Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications
Abstract
:1. Introduction
2. Preparation of Magnetic Carbon Nanotubes
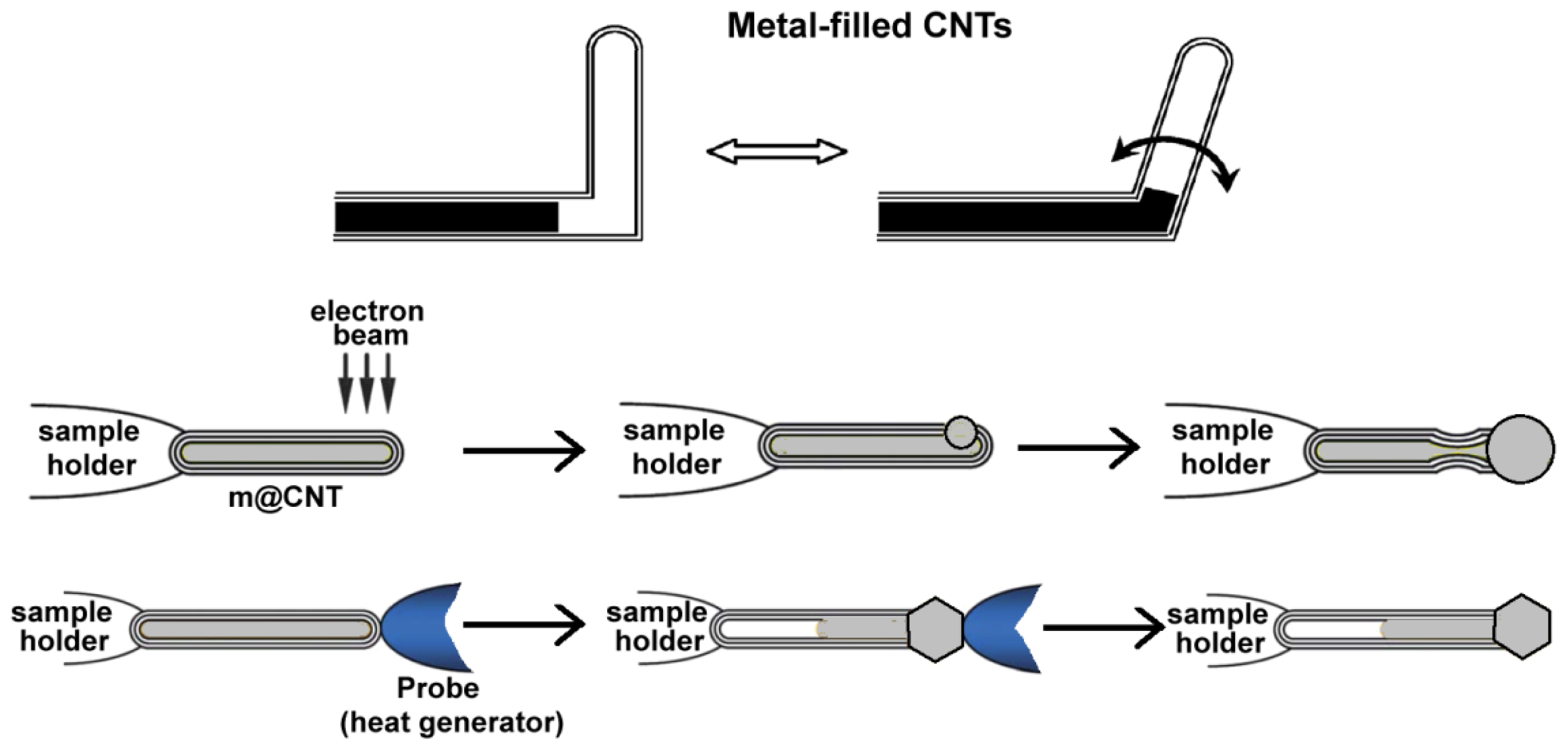
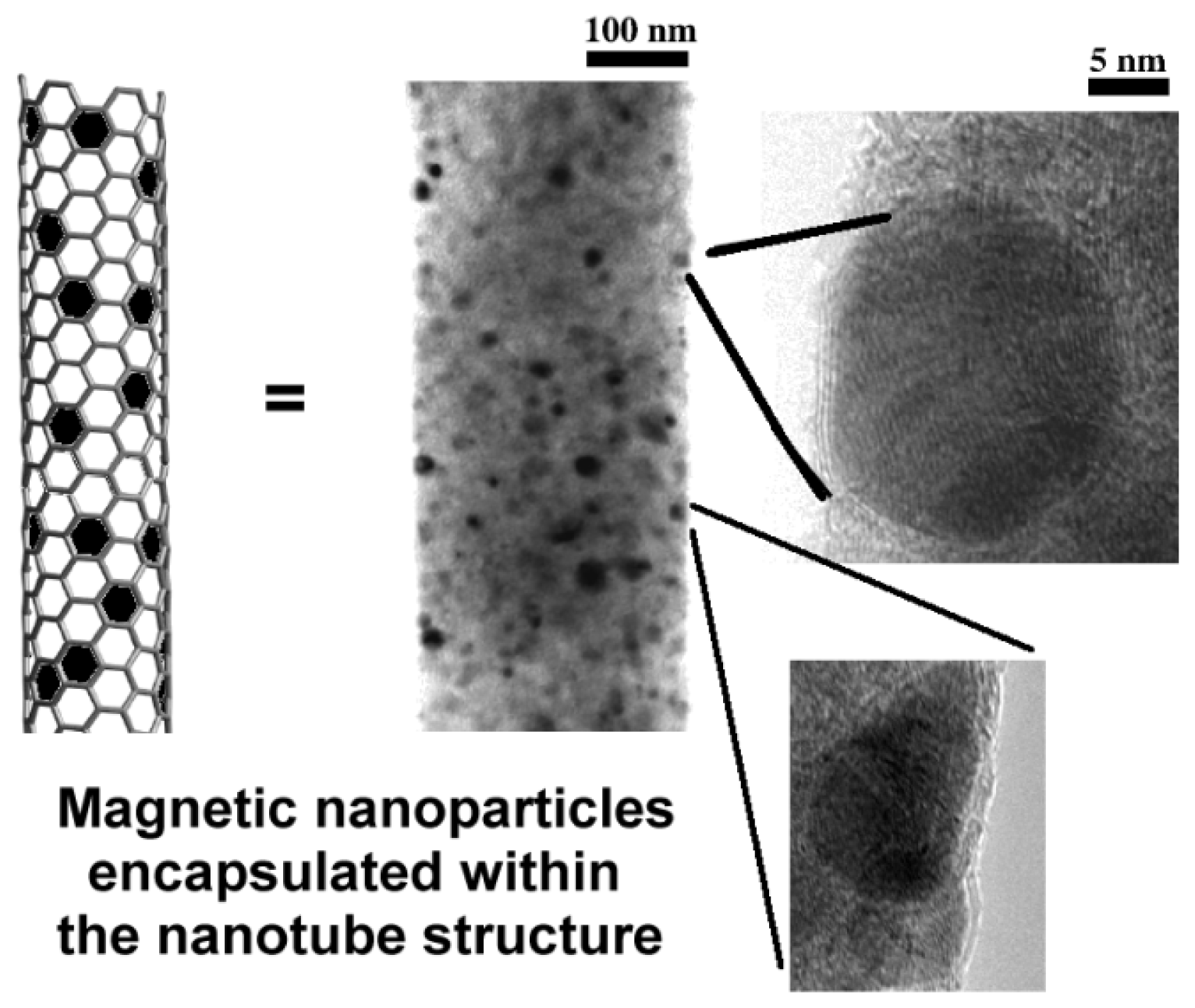
2.1. Carbon Nanotubes Filled with Metals
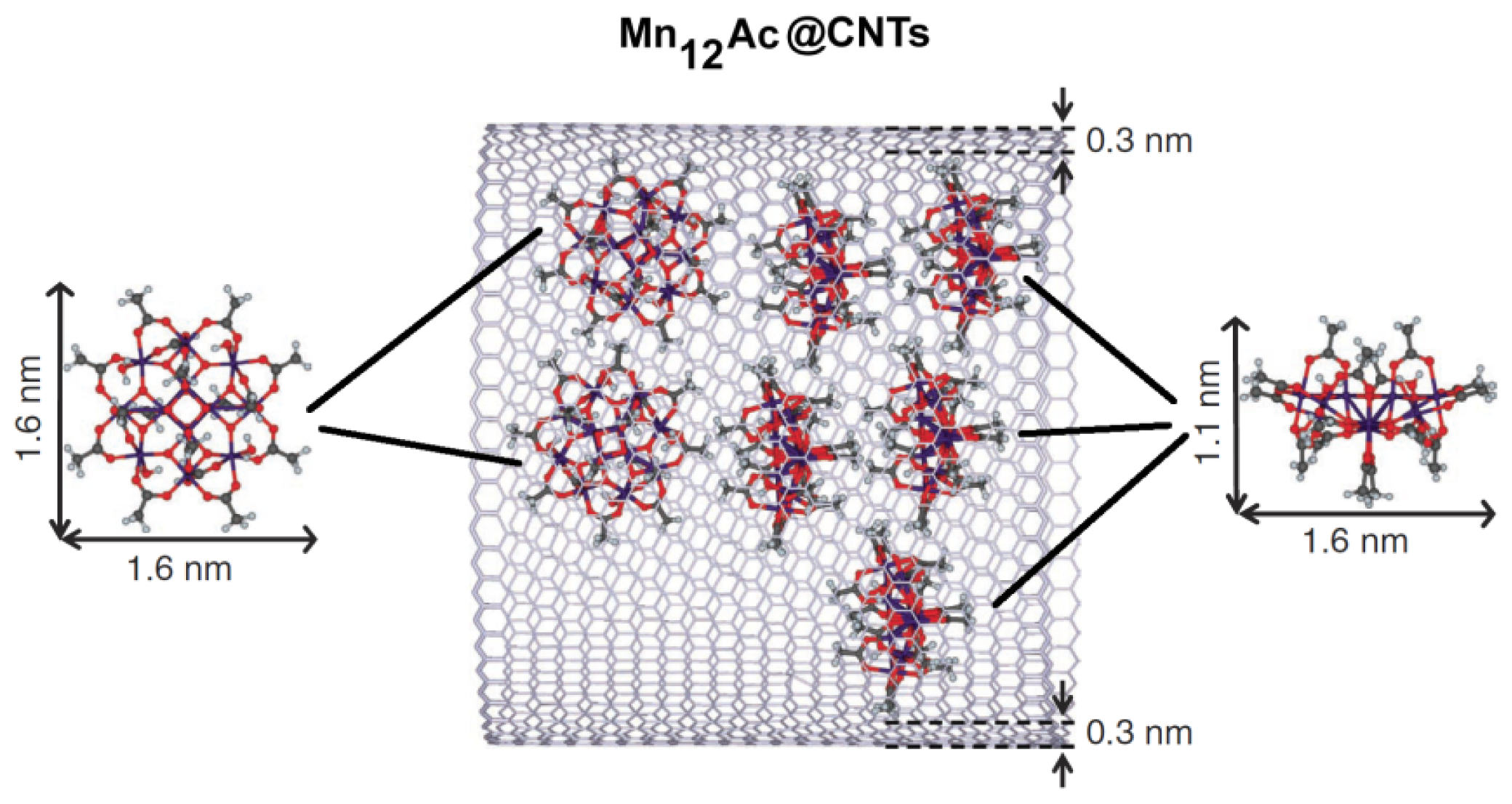
2.2. Endohedral Functionalization of CNTs
2.3. Exohedral Functionalization of CNTs
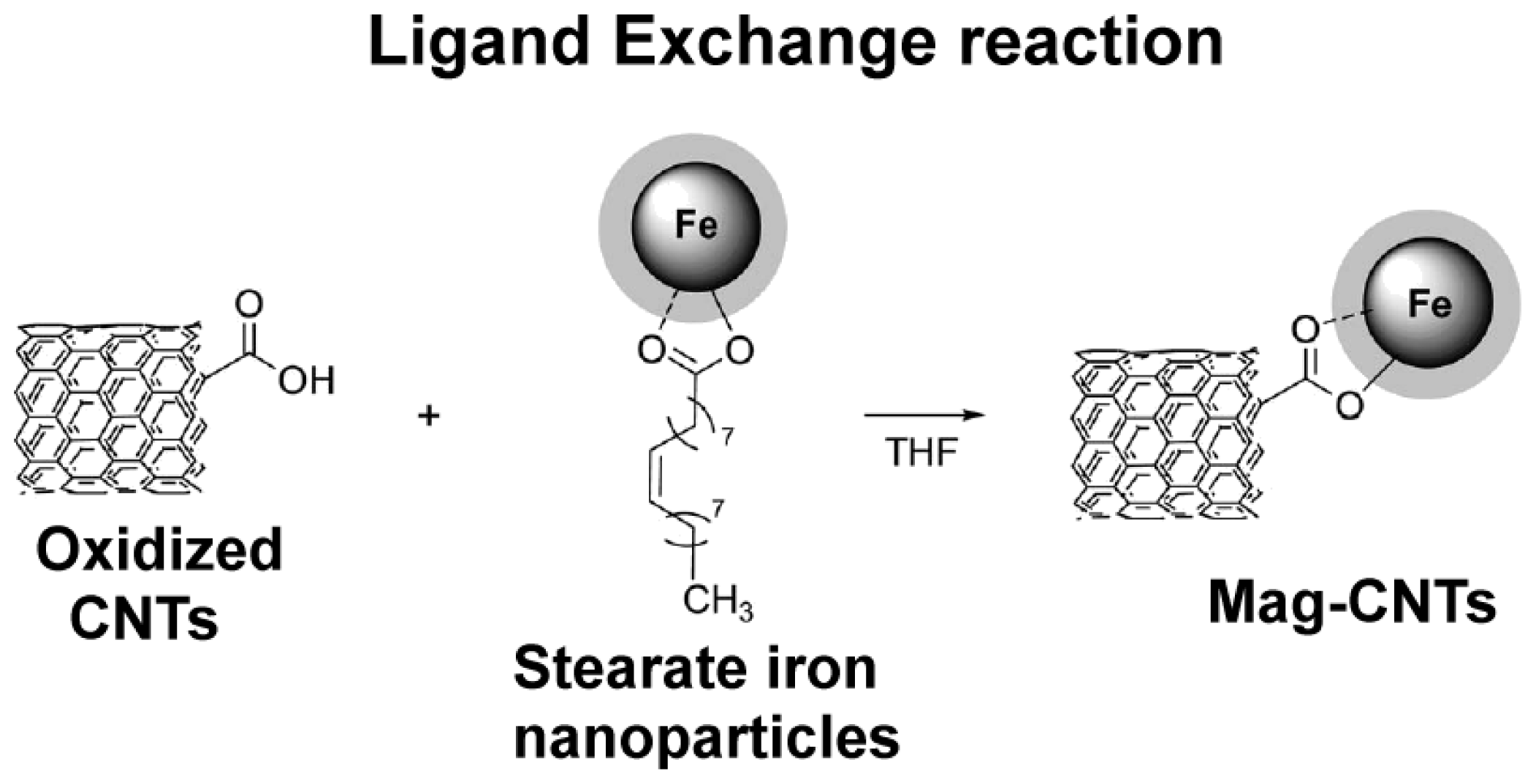
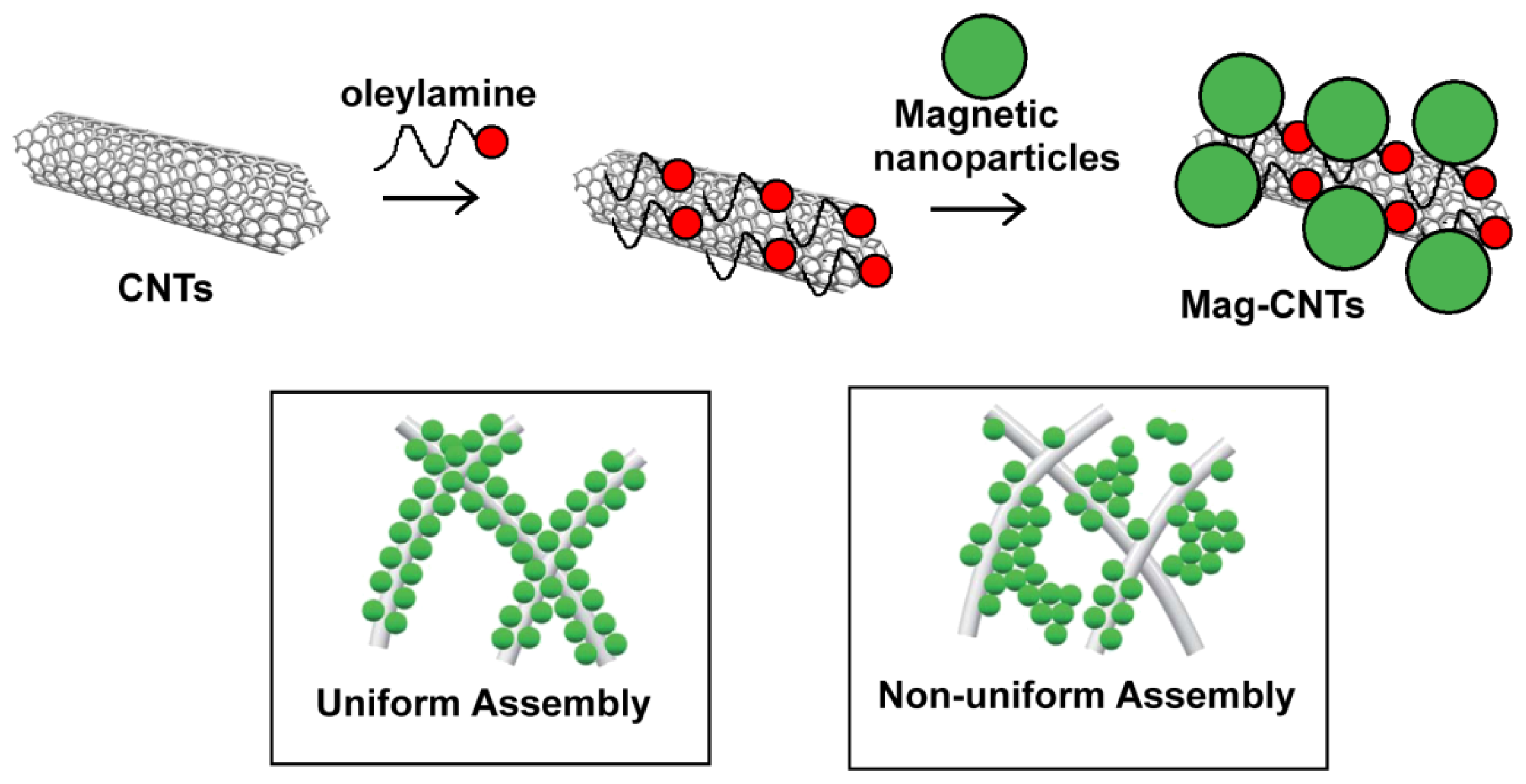
2.3.1. Decoration of the Carbon nanotubes’ Surface with Magnetic Nanoclusters
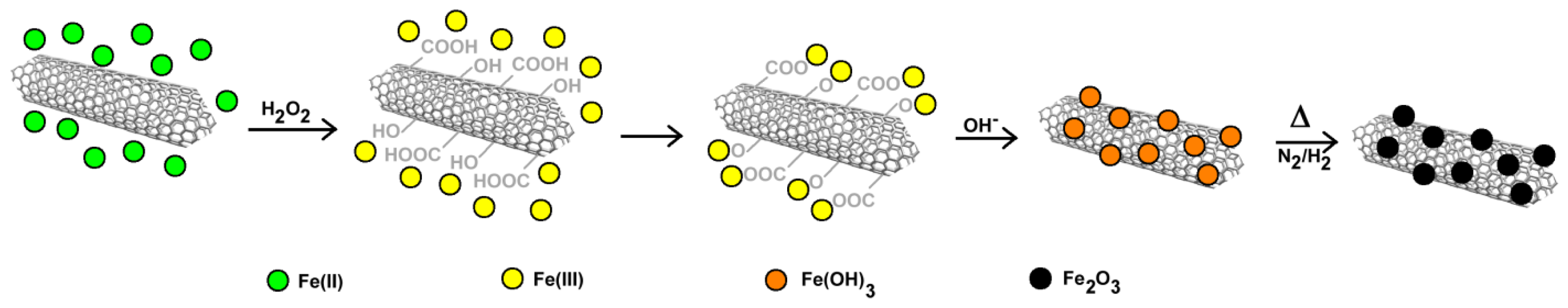
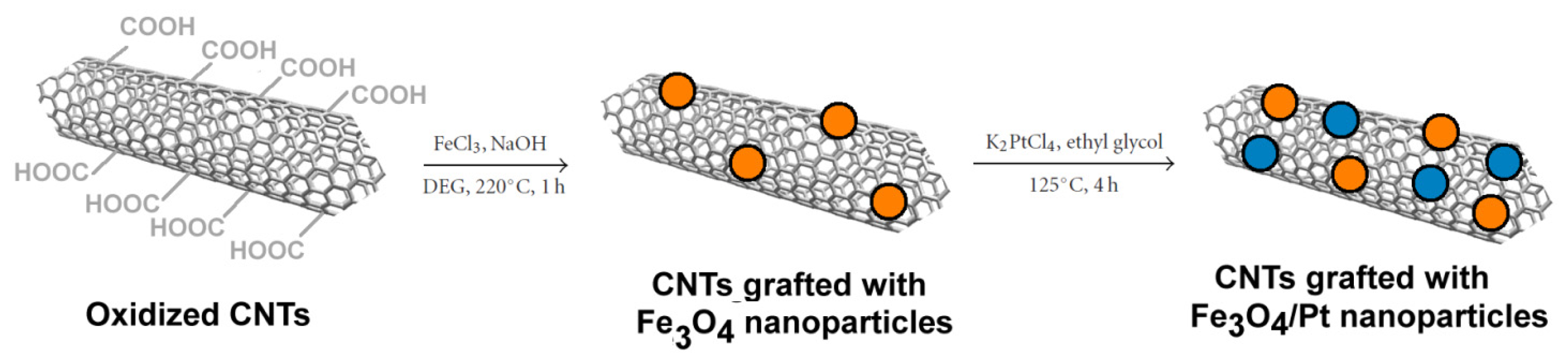
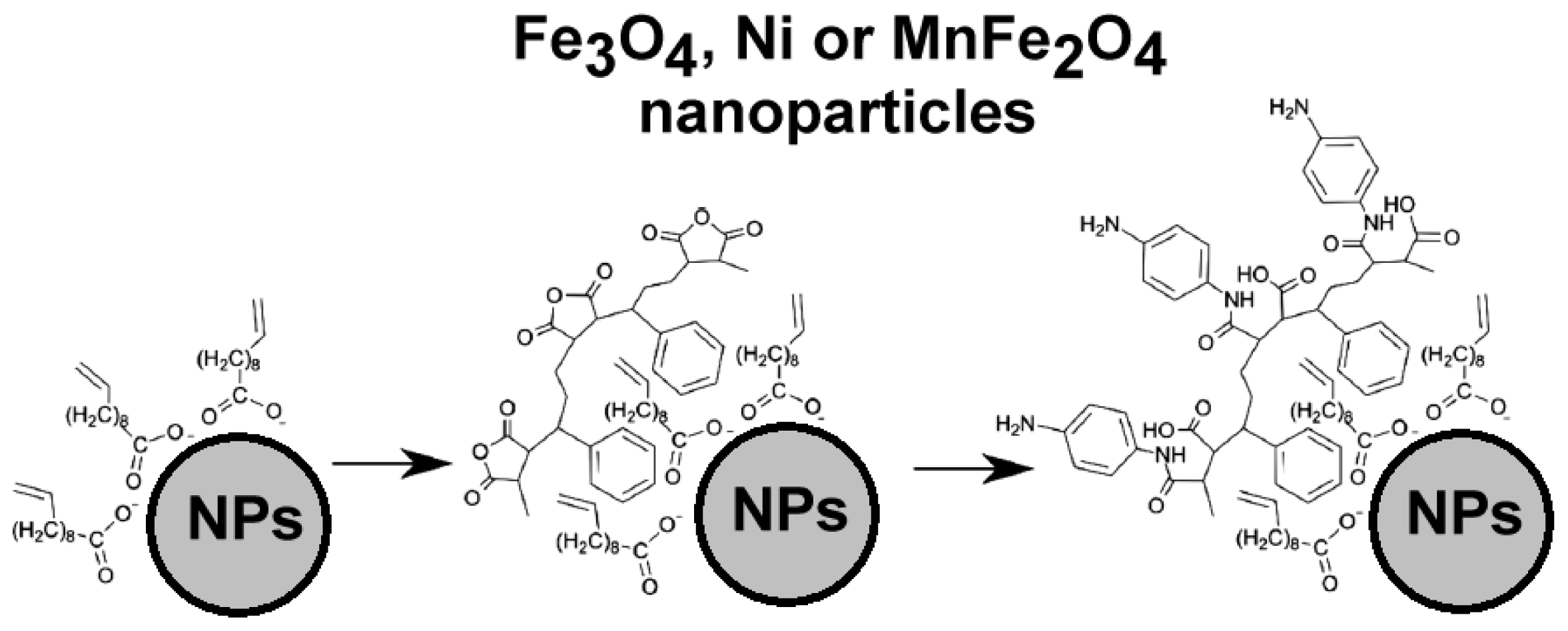
2.3.2. Carbon Nanotubes Decorated with Magnetic Nanoparticles
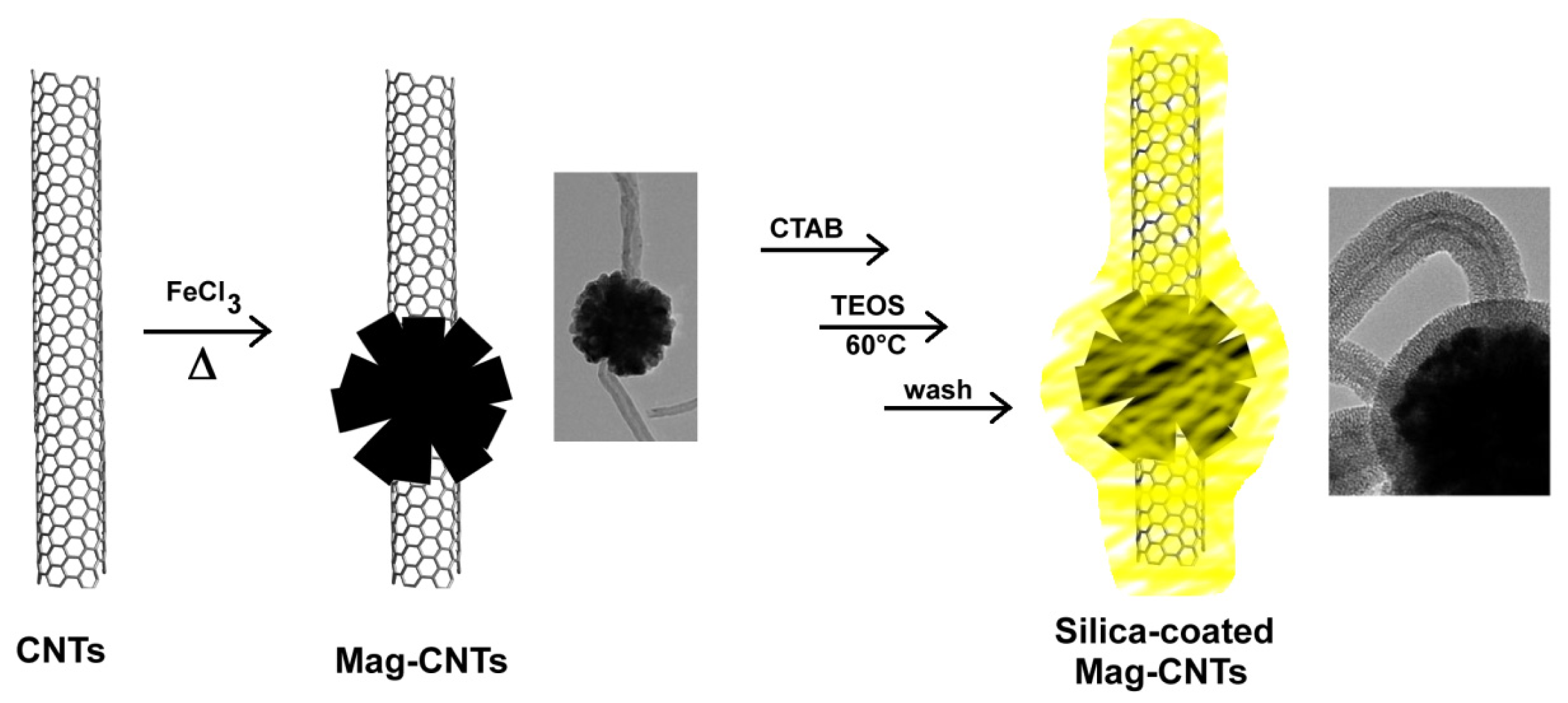
2.3.3. Coating of Magnetic Carbon Nanotubes with Mesoporous Silica
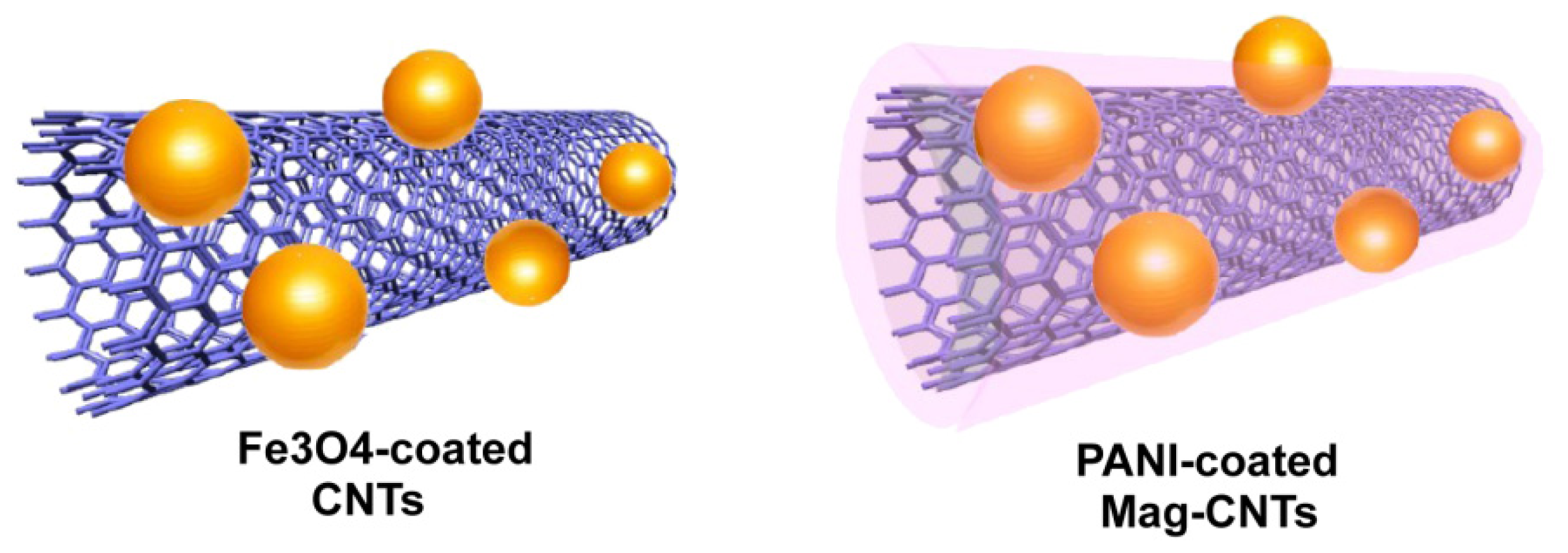
2.3.4. Coating of Carbon Nanotubes Prior to Incubation with Magnetic Nanoparticles
2.3.5. Coating of Magnetic Particles with Polymers prior to Incubation with CNTs
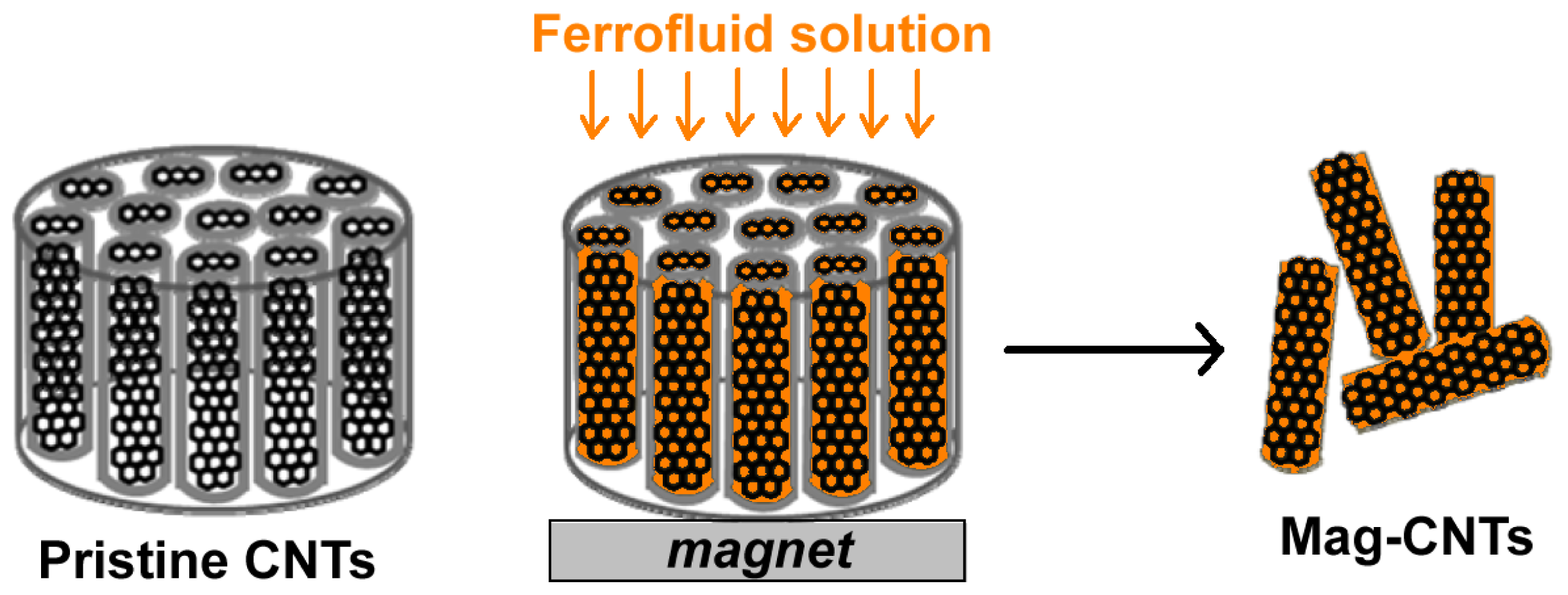
2.4. Magnetic Nanoparticles Embedded in the Carbon Nanotube’s Structure
3. General Discussion about the Preparation of Mag-CNTs
4. Applications
4.1. Applications in Biomedicine
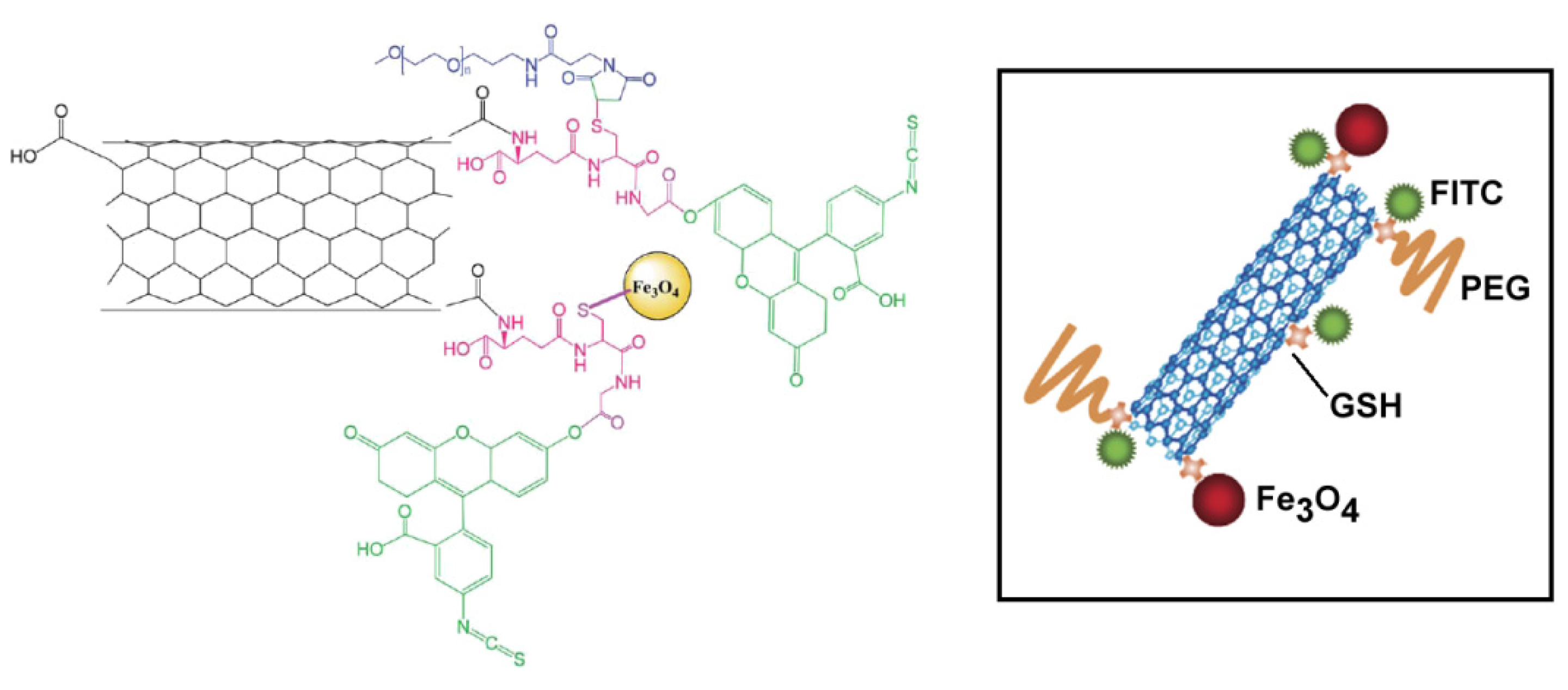
4.2. Cellular Imaging
4.3. Cell Tracking
4.4. Lymphatic Targeting
4.5. Cancer Lymph Node Metastasis Treatment
4.6. Human Monocytic Cells: Implications for Cell-Based Cancer Gene Therapy
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol 2008, 42, 5843–5859. [Google Scholar]
- Wu, H.; Chang, X.; Liu, L.; Zhao, F.; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem 2010, 20, 1036–1052. [Google Scholar]
- Valcàrcel, M.; Càrdenas, S.; Simonet, B.M.; Moliner-Martìnez, Y.; Lucena, R. Carbon nanostructures as sorbent materials in analytical processes. TrAC Trends Anal. Chem 2008, 27, 34–43. [Google Scholar]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol 2009, 4, 627–633. [Google Scholar]
- Bhirde, A.A.; Patel, V.; Gavard, J.; Zhang, G.; Sousa, A.A.; Masedunskas, A.; Leapman, R.D.; Weigert, R.; Gutkind, J.S.; Rusling, J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 2009, 3, 307–316. [Google Scholar]
- Burke, A.; Ding, X.; Singh, R.; Kraft, R.A.; Levi-Polyachenko, N.; Rylander, M.N.; Szot, C.; Buchanan, C.; Whitney, J.; Fisher, J.; et al. Long-term survival following a single treatment of kidney tumors with multiwalled carbon nanotubes and near-infrared radiation. Proc. Natl. Acad. Sci. USA 2009, 106, 12897–12902. [Google Scholar]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug delivery with carbon nanotubes for in vivo cancer treatment. Cancer Res 2008, 68, 6652–6660. [Google Scholar]
- Guo, S.; Dong, S.; Wang, E. Constructing carbon nanotube/Pt nanoparticle hybrids using an imidazolium-salt-based ionic liquid as a linker. Adv. Mater 2010, 22, 1269–1272. [Google Scholar]
- Singh, P.; Kumar, J.; Toma, F.M.; Raya, J.; Prato, M.; Fabre, B.; Verma, S.; Bianco, A. Synthesis and characterization of nucleobase-carbon nanotube hybrids. J. Am. Chem. Soc 2009, 131, 13555–13562. [Google Scholar]
- Kauffman, D.R.; Star, A. Carbon nanotube gas and vapor sensors. Angew. Chem. Int. Ed. Engl 2008, 47, 6550–6570. [Google Scholar]
- Wildgoose, G.G.; Banks, C.E.; Compton, R.G. Metal nanoparticles and related materials supported on carbon nanotubes: Methods and applications. Small 2006, 2, 182–193. [Google Scholar]
- Choi, J.H.; Nguyen, F.T.; Barone, P.W.; Heller, D.A.; Moll, A.E.; Patel, D.; Boppart, S.A.; Strano, M.S. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett 2007, 7, 861–867. [Google Scholar]
- Gao, C.; Li, W.; Morimoto, H.; Nagaoka, Y.; Maekawa, T. Magnetic carbon nanotubes: Synthesis by electrostatic self-assembly approach and application in biomanipulations. J. Phys. Chem. B 2006, 110, 7213–7220. [Google Scholar]
- Yang, D.; Yang, F.; Hu, J.; Long, J.; Wang, C.; Fu, D.; Ni, Q. Hydrophilic multi-walled carbon nanotubes decorated with magnetite nanoparticles as lymphatic targeted drug delivery vehicles. Chem. Commun 2009, 29, 4447–4449. [Google Scholar]
- Chen, C.L.; Wang, X.K.; Nagatsu, M. Europium adsorption on multiwall carbon nanotube/iron oxide magnetic composite in the presence of polyacrylic acid. Environ. Sci. Technol 2009, 43, 2362–2367. [Google Scholar]
- Zhao, X.; Johnston, C.; Grant, P.S. A novel hybrid supercapacitor with a carbon nanotube cathode and an iron oxide/carbon nanotube composite anode. J. Mater. Chem 2009, 19, 8755–8760. [Google Scholar]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater 2009, 21, 2133–2148. [Google Scholar]
- Metin, O.; Mazumder, V.; Ozkar, S.; Sun, S. Monodisperse nickel nanoparticles and their catalysis in hydrolytic dehydrogenation of ammonia borane. J. Am. Chem. Soc 2010, 132, 1468–1469. [Google Scholar]
- Gonzalez-Fernandez, M.A.; Torres, T.E.; Andres-Verges, M.; Costo, R.; Presa, P.D.L.; Serna, C.J.; Morales, M.P.; Marquina, C.; Ibarra, M.R.; Goya, G.F. Magnetic nanoparticles for power absorption: Optimizing size, shape and magnetic properties. J. Solid State Chem 2009, 182, 2779–2784. [Google Scholar]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev 2008, 108, 2064–2110. [Google Scholar]
- Dames, P.; Gleich, B.; Flemmer, A.; Hajek, K.; Seidl, N.; Wiekhorst, F.; Eberbeck, D.; Bittmann, I.; Bergemann, C.; Weyh, T.; et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol 2007, 2, 495–499. [Google Scholar]
- Ugarte, D.; Chatelain, A.; de Heer, W.A. Nanocapillarity and chemistry in carbon nanotubes. Science 1996, 274, 1897–1899. [Google Scholar]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening carbon nanotubes with oxygen and implications for filling. Nature 1993, 362, 522–525. [Google Scholar]
- Svensson, K.; Olin, H.; Olsson, E. Nanopipettes for metal transport. Phys. Rev. Lett 2004, 93, 145901. [Google Scholar]
- He, H.; Zhang, Y.; Gao, C.; Wu, J. “Clicked” magnetic nanohybrids with a soft polymer interlayer. Chem. Commun 2009, 13, 1655–1657. [Google Scholar]
- Jiang, K.; Eitan, A.; Schadler, L.S.; Ajayan, P.M.; Siegel, R.W.; Grobert, N.; Mayne, M.; Reyes-Reyes, M.; Terrones, H.; Terrones, M. Selective Attachment of gold nanoparticles to nitrogen-doped carbon nanotubes. Nano Lett 2003, 3, 275–277. [Google Scholar]
- Wang, Z.; Li, M.; Zhang, Y.; Yuan, J.; Shen, Y.; Niu, L.; Ivaska, A. Thionine-interlinked multi-walled carbon nanotube/gold nanoparticle composites. Carbon 2007, 45, 2111–2115. [Google Scholar]
- Rahman, G.M.; Guldi, D.M.; Zambon, E.; Pasquato, L.; Tagmatarchis, N.; Prato, M. Dispersable carbon nanotube/gold nanohybrids: Evidence for strong electronic interactions. Small 2005, 1, 527–530. [Google Scholar]
- Elias, A.L.; Rodriguez-Manzo, J.A.; McCartney, M.R.; Golberg, D.; Zamudio, A.; Baltazar, S.E.; Lopez-Urias, F.; Munoz-Sandoval, E.; Gu, L.; Tang, C.C.; et al. Production and characterization of single-crystal FeCo nanowires inside carbon nanotubes. Nano Lett 2005, 5, 467–472. [Google Scholar]
- Tao, X.; Dong, L.; Zhang, W.; Zhang, X.; Cheng, J.; Huang, H.; Gan, Y. Controllable melting and flow of Î2-Sn in flexible amorphous carbon nanotubes. Carbon 2009, 47, 3122–3127. [Google Scholar]
- Gimenez-Lopez Mdel, C.; Moro, F.; la Torre, A.; Gomez-Garcia, C.J.; Brown, P.D.; van Slageren, J.; Khlobystov, A.N. Encapsulation of single-molecule magnets in carbon nanotubes. Nat. Commun 2011, 2. [Google Scholar] [CrossRef]
- Lis, T. Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate. Acta Crystallogr. Sect. B 1980, 36, 2042–2046. [Google Scholar]
- Rossella, F.; Soldano, C.; Bellani, V.; Tommasini, M. Metal-filled carbon nanotubes as a novel class of photothermal nanomaterials. Adv. Mater 2012, 24, 2453–2458. [Google Scholar]
- Soldano, C.; Kar, S.; Talapatra, S.; Nayak, S.; Ajayan, P.M. Detection of nanoscale magnetic activity using a single carbon nanotube. Nano Lett 2008, 8, 4498–4505. [Google Scholar]
- Zambrano, H.A.; Walther, J.H.; Koumoutsakos, P.; Sbalzarini, I.F. Thermophoretic motion of water nanodroplets confined inside carbon nanotubes. Nano Lett 2009, 9, 66–71. [Google Scholar]
- Barreiro, A.; Rurali, R.; Hernandez, E.R.; Moser, J.; Pichler, T.; Forro, L.; Bachtold, A. Subnanometer motion of cargoes driven by thermal gradients along carbon nanotubes. Science 2008, 320, 775–778. [Google Scholar]
- Korneva, G.; Ye, H.; Gogotsi, Y.; Halverson, D.; Friedman, G.; Bradley, J.C.; Kornev, K.G. Carbon nanotubes loaded with magnetic particles. Nano Lett 2005, 5, 879–884. [Google Scholar]
- Pal, S.; Chandra, S.; Phan, M.H.; Mukherjee, P.; Srikanth, H. Carbon nanostraws: Nanotubes filled with superparamagnetic nanoparticles. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Charron, G.; Giusti, A.; Mazerat, S.; Mialane, P.; Gloter, A.; Miserque, F.; Keita, B.; Nadjo, L.; Filoramo, A.; Riviere, E.; et al. Assembly of a magnetic polyoxometalate on SWNTs. Nanoscale 2010, 2, 139–144. [Google Scholar]
- Zanolli, Z.; Charlier, J.C. Single-molecule sensing using carbon nanotubes decorated with magnetic clusters. ACS Nano 2012, 6, 10786–10791. [Google Scholar]
- Gupta, V.K.; Mittal, A.; Kurup, L.; Mittal, J. Adsorption of a hazardous dye, erythrosine, over hen feathers. J. Colloid Interface Sci 2006, 304, 52–57. [Google Scholar]
- Gupta, V.K.; Ali, I.; Saini, V.K. Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J. Colloid Interface Sci 2007, 315, 87–93. [Google Scholar]
- Yu, F.; Chen, J.; Chen, L.; Huai, J.; Gong, W.; Yuan, Z.; Wang, J.; Ma, J. Magnetic carbon nanotubes synthesis by Fenton’s reagent method and their potential application for removal of azo dye from aqueous solution. J. Colloid Interface Sci 2012, 378, 175–183. [Google Scholar]
- Jia, B.; Gao, L.; Sun, J. Self-assembly of magnetite beads along multiwalled carbon nanotubes via a simple hydrothermal process. Carbon 2007, 45, 1476–1481. [Google Scholar]
- Liu, Y.; Jiang, W.; Wang, Y.; Zhang, X.J.; Song, D.; Li, F.S. Synthesis of Fe3O4/CNTs magnetic nanocomposites at the liquidâ liquid interface using oleate as surfactant and reactant. J. Magn. Magn. Mater 2009, 321, 408–412. [Google Scholar]
- Huiqun, C.; Meifang, Z.; Yaogang, L. Novel carbon nanotube iron oxide magnetic nanocomposites. J. Magn. Magn. Mater 2006, 305, 321–324. [Google Scholar]
- Ensafi, A.A.; Allafchian, A.R. Multiwall carbon nanotubes decorated with NiFe2O4 magnetic nanoparticles, a new catalyst for voltammetric determination of cefixime. Colloids Surf. B Biointerfaces 2013, 102, 687–693. [Google Scholar]
- Jain, R.; Gupta, V.K.; Jadon, N.; Radhapyari, K. Voltammetric determination of cefixime in pharmaceuticals and biological fluids. Anal. Biochem 2010, 407, 79–88. [Google Scholar]
- Lamanna, G.; Garofalo, A.; Popa, G.; Wilhelm, C.; Begin-Colin, S.; Felder-Flesch, D.; Bianco, A.; Gazeau, F.; Menard-Moyon, C. Endowing carbon nanotubes with superparamagnetic properties: Applications for cell labeling, MRI cell tracking and magnetic manipulations. Nanoscale 2013, 5, 4412–4421. [Google Scholar]
- Hongkun, H.; Chao, G. Synthesis of Fe3O4/Pt nanoparticles decorated carbon nanotubes and their use as magnetically recyclable catalysts. J. Nanomater 2011, 2011. [Google Scholar] [CrossRef]
- Gao, C.; He, H.; Zhou, L.; Zheng, X.; Zhang, Y. Scalable functional group engineering of carbon nanotubes by improved one-step nitrene chemistry. Chem. Mater 2009, 21, 360–370. [Google Scholar]
- Liu, W.; Wu, H.; Li, B.; Dong, C.; Choi, M.M.F.; Shuang, S. Immobilization of platinum nanoparticles and glucose oxidase on eggshell membrane for glucose detection. Anal. Methods 2013, 5, 5154–5160. [Google Scholar]
- Deng, Y.; Deng, C.; Yang, D.; Wang, C.; Fu, S.; Zhang, X. Preparation, characterization and application of magnetic silica nanoparticle functionalized multi-walled carbon nanotubes. Chem. Commun 2005, 44, 5548–5550. [Google Scholar]
- Lee, P.; Chiu, Y.; Sun, Y.; Ling, Y. Synthesis of a hybrid material consisting of magnetic iron-oxide nanoparticles and carbon nanotubes as a gas adsorbent. Carbon 2010, 48, 1397–1404. [Google Scholar]
- Li, W.; Gao, C.; Qian, H.; Ren, J.; Yan, D. Multiamino-functionalized carbon nanotubes and their applications in loading quantum dots and magnetic nanoparticles. J. Mater. Chem 2006, 16, 1852–1859. [Google Scholar]
- Liu, Z.; Wang, J.; Xie, D.; Chen, G. Polyaniline-coated Fe3O4 nanoparticle-carbon-nanotube composite and its application in electrochemical biosensing. Small 2008, 4, 462–466. [Google Scholar]
- Zhang, Q.; Zhu, M.; Zhang, Q.; Li, Y.; Wang, H. The formation of magnetite nanoparticles on the sidewalls of multi-walled carbon nanotubes. Compos. Sci. Technol 2009, 69, 633–638. [Google Scholar]
- Morales-Cid, G.; Fekete, A.; Simonet, B.M.; Lehmann, R.; Cardenas, S.; Zhang, X.; Valcarcel, M.; Schmitt-Kopplin, P. In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro)quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal. Chem 2010, 82, 2743–2752. [Google Scholar]
- Lu, X.; Liu, H.; Deng, C.; Yan, X. Facile synthesis and application of mesoporous silica coated magnetic carbon nanotubes. Chem. Commun 2011, 47, 1210–1212. [Google Scholar]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. Novel carbon nanotube-polystyrene foam composites for electromagnetic interference shielding. Nano Lett 2005, 5, 2131–2134. [Google Scholar]
- Li, N.; Huang, Y.; Du, F.; He, X.; Lin, X.; Gao, H.; Ma, Y.; Li, F.; Chen, Y.; Eklund, P.C. Electromagnetic interference (EMI) shielding of single-walled carbon nanotube epoxy composites. Nano Lett 2006, 6, 1141–1145. [Google Scholar]
- Mahmoodi, M.; Arjmand, M.; Sundararaj, U.; Park, S. The electrical conductivity and electromagnetic interference shielding of injection molded multi-walled carbon nanotube/polystyrene composites. Carbon 2012, 50, 1455–1464. [Google Scholar]
- Cao, M.; Song, W.; Hou, Z.; Wen, B.; Yuan, J. The effects of temperature and frequency on the dielectric properties, electromagnetic interference shielding and microwave-absorption of short carbon fiber/silica composites. Carbon 2010, 48, 788–796. [Google Scholar]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric oxide/multiwalled carbon nanotube vs polyaniline/ferroferric oxide/multiwalled carbon nanotube multiheterostructures for highly effective microwave absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar]
- Li, X.; Thompson, J.D.; Zhang, Y.; Brady, C.I.; Zou, G.; Mack, N.H.; Williams, D.; Duque, J.G.; Jia, Q.; Doorn, S.K. Efficient synthesis of tailored magnetic carbon nanotubes via a noncovalent chemical route. Nanoscale 2011, 3, 668–673. [Google Scholar]
- Bear, J.C.; McNaughter, P.D.; Jurkschat, K.; Crossley, A.; Aldous, L.; Compton, R.G.; Mayes, A.G.; Wildgoose, G.G. Synthesis and characterization of carbon nanotubes covalently functionalized with amphiphilic polymer coated superparamagnetic nanocrystals. J. Colloid Interface Sci 2012, 383, 110–117. [Google Scholar]
- Sun, C.; Liu, Y.; Ding, W.; Gou, Y.; Xu, K.; Xia, G.; Ding, Q. Synthesis and characterization of superparamagnetic CoFe2O4/MWCNT hybrids for tumor-targeted therapy. J. Nanosci. Nanotechnol 2013, 13, 236–241. [Google Scholar]
- Vermisoglou, E.C.; Pilatos, G.; Romanos, G.E.; Devlin, E.; Kanellopoulos, N.K.; Karanikolos, G.N. Magnetic carbon nanotubes with particle-free surfaces and high drug loading capacity. Nanotechnology 2011, 22. [Google Scholar] [CrossRef]
- Atamna, H.; Nguyen, A.; Schultz, C.; Boyle, K.; Newberry, J.; Kato, H.; Ames, B.N. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J 2008, 22, 703–712. [Google Scholar]
- Masotti, A. Multifunctional nanoparticles, nanocages and degradable polymers as a potential novel generation of non-invasive molecular and cellular imaging systems. Recent Pat. Nanotechnol 2011, 5, 163–177. [Google Scholar]
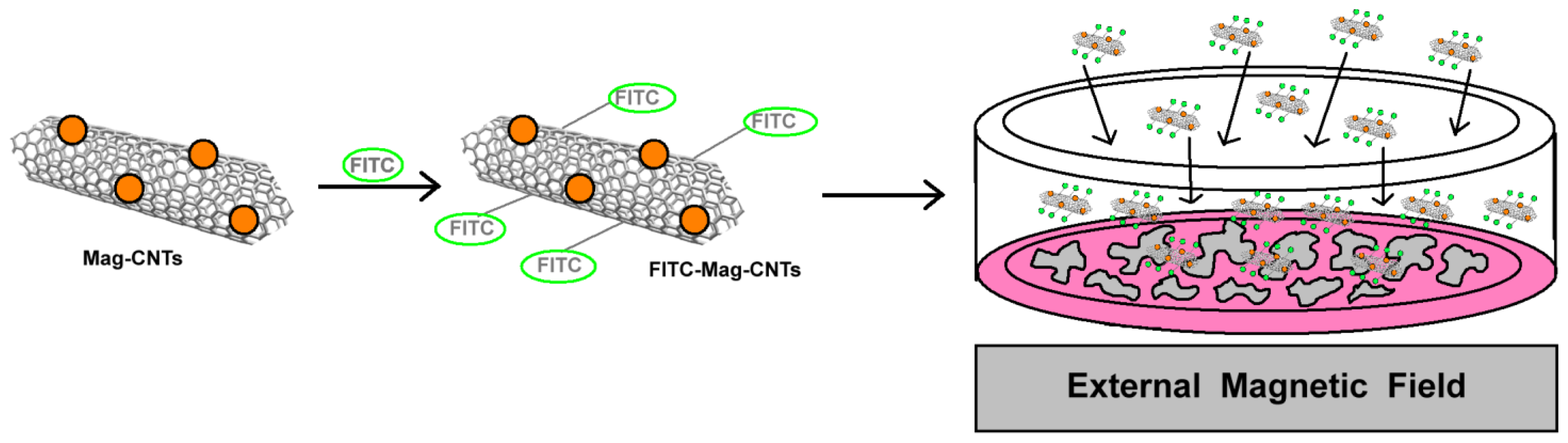
- Khandare, J.J.; Jalota-Badhwar, A.; Satavalekar, S.D.; Bhansali, S.G.; Aher, N.D.; Kharas, F.; Banerjee, S.S. PEG-conjugated highly dispersive multifunctional magnetic multi-walled carbon nanotubes for cellular imaging. Nanoscale 2012, 4, 837–844. [Google Scholar]
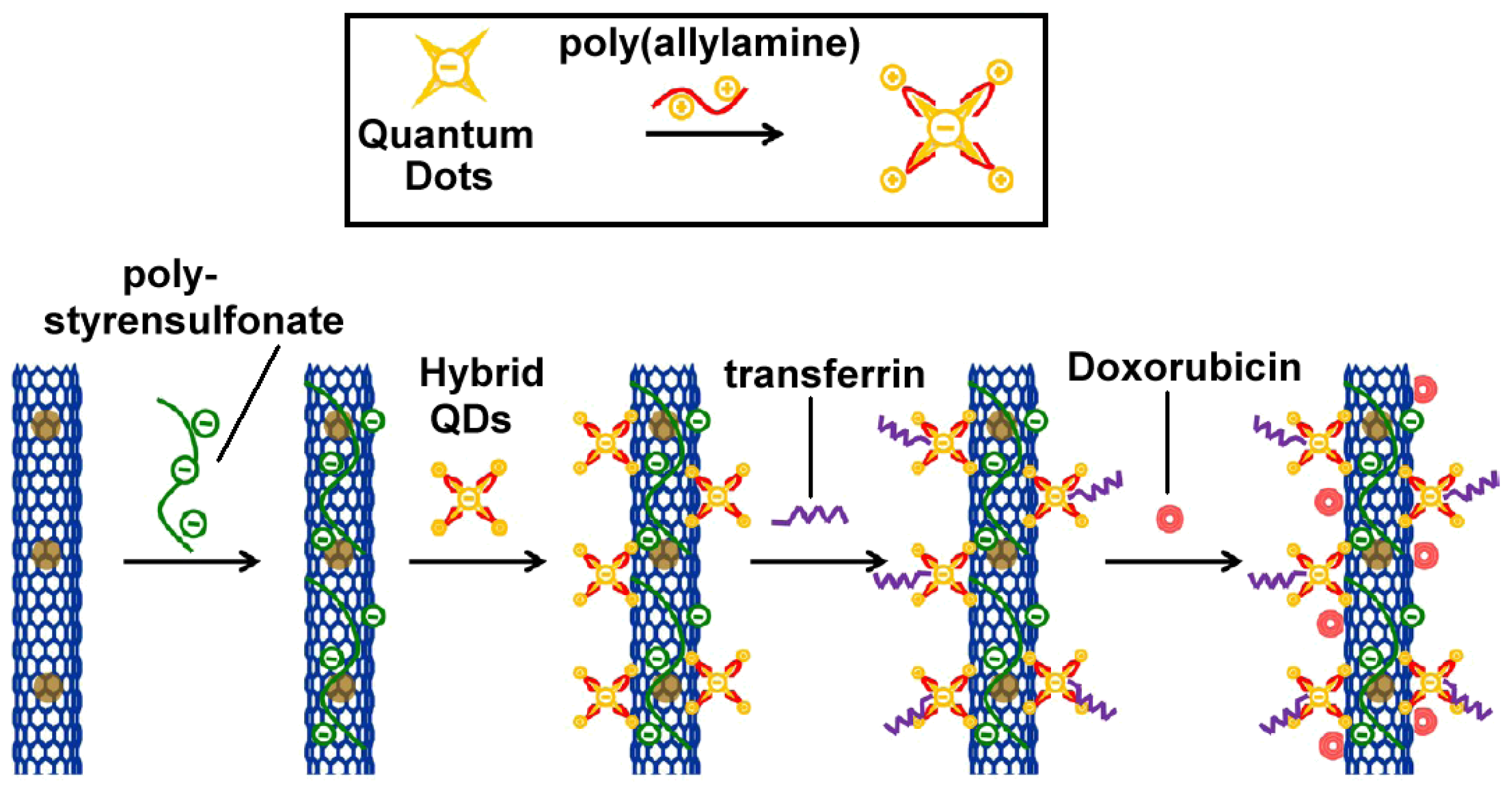
- Chen, M.L.; He, Y.J.; Chen, X.W.; Wang, J.H. Quantum dots conjugated with Fe3O4-filled carbon nanotubes for cancer-targeted imaging and magnetically guided drug delivery. Langmuir 2012, 28, 16469–16476. [Google Scholar]
- Gul, H.; Lu, W.; Xu, P.; Xing, J.; Chen, J. Magnetic carbon nanotube labelling for haematopoietic stem/progenitor cell tracking. Nanotechnology 2010, 21. [Google Scholar] [CrossRef]
- Cai, D.; Mataraza, J.M.; Qin, Z.H.; Huang, Z.; Huang, J.; Chiles, T.C.; Carnahan, D.; Kempa, K.; Ren, Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat. Methods 2005, 2, 449–454. [Google Scholar]
- Shayan, R.; Achen, M.G.; Stacker, S.A. Lymphatic vessels in cancer metastasis: Bridging the gaps. Carcinogenesis 2006, 27, 1729–1738. [Google Scholar]
- Jain, R.K.; Padera, T.P. Prevention and treatment of lymphatic metastasis by antilymphangiogenic therapy. J. Natl. Cancer Inst 2002, 94, 785–787. [Google Scholar]
- Yang, F.; Fu de, L.; Long, J.; Ni, Q.X. Magnetic lymphatic targeting drug delivery system using carbon nanotubes. Med. Hypotheses 2008, 70, 765–767. [Google Scholar]
- Yang, F.; Jin, C.; Yang, D.; Jiang, Y.; Li, J.; Di, Y.; Hu, J.; Wang, C.; Ni, Q.; Fu, D. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur. J. Cancer 2011, 47, 1873–1882. [Google Scholar]
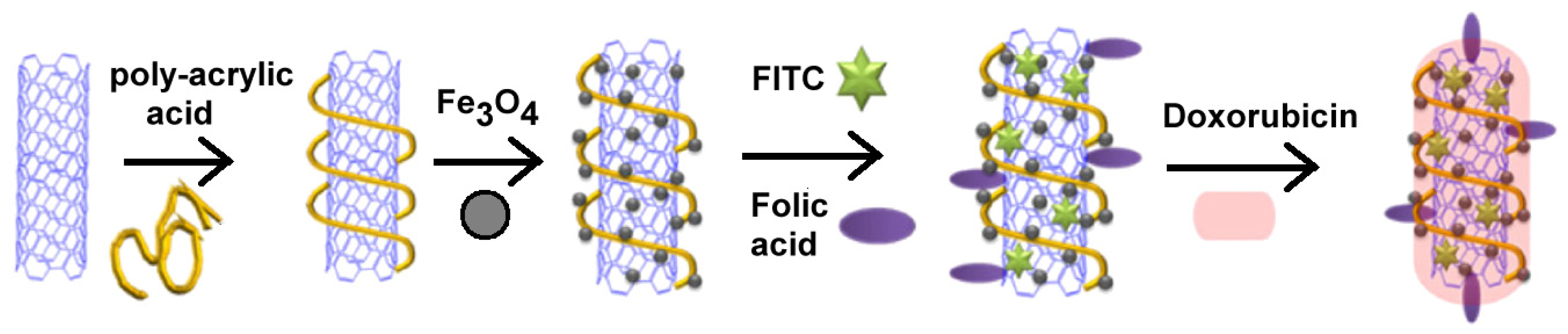
- Lu, Y.J.; Wei, K.C.; Ma, C.C.; Yang, S.Y.; Chen, J.P. Dual targeted delivery of doxorubicin to cancer cells using folate-conjugated magnetic multi-walled carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 89, 1–9. [Google Scholar]
- Schnoor, M.; Buers, I.; Sietmann, A.; Brodde, M.F.; Hofnagel, O.; Robenek, H.; Lorkowski, S. Efficient non-viral transfection of THP-1 cells. J. Immunol. Methods 2009, 344, 109–115. [Google Scholar]
- Muthana, M.; Scott, S.D.; Farrow, N.; Morrow, F.; Murdoch, C.; Grubb, S.; Brown, N.; Dobson, J.; Lewis, C.E. A novel magnetic approach to enhance the efficacy of cell-based gene therapies. Gene Ther 2008, 15, 902–910. [Google Scholar]
- Gul-Uludag, H.; Lu, W.; Xu, P.; Xing, J.; Chen, J. Efficient and rapid uptake of magnetic carbon nanotubes into human monocytic cells: Implications for cell-based cancer gene therapy. Biotechnol. Lett 2012, 34, 989–993. [Google Scholar]



© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Masotti, A.; Caporali, A. Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications. Int. J. Mol. Sci. 2013, 14, 24619-24642. https://doi.org/10.3390/ijms141224619
Masotti A, Caporali A. Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications. International Journal of Molecular Sciences. 2013; 14(12):24619-24642. https://doi.org/10.3390/ijms141224619
Chicago/Turabian StyleMasotti, Andrea, and Andrea Caporali. 2013. "Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications" International Journal of Molecular Sciences 14, no. 12: 24619-24642. https://doi.org/10.3390/ijms141224619
APA StyleMasotti, A., & Caporali, A. (2013). Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications. International Journal of Molecular Sciences, 14(12), 24619-24642. https://doi.org/10.3390/ijms141224619




