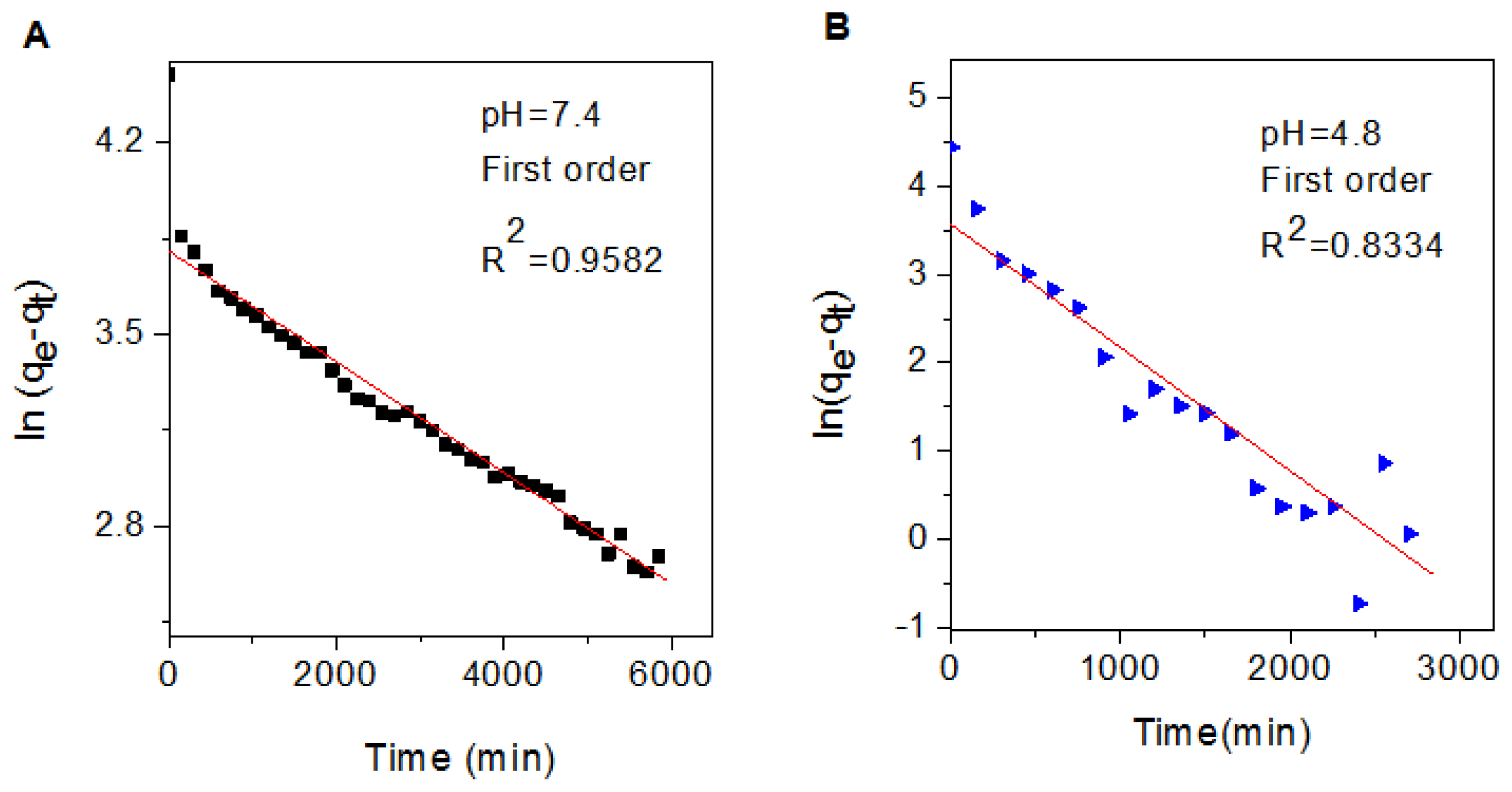
2.7. Release Kinetics of Perindopril Erbumine from the Nanocomposite
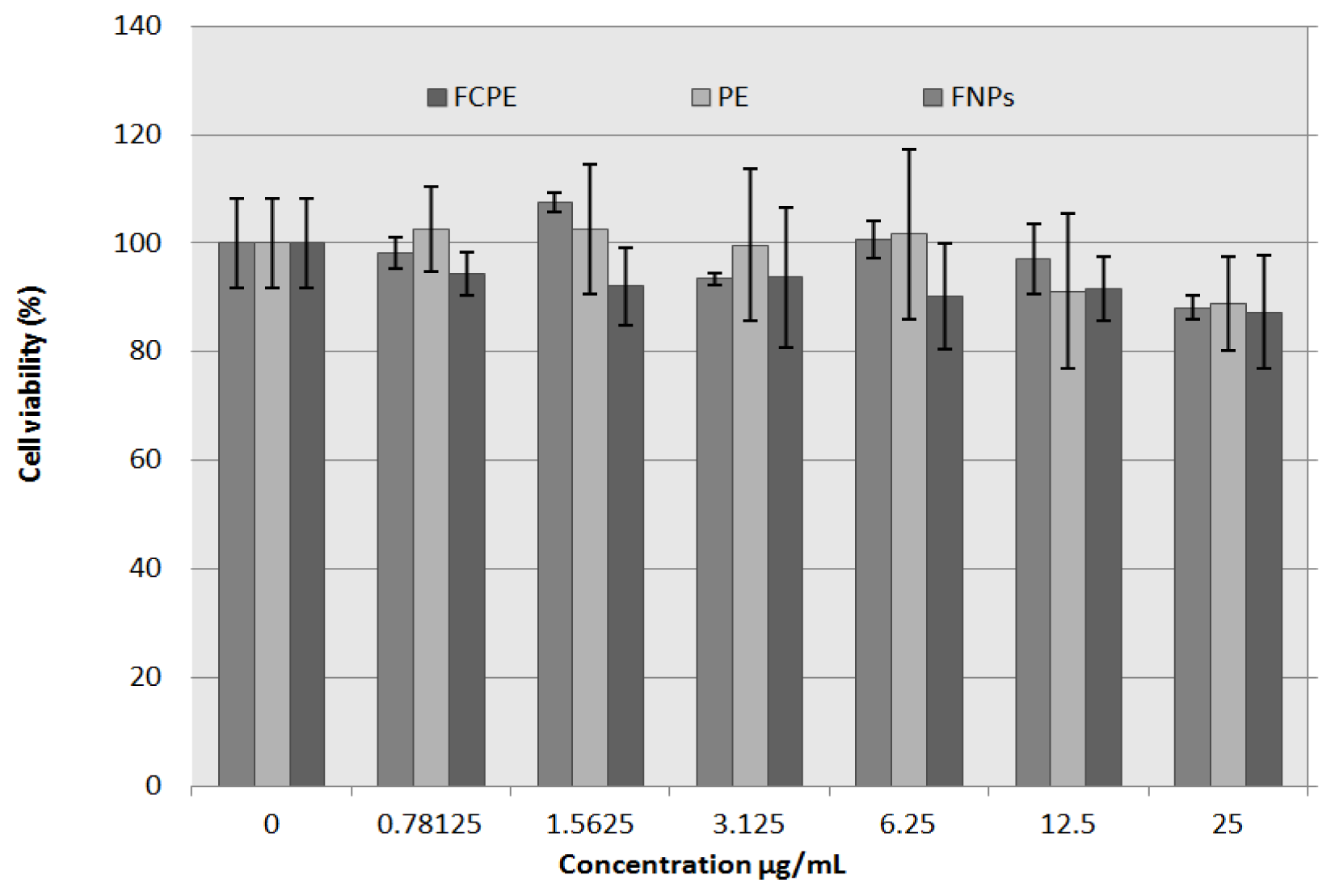
2.8. In Vitro Bioassay
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of Magnetic Nanoparticles and Coating Procedure
3.3. Cell Culture
3.4. Cytotoxicity Study
3.5. Drug Releasing Procedure
3.6. Characterization
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Perrone Donnorso, M.; Miele, E.; de Angelis, F.; la Rocca, R.; Limongi, T.; Cella Zanacchi, F.; Marras, S.; Brescia, R.; di Fabrizio, E. Nanoporous silicon nanoparticles for drug delivery applications. Microelectron. Eng 2012, 98, 626–629. [Google Scholar]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discovery 2010, 9, 615–627. [Google Scholar]
- Li, L.; Mak, K.Y.; Shi, J.; Leung, C.H.; Wong, C.M.; Leung, C.W.; Mak, C.S.K.; Chan, K.Y.; Chan, N.M.M.; Wu, E.X. Sterilization on dextran-coated iron oxide nanoparticles: Effects of autoclaving, filtration, UV irradiation, and ethanol treatment. Microelectron. Eng 2013, 111, 310–313. [Google Scholar]
- Lind, K.; Kresse, M.; Debus, N.P.; Müller, R.H. A novel formulation for superparamagnetic iron oxide (SPIO) particles enhancing MR lymphography: Comparison of physicochemical properties and the in vivo behaviour. J. Drug Target 2002, 10, 221–230. [Google Scholar]
- Qu, J.-B.; Shao, H.-H.; Jing, G.-L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloids Surf. B 2013, 102, 37–44. [Google Scholar]
- Kong, X.; Li, X.; Wang, X.; Liu, T.; Gu, Y.; Guo, G.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. Synthesis and characterization of a novel MPEG-chitosan diblock copolymer and self-assembly of nanoparticles. Carbohydr. Polym 2010, 79, 170–175. [Google Scholar]
- Schweiger, C.; Pietzonka, C.; Heverhagen, J.; Kissel, T. Novel magnetic iron oxide nanoparticles coated with poly(ethylene imine)-g-poly(ethylene glycol) for potential biomedical application: Synthesis, stability, cytotoxicity and MR imaging. Int. J. Pharm 2011, 408, 130–137. [Google Scholar]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ 2001, 9, 63–84. [Google Scholar]
- Neu, M.; Fischer, D.; Kissel, T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med 2005, 7, 992–1009. [Google Scholar]
- Pardoe, H.; Chua-Anusorn, W.; St Pierre, T.G.; Dobson, J. Structural and magnetic properties of nanoscale iron oxide particles synthesized in the presence of dextran or polyvinyl alcohol. J. Magn. Magn. Mater 2001, 225, 41–46. [Google Scholar]
- Lehr, C.-M.; Bouwstra, J.A.; Schacht, E.H.; Junginger, H.E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int. J. Pharm 1992, 78, 43–48. [Google Scholar]
- Jain, T.K.; Morales, M.A.; Sahoo, S.K.; Leslie-Pelecky, D.L.; Labhasetwar, V. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol. Pharm 2005, 2, 194–205. [Google Scholar]
- Tsai, Z.-T.; Wang, J.-F.; Kuo, H.-Y.; Shen, C.-R.; Wang, J.-J.; Yen, T.-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: A potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater 2010, 322, 208–213. [Google Scholar]
- Qu, J.; Liu, G.; Wang, Y.; Hong, R. Preparation of Fe3O4-chitosan nanoparticles used for hyperthermia. Adv. Powder Technol 2010, 21, 461–467. [Google Scholar]
- Ravi Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym 2000, 46, 1–27. [Google Scholar]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar]
- Burt, V.L.; Cutler, J.A.; Higgins, M.; Horan, M.J.; Labarthe, D.; Whelton, P.; Brown, C.; Roccella, E.J. Trends in the prevalence, awareness, treatment, and control of hypertension in the adult US population data from the health examination surveys, 1960 to 1991. Hypertension 1995, 26, 60–69. [Google Scholar]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar]
- Clark, L.T. Safety profile of perindopril. Am. J. Cardiol 2001, 88, 36–40. [Google Scholar]
- Wong, J.; Patel, R.A.; Kowey, P.R. The clinical use of angiotensin-converting enzyme inhibitors. Prog. Cardiovasc. Dis 2004, 47, 116–130. [Google Scholar]
- Bertrand, M.E. Provision of cardiovascular protection by ACE inhibitors: A review of recent trials. Curr. Med. Res. Opin 2004, 20, 1559–1569. [Google Scholar]
- Laurent, S.P. Evidence for benefits of perindopril in hypertension and its complications. Am. J. hypertens 2005, 18, 155S–162S. [Google Scholar]
- Remková, A.; Kratochvil’ova, H. Impact of the therapy by renin-angiotensin system targeting antihypertensive agents perindopril versus telmisartan on prothrombotic state in essential hypertension. J. Hum. Hypertens 2008, 22, 338–345. [Google Scholar]
- Pascard, C.; Guilhem, J.; Vincent, M.; Remond, G.; Portevin, B.; Laubie, M. Configuration and preferential solid-state conformations of perindoprilat (S-9780). Comparison with the crystal structures of other ACE inhibitors and conclusions related to structure-activity relationships. J. Med. Chem 1991, 34, 663–669. [Google Scholar]
- Al Ali, S.H.H.; Al-Qubaisi, M.; Hussein, M.Z.; Ismail, M.; Zainal, Z.; Hakim, M.N. Controlled release and angiotensin-converting enzyme inhibition properties of an antihypertensive drug based on a perindopril erbumine-layered double hydroxide nanocomposite. Int. J. Nanomed 2012, 7, 2129–2141. [Google Scholar]
- Zhang, B.; Wang, D.-F.; Li, H.-Y.; Xu, Y.; Zhang, L. Preparation and properties of chitosan-soybean trypsin inhibitor blend film with anti-Aspergillus flavus activity. Ind. Crop. Prod 2009, 29, 541–548. [Google Scholar]
- Fan, M.; Hu, Q.; Shen, K. Preparation and structure of chitosan soluble in wide pH range. Carbohydr. Polym 2009, 78, 66–71. [Google Scholar]
- Zhao, Y.; Qiu, Z.; Huang, J. Preparation and analysis of Fe3O4 magnetic nanoparticles used as targeted-drug carriers. Chin. J. Chem. Eng 2008, 16, 451–455. [Google Scholar]
- Kuo, C.-H.; Liu, Y.-C.; Chang, C.-M.J.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym 2012, 87, 2538–2545. [Google Scholar]
- Kipkemboi, P.K.; Kiprono, P.C.; Sanga, J.J. Vibrational spectra of t-butyl alcohol, t-butylamine and t-butyl alcohol + t-butylamine binary liquid mixtures. Bull. Chem. Soc. Ethiop 2003, 17, 211–218. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Tronto, J.; dos Reis, M.J.; Silvério, F.; Balbo, V.R.; Marchetti, J.M.; Valim, J.O.B. In vitro release of citrate anions intercalated in magnesium aluminium layered double hydroxides. J. Phys. Chem. Solids 2004, 65, 475–480. [Google Scholar]
- Yasin, Y.; Ismail, N.M.; Hussein, M.Z.; Aminudin, N. Synthesis and characterization of lawsone-intercalated ZnAl layered double hydroxides. J. Biomed. Nanotechnol 2011, 7, 486–488. [Google Scholar]
- Ge, Y.; Zhang, Y.; Xia, J.; Ma, M.; He, S.; Nie, F.; Gu, N. Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake. in vitro. Colloids Surf. B 2009, 73, 294–301. [Google Scholar]
- Li, G.-Y.; Jiang, Y.-R.; Huang, K.-L.; Ding, P.; Chen, J. Preparation and properties of magnetic Fe3O4-chitosan nanoparticles. J. Alloy. Compd 2008, 466, 451–456. [Google Scholar]
- Macêdo, R.O.; do Nascimento, T.G.; Aragăo, C.F.S.; Gomes, A.P.B. Application of thermal analysis in the characterization of anti-hypertensive drugs. J. Therm. Anal. Calorim 2000, 59, 657–661. [Google Scholar]
- Zhang, H.; Zou, K.; Sun, H.; Duan, X. A magnetic organic-inorganic composite: Synthesis and characterization of magnetic 5-aminosalicylic acid intercalated layered double hydroxides. J. Solid State Chem 2005, 178, 3485–3493. [Google Scholar]
- Ambrogi, V.; Fardella, G.; Grandolini, G.; Perioli, L.; Tiralti, M.C. Intercalation compounds of hydrotalcite-like anionic clays with anti-inflammatory agents, II: Uptake of diclofenac for a controlled release formulation. AAPS PharmSciTech 2002, 3, 77–82. [Google Scholar]
- Saifullah, B.; Hussein, M.Z.; Hussein-Al-Ali, S.H.; Fakurazi, S. Sustained release formulation of an anti-tuberculosis drug based on para-amino salicylic acid-zinc layered hydroxide nanocomposite. Chem. Cent. J 2013, 7, 72. [Google Scholar] [Green Version]
- Kura, A.U.; Al Ali, S.H.H.; Hussein, M.Z.; Fakurazi, S.; Arulselvan, P. Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int. J. Nanomed 2013, 8, 1103–1110. [Google Scholar]
- Ho, Y.-S.; Ofomaja, A.E. Pseudo-second-order model for lead ion sorption from aqueous solutions onto palm kernel fiber. J. Hazard. Mater 2006, 129, 137–142. [Google Scholar]
- Dong, L.; Yan, L.; Hou, W.-G.; Liu, S.-J. Synthesis and release behavior of composites of camptothecin and layered double hydroxide. J. Solid State Chem 2010, 183, 1811–1816. [Google Scholar]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsiao, M.; Chen, C.H.; Hwu, Y.K. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 2010, 21, 075102. [Google Scholar]
- Lee, H.; Shao, H.; Huang, Y.; Kwak, B. Synthesis of MRI contrast agent by coating superparamagnetic iron oxide with chitosan. Magn. IEEE Trans 2005, 41, 4102–4104. [Google Scholar]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe3O4 magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed 2012, 7, 5745–5756. [Google Scholar]



| Sample | Ms (emu/g) | Mr (emu/g) | Hci (G) |
|---|---|---|---|
| Fe3O4 | 44.655 | 1.5714 | 21.955 |
| FC | 38.573 | 0.6971 | 24.065 |
| FCPE | 27.664 | 1.1869 | 27.002 |
| Aqueous Solution | Saturated Release (%) | R2 | Rate constant (k) a (mg/min) | t1/2a (min) | ||
|---|---|---|---|---|---|---|
| First-order | Pseudo-second order | Parabolic diffusion | ||||
| pH 7.4 | 85.8 | 0.9582 | 0.4776 | 0.9054 | 2.02 × 10−4 | 3431 |
| pH 4.8 | 72.2 | 0.8334 | 0.6760 | 0.7367 | 1.4 × 10−3 | 495 |
© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Sustained Release of Prindopril Erbumine from Its Chitosan-Coated Magnetic Nanoparticles for Biomedical Applications. Int. J. Mol. Sci. 2013, 14, 23639-23653. https://doi.org/10.3390/ijms141223639
Dorniani D, Hussein MZB, Kura AU, Fakurazi S, Shaari AH, Ahmad Z. Sustained Release of Prindopril Erbumine from Its Chitosan-Coated Magnetic Nanoparticles for Biomedical Applications. International Journal of Molecular Sciences. 2013; 14(12):23639-23653. https://doi.org/10.3390/ijms141223639
Chicago/Turabian StyleDorniani, Dena, Mohd Zobir Bin Hussein, Aminu Umar Kura, Sharida Fakurazi, Abdul Halim Shaari, and Zalinah Ahmad. 2013. "Sustained Release of Prindopril Erbumine from Its Chitosan-Coated Magnetic Nanoparticles for Biomedical Applications" International Journal of Molecular Sciences 14, no. 12: 23639-23653. https://doi.org/10.3390/ijms141223639





