2. Results and Discussion
In this study the response surface methodology was used to simultaneously optimize the production of two lignin-modifying enzymes (Lac and MnP) produced by C. subvermispora. For each enzyme, the optimization was performed using the same methodology for both the enzyme activity and its corresponding production rate. In order to optimize the model consisting of two factors that needed to be improved simultaneously (the maximal enzyme activity and its maximal production rate) as a single system response, the Objective Functions (OFLac and OFMnP) for batch experiments were expressed by:
where ([
ELac])
max and ([
EMnP])
max are the highest values of the experimentally obtained maximal Lac and MnP activities ([
ELac] and [
EMnP] in U L
−1) for all settings presented in
Table 1,
w is the weighting factor (
i.e., the fraction of the normalized maximal enzyme activity contribution in the overall
OF), (d[
ELac]/d
t)
max and (d[
EMnP]/d
t)
max are the highest values of experimentally obtained maximal Lac and MnP production rate (d[
ELac]/d
t and d[
EMnP]/d
t in U L
−1 day
−1) for all settings presented in
Table 1.
OFMnP could not be optimized due to the absence of local maxima within reasonable substrate concentration values (the combined measurement and estimation error was too large). Nonetheless, a new Objective Function (
OFLac/MnP) for batch experiments was defined as:
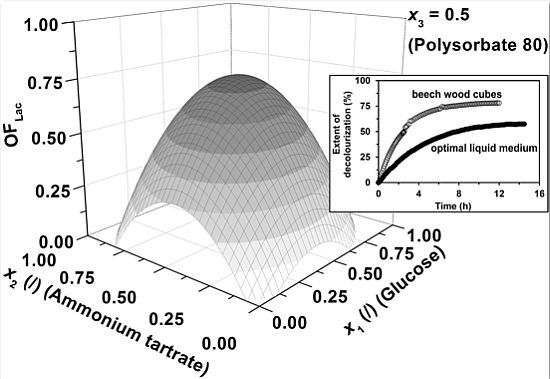
In order not to introduce too many variables to the design of experiments, as the three crucial independent variables, pertinent to enzyme production, two principal nutrients (carbon and nitrogen source—glucose (1) and ammonium tartrate (2), respectively) and Polysorbate 80 (3), a non-ionic surfactant and emulsifier, were chosen. In
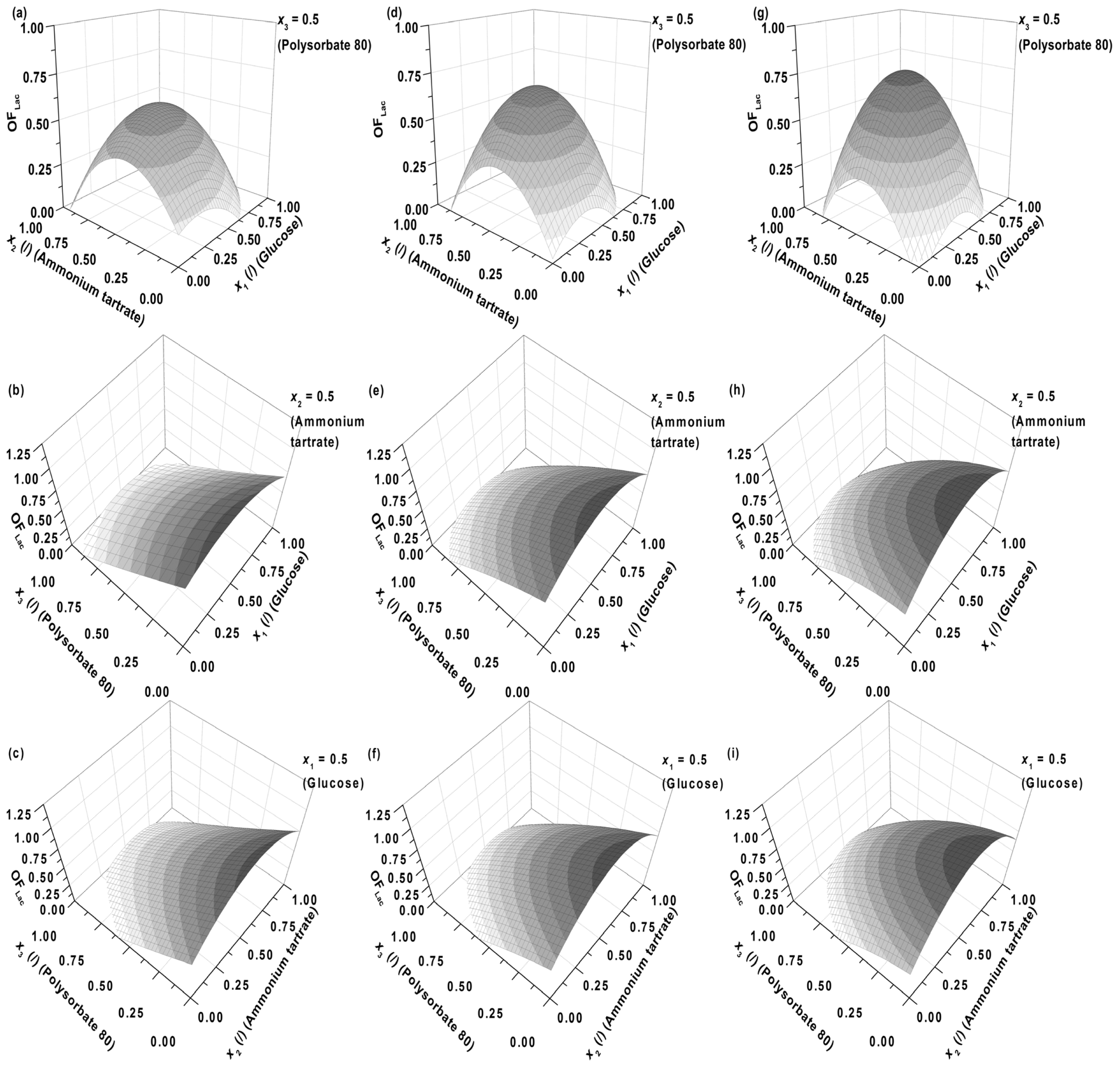
Figure 1, the influence of medium components specifically; glucose (
x1), ammonium tartrate (
x2) and Polysorbate 80 (
x3), on the combined activity and productivity of Lac (
OFLac) is presented. In
Figure 1a–c the Lac activity has a lower contribution (0.25) to the overall objective function (
Equation 1) than the Lac productivity (0.75), whereas its impact gradually increases through
Figure 1d–f (
w = 0.50) and 1g–i (
w = 0.75).
The first observation which can be drawn is that the general shape of the response surfaces remains approximately the same, regardless of the fraction of the Lac activity (
w) and productivity (1 −
w) in the overall response (
OFLac), the latter of course being dependent on
w. Correspondingly, there is an optimum in Lac activity ([
ELac])/productivity (d[
ELac]/d
t) at the specific glucose (
x1) and ammonium tartrate (
x2) concentrations at the fixed Polysorbate 80 level (
x3 = 0.5,
CP = 0.55 g L
−1), meaning that at lower concentrations both medium components promote enzyme production, whereas an additional increase in concentrations results in an inhibitory effect. The research of
C. subvermispora in biotechnological applications revealed the obtained Lac activities around 75 U L
−1 and MnP activities around 40 U L
−1 after 5 and 14 days of incubation, respectively, in a MPG (malt extract, peptone, and glucose) medium at 25 °C [
22]. Enzyme production by
C. subvermispora is regulated by nitrogen and glucose concentrations in the medium [
23–
25]. With a growth limiting amount of nitrogen source (0.2 g L
−1), the fungus
C. subvermispora produces low levels of both enzymes compared to a medium with a sufficient nitrogen source concentration (2.0 g L
−1). The same pattern was found with a carbon source. The fungus produced high levels of both enzymes (about 500 U L
−1 of MnP and 400 U L
−1 of Lac, respectively, between two and three weeks of cultivation at 30 °C) at high carbon concentrations (10 g L
−1 glucose) and low levels at 1.0 g L
−1 of glucose in a medium with sufficient nitrogen concentration (2.0 g L
−1) [
23]. The enzyme activities obtained by Ruttimann-Johnson
et al. were higher in comparison to the ones, presented in this study, nonetheless; the enzyme production rates in this study are comparable if not even higher [
23]. For a technological enzyme production process the productivity is of a primordial interest, most frequently it is at least comparably important to the enzyme activity itself. This observation is somewhat different from the one indicated in
Figure 1. An explanation might be that the above mentioned authors did not examine the effect of carbon source (glucose) concentration over the entire range, but were limited to either its low or high values. Considering
Figure 1a,d,g, one could reach the same conclusion, if only two points were compared on the response surface at a fixed nitrogen source concentration (ammonium tartrate) level (
x2). On the other hand, the experimental conditions were not exactly the same in the present study as in the work reported by Ruttimann-Johnson
et al. [
23], as the latter omitted a surfactant in the growth medium. Thus, the concentration of glucose which would suppress enzyme production was not established; nonetheless, it might well exist within their studied range of concentrations of 1–10 g L
−1 [
23] or be even higher. Considering our results, the locus of optimal Lac activity/productivity (
OFLac) in terms of glucose and ammonium tartrate concentration varies with the contribution of Lac activity to the response, but not significantly (
Figure 1a,d,g), implying that medium levels of glucose (2–6 g L
−1) and tartrate (0.8–1.2 g L
−1) are favourable for both the activity and productivity of Lac. The latter is pertinent to industrial processes where relatively high yield and rate need to be obtained concurrently. However, extremely high yield and rate cannot be achieved simultaneously, as
OFLac in
Figure 1a,d,g does not exceed unity, meaning that there is a certain reduction in Lac productivity on account of its higher activity, and vice versa.
In the production of enzymes, especially in large-scale processing, antifoaming agents and surfactants are normally used. Polysorbate 80 (Tween 80) is a non-ionic surfactant that is helpful in releasing fungal enzymes to the external environment. Although Polysorbate 80 is of low toxicity to the cellular membrane, it can alter the structure and morphology of fungi and the bacterial cell wall, leading to an increase of protein secretion [
26].
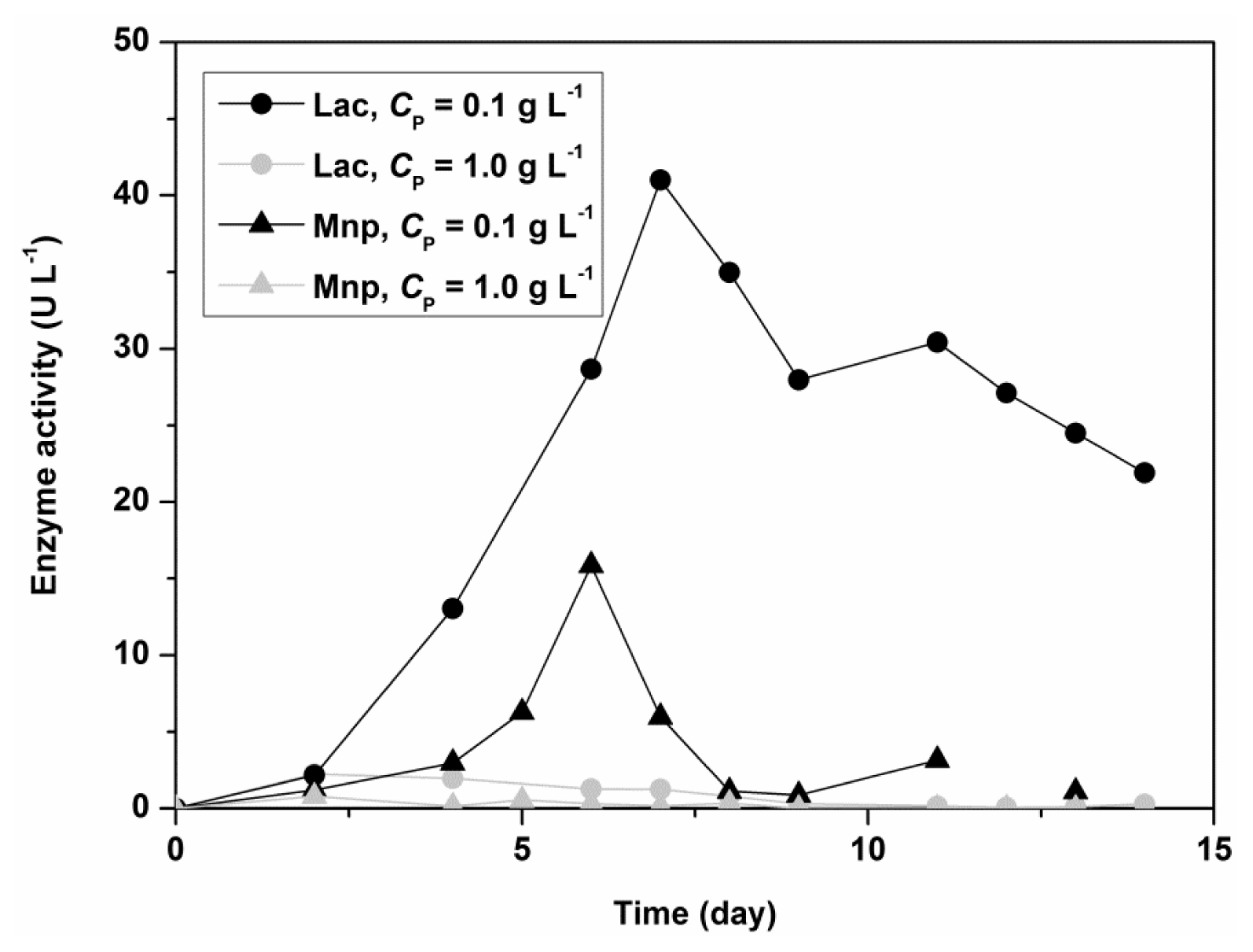
Figure 2 shows the influence of Polysorbate 80 on the activity of Lac and MnP for the fungus
C. subvermispora in a shaken flask experiment. The initial concentrations of nitrogen and carbon sources were 5.50 g L
−1 for glucose and 1.05 g L
−1 for ammonium tartrate. Lac and MnP activities in the filtrate are 10 times higher in the presence of a low concentration of Polysorbate 80 (0.1 g L
−1). The results thus showed that Polysorbate 80 could be used as a stimulatory agent in submerged fungal cultivation, but in low concentrations.
Figure 1b,c,e,f,h,i (model predictions) also show that either at fixed glucose (
x1 = 0.5,
CG = 5.50 g L
−1) or ammonium tartrate concentrations (
x2 = 0.5,
CDT = 1.05 g L
−1), the concentration of Polysorbate 80 (
x3) should be as low as possible. The dependence on nitrogen or carbon source exhibits a maximum. The latter is again within medium levels of glucose (2–6 g L
−1) and ammonium tartrate (0.8–1.2 g L
−1).
The response surface methodology was less successful in describing the activity/productivity of MnP, e.g., the results implied that the optimal MnP activity/productivity would be obtained in the absence of a nitrogen source. Error analysis suggested that this was not a result of the modelling step of the RSM procedure (configure Experimental Section), but of the experimental phase—the production of MnP by
C. subvermispora was poorly repeatable. However, there was a certain correlation with Lac production, and hence the corrected objective function (
OFLac/MnP) was defined through
Equation 3 for simultaneous optimization of both Lac and MnP activity. In addition to this, even though a poorer predictability of the model for MnP activity and productivity may be observed through lower values of
R2 (the latter indicates explained variability) and
Q2 (the latter indicates predicted variability) coefficients (presented in the continuation), the latter may be considered acceptable. Moreover, the predictability of the new (corrected) Objective Function for the simultaneous optimization of both Lac and MnP activity is more than acceptable on account of excellent predictability for Lac and an average one for MnP.
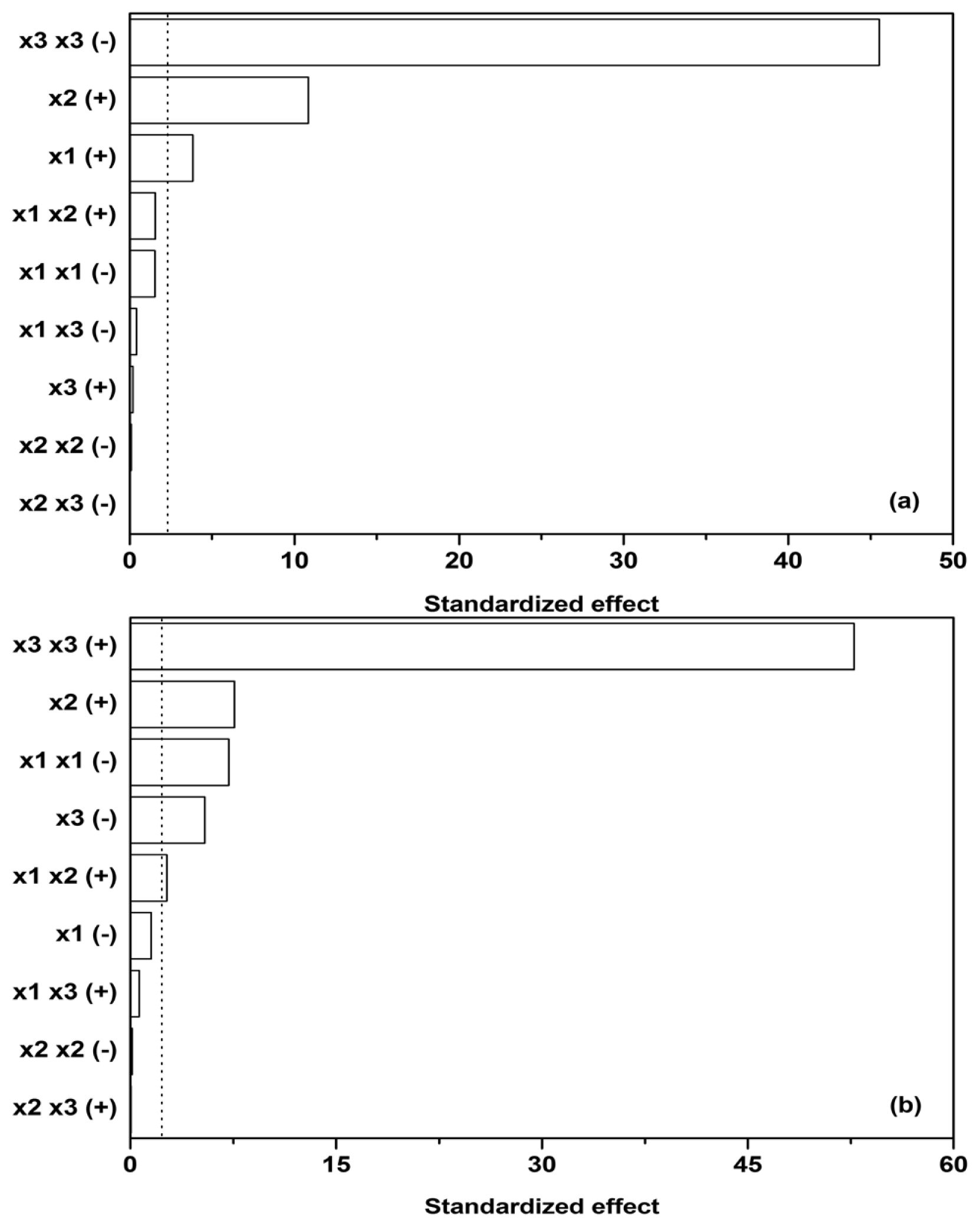
Subsequently, sensitivity analysis was performed on the quadratic model utilized and its corresponding parameters (the parameters in
Equation 2 and
Table 2), estimated by linear regression. Cohen’s
f2 was used to measure the effect size of individual contributions in the model (
x1, the initial glucose,
x2, ammonium tartrate, and
x3, Polysorbate 80 concentration,
y1,
y2,
y3, and
y3 dependent variables), while the
F-test was used as a statistical test to determine which effects were within the 95% confidence interval margin (dotted line in
Figure 3).
Figure 3a reveals that glucose as a carbon source (
x1) and ammonium tartrate as a nitrogen source (
x2) both exhibit a positive effect on Lac activity (
y1, [
ELac]), the latter larger. The effect of the Polysorbate 80 quadratic term (
x3 x3) on Lac activity, on the other hand, is negative and predominant. The quadratic term would imply a stationary point in the dependence of [
ELac] on
x3, but
Figure 1b,c,e,f, 1h, and 1i show no such occurrences. The stationary point thus probably lies outside the presented range, and therefore the joint effect of the linear and quadratic term of
x3 could have been approximated by linearization. Another feature of interest lies in the fact that the influences of carbon source, nitrogen source, and surfactant seem to be independent, as the interdependence terms (
x1 x2,
x1 x3, and
x2 x3) in
Figure 3a are negligible, the synergistic effect of glucose and ammonium tartrate (
x1 x2 (+)) being the largest of them. The dependences on glucose (
x1) and ammonium tartrate (
x2) in
Figure 1 nevertheless reveal local maxima.
The maxima in Lac activity/productivity (
OFLac) as a function of glucose (
x1) and ammonium tartrate (
x2) concentrations in
Figure 1 may therefore be predominantly ascribed to the contribution of Lac productivity (
y2, d[
ELac]/d
t). Lac productivity is thus noticeably affected by the inhibitory effect of glucose (
x1 x1 (−)) and the positive combined glucose/ammonium tartrate effect (
x1 x2 (+)) (
Figure 3b). The latter two, along with the linear terms, are responsible for the maxima in the Lac activity/productivity. The effect of ammonium tartrate on Lac productivity is analogous to that which it has on its activity; specifically, it is positive and notable in its influence. Lastly, Polysorbate 80 (
x3) principally affects productivity, as well as activity; the productivity both in the linear (−) and quadratic (+) terms. The overall effect of the surfactant on productivity is the same as that which it asserts on activity, namely negative. Other effects had negligible influence.
The optimization of the quadratic model utilized ensued, where the model parameters were estimated by linear regression. The objective function, constituted from enzyme (Lac and MnP) activity and production rate, was maximized for variable portions of either, w. Parameters (the optimal initial medium concentrations x1, x2, and x3) were estimated by nonlinear regression, namely the Levenberg–Marquardt algorithm (10−5 tolerance).
Figure 4 shows the optimal values of two objective functions, (
OFLac) Lac activity and productivity, and (
OFLac/MnP) Lac and MnP activities, which were maximized simultaneously, with variable contributions of Lac activity (
w) to the objective function. In the first case one may observe that although the overall objective function
OFLac assumes its greatest value (1.17) at
w = 0 due to high Lac productivity, Lac activity is greatly decreased (only 40% of the highest experimental value). As the contribution to Lac activity in
OFLac,
w, increases, Lac activity (
y1,Lac) first abruptly and then gradually increases towards 1.14, while concurrently Lac productivity (
y2,Lac) exhibits a rather small decrease, from 1.17 to 1.00. This would imply that, with the exception of very rare cases when the maximal productivity would be attained regardless of the relatively low activity (
w = 0,
OFLac = 1.17), continuous systems should preferably be operated in conditions of the highest activity and only slightly decreased productivity (
w = 1,
OFLac = 1.14), or at least in this vicinity. Of course, another aspect to consider is the concentration of medium components at which distinct levels of activity or productivity may be achieved.
Simultaneously optimizing Lac and MnP activities (OFLac/MnP) reveals that it is difficult to obtain high levels of both Lac and MnP, respectively, since for high MnP concentrations (w→0), Lac activity decreases basically to zero. On the other hand, upon obtaining high Lac concentrations (114% of the highest experimental value), MnP activity may be maintained at an intermediate level (63% of the highest experimental value).
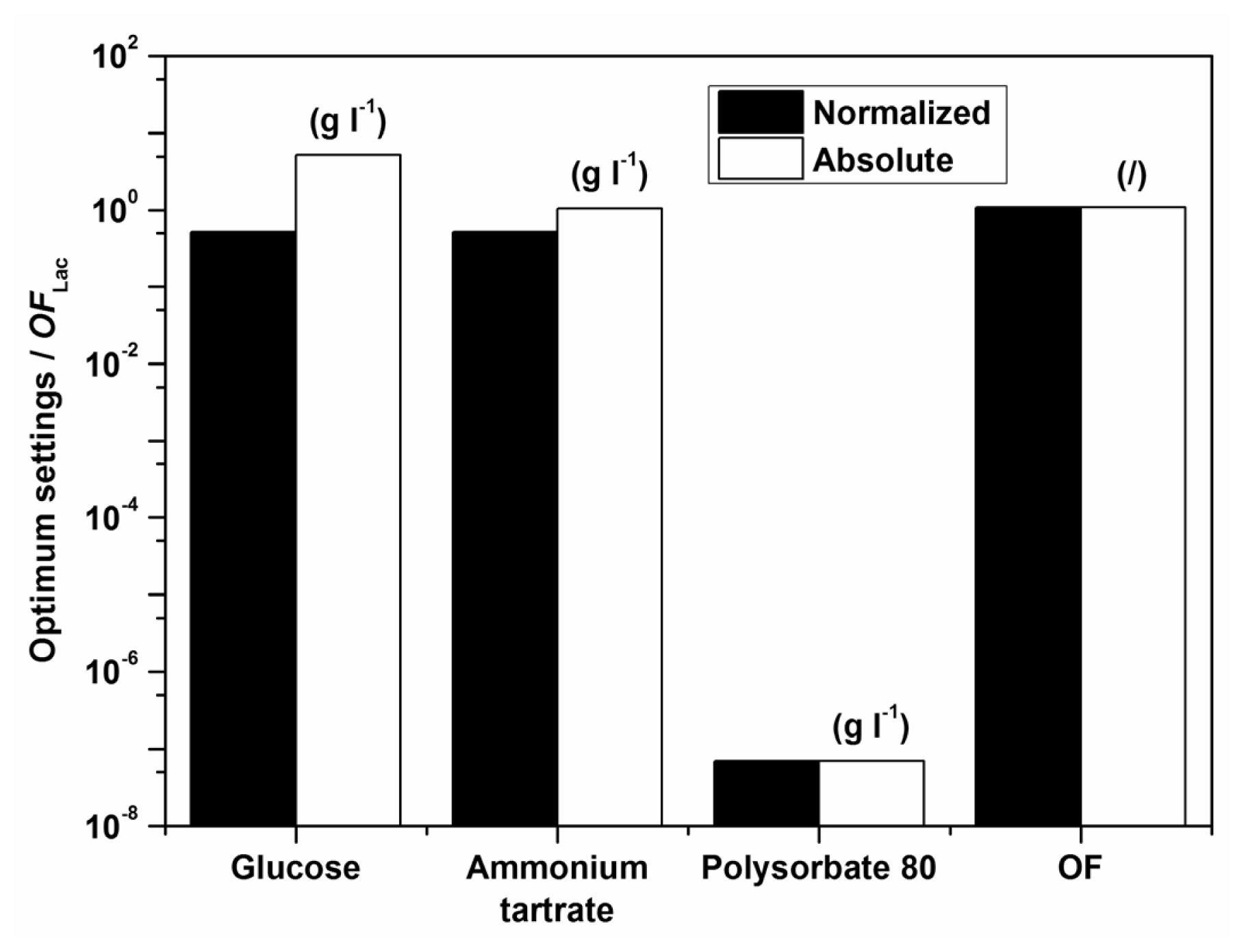
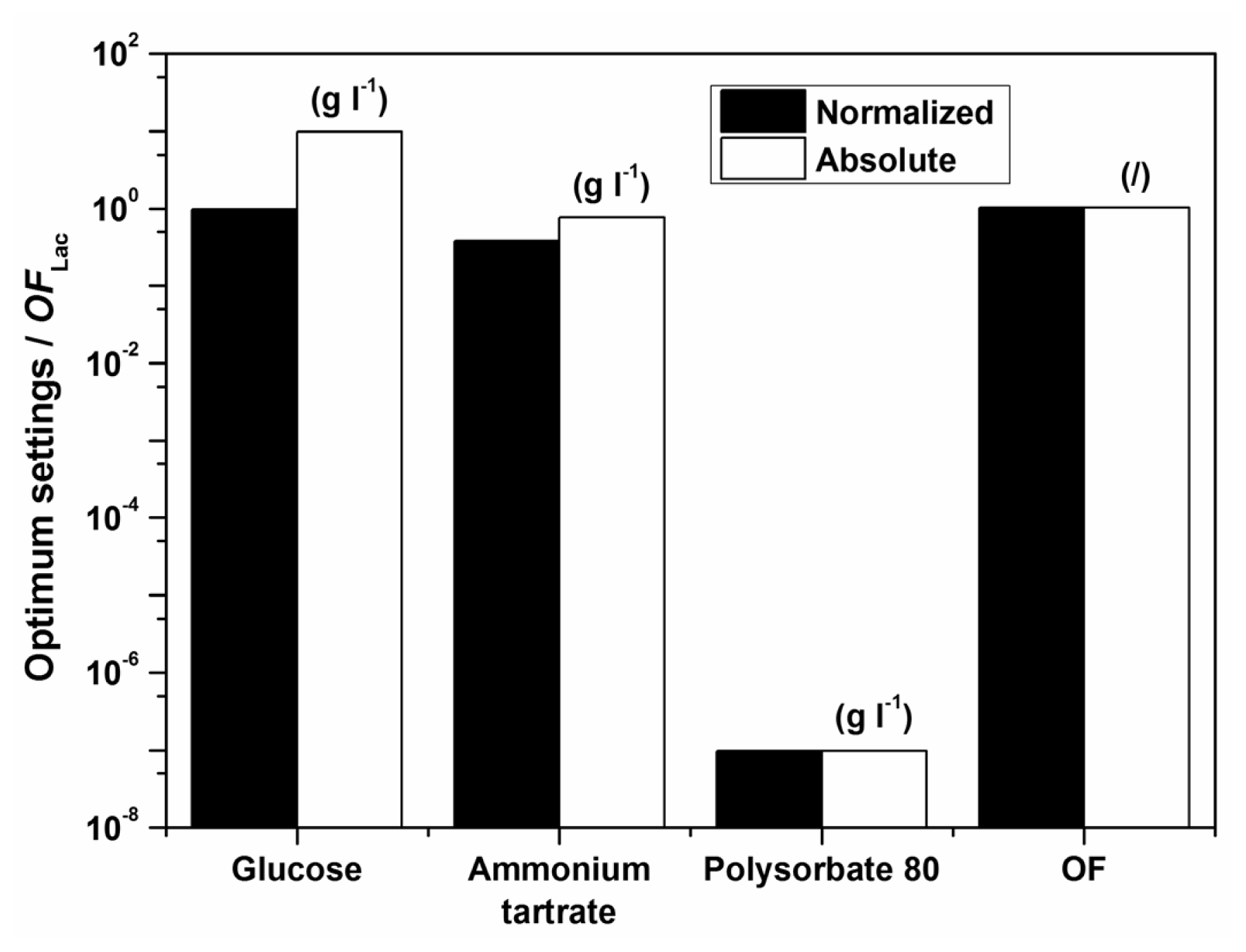
Examples of initial glucose, ammonium tartrate, and Polysorbate 80 concentrations for the optimal Lac activity and productivity (
OFLac) and Lac and MnP activities (
OFLac/MnP) are presented in
Tables 3–
5 and
Figures 5 and
6. In all cases the absence of the surfactant should increase both enzyme activity and productivity. The intermediate concentrations of carbon and nitrogen sources corresponded to the maxima exhibited by the response surfaces in
Figure 1. The optimization results had to be verified (Experimental Section) to validate if omitting the surfactant in the medium formulation was indeed favourable for enzyme activity and productivity.
According to the model there should preferably not be any surfactant in the medium. The implication of the fact that the highest Lac activity and productivity are predicted by the model in the absence of Polysorbate 80 is on the one hand favourable, thus avoiding an additional component in the medium and directly decreases cost; furthermore, this could decrease downstream processing and potential contamination of crude filtrates as well. Nonetheless, verification experiments showed a discrepancy between model and experimental data, implying that the range of Polysorbate 80 concentrations examined may have been too broad. Consequently, a series of experiments was undertaken varying only the surfactant concentration in the region below the lowest concentration of 0.3 g L
−1. The results, which were obtained with optimum settings for
OFLac/MnP at
w = 0.5 (
Table 6), clearly indicate an extremely low optimal surfactant concentration, which was determined as 0.05 g L
−1. Accordingly, the following conclusion may be drawn. The model describes well the dependence of Lac activity and productivity within the examined broad range of concentrations examined; nonetheless it would be prudent to repeat the procedure, illustrated in the Experimental Section, for a narrow window of Polysorbate 80 concentrations in order to achieve better accuracy, especially as far as the decisive surfactant concentration is concerned. Thus, the differences between the measured and the predicted values in
Table 6 save for ([
EMnP])
max at
CP = 0 g L
−1 arise because of the broadly examined range of factors (glucose, ammonium tartrate, and Polysorbate 80 in
Table 1)—If parameters were estimated in a narrower range, the optimization would be more accurate; nonetheless, the overall accuracy in the broader range of concentrations would be poorer. Even so, the discrepancy between predicted and experimental laccase production does not confirm the low reliability for this enzyme production, even more the reliability of the predicted laccase production is very high for the studied broad range of nutrient concentrations; only for a more accurate optimization were the additional measurements conducted. In general, the good predictability of the model for Lac activity and productivity may be observed through relatively high values of
R2 (the latter indicates explained variability) and
Q2 (the latter indicates predicted variability) coefficients, which were 0.875 and 0.872 (
R2), and 0.608 and 0.623 (
Q2) for Lac activity and productivity, correspondingly. A poorer predictability of the model for MnP activity and productivity may be observed through lower values of
R2 and
Q2 parameters, which were 0.686 and 0.795 (
R2), and 0.339 and 0.468 (
Q2) for MnP activity and productivity, correspondingly. The objective function (used in the model), using which we aimed to optimize the production, is indeed extremely useful, but in point of fact implies good predictability of the model (e.g., high
Q2). The reported coefficients demonstrate this for Lac activity and productivity, but not for the case of MnP.
The second phase of the study continued with
C. subvermispora cultivation experiments on various immobilization supports using the optimized liquid medium (OLM). Beech wood cubes (BWCM) and pine wood cubes (PWCM) containing natural lignin as inducer and inert polyurethane foam cubes (PUFCM) were used to immobilize the culture growth and to control the immobilization effect in comparison to the combined effect of the other supports that act as carriers as well as inducers. The composition of the liquid media was prepared according to the optimum settings for
OFLac/MnP at
w = 0.5 obtained from the response surface methodology experiment. In addition, the OLM was used as a reference medium with free mycelium. The results are presented in
Figure 7. The maximal enzyme activities with free mycelium in the OLM without immobilization support were achieved after eight days, namely 108 U L
−1 for MnP and 19 U L
−1 for Lac. It is evident that the use of wood supports affected the enzyme activities. Beech wood and pine wood induced higher Lac activities. Maximal values using BWCM and PWCM, achieved after eight days of cultivation, were 150 U L
−1 and 135 U L
−1, respectively. In contrast, MnP activities using wood supports were lower than the results with only liquid medium OLM, namely 60 U L
−1 for BWCM and 20 U L
−1 for PWCM. Production of enzymes was the lowest under PUFCM cultivation conditions: MnP activity was only 40 U L
−1, while Lac activity was close to zero (2 U L
−1). In the literature it was reported that inert materials such as PUF affect fungal growth as an immobilization surface, but alone cannot increase enzyme synthesis [
16,
20]. In contrast, natural materials such as straw or wood with structural cell-wall components like cellulose, hemicelluloses and lignin form carbon sources available for fungal growth. In addition, wood contains small amounts of soluble sugars, lipids, peptides and starch as well as minerals and a wide range of extractives and volatiles. All these compounds vary with tree species like pine, beech or oak and therefore affect fungal growth and enzyme production in different ways [
27]. Hence, they can be utilized both as an immobilization surface and a natural inducer [
20,
21]. Present results are also in agreement with our previous investigation, where the synthesis of ‘tailor made’ enzyme composition, that is excess Lac or excess MnP, was proposed by variation of the carbon and nitrogen source in the medium and the use of immobilization supports of different types of wood [
21].
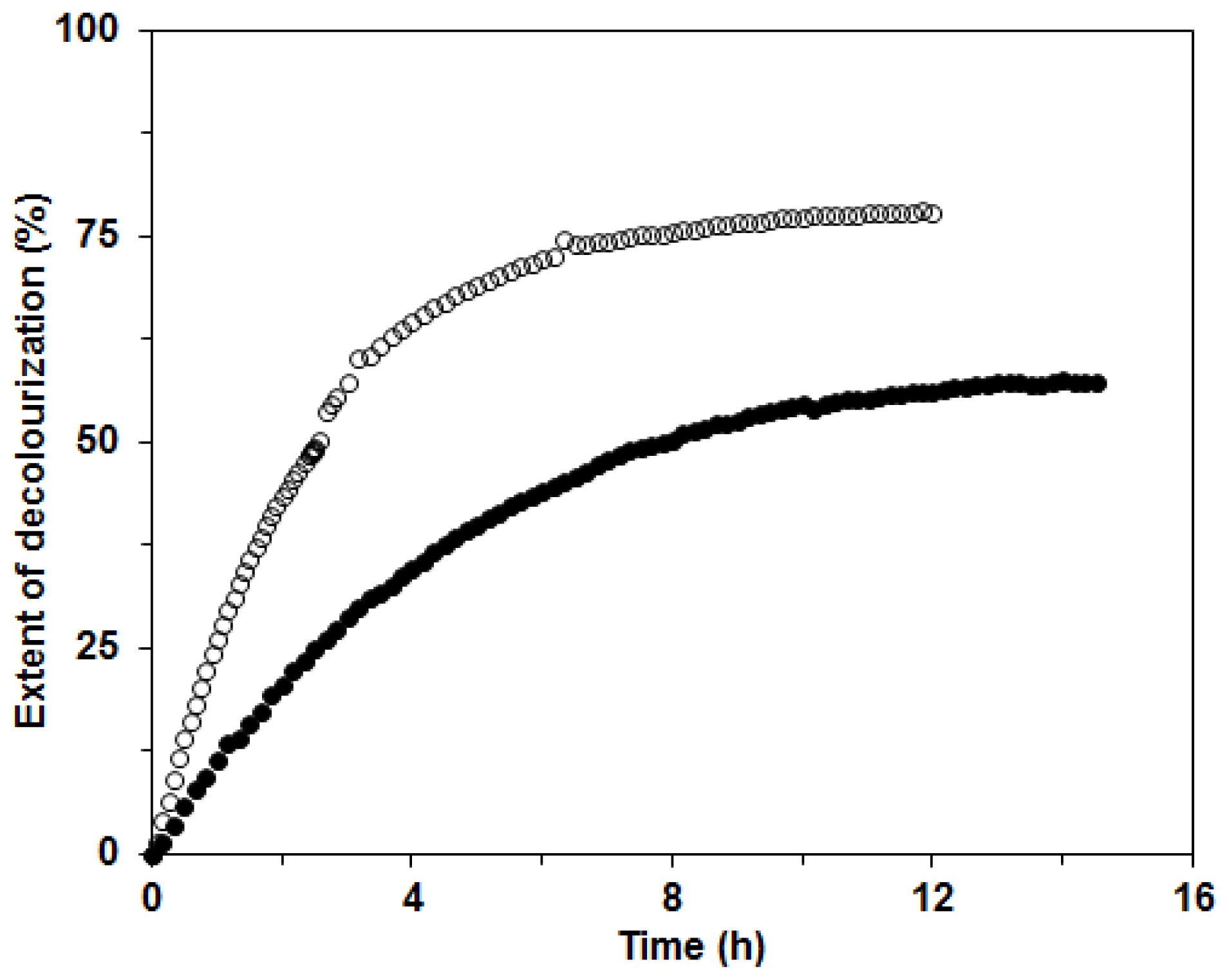
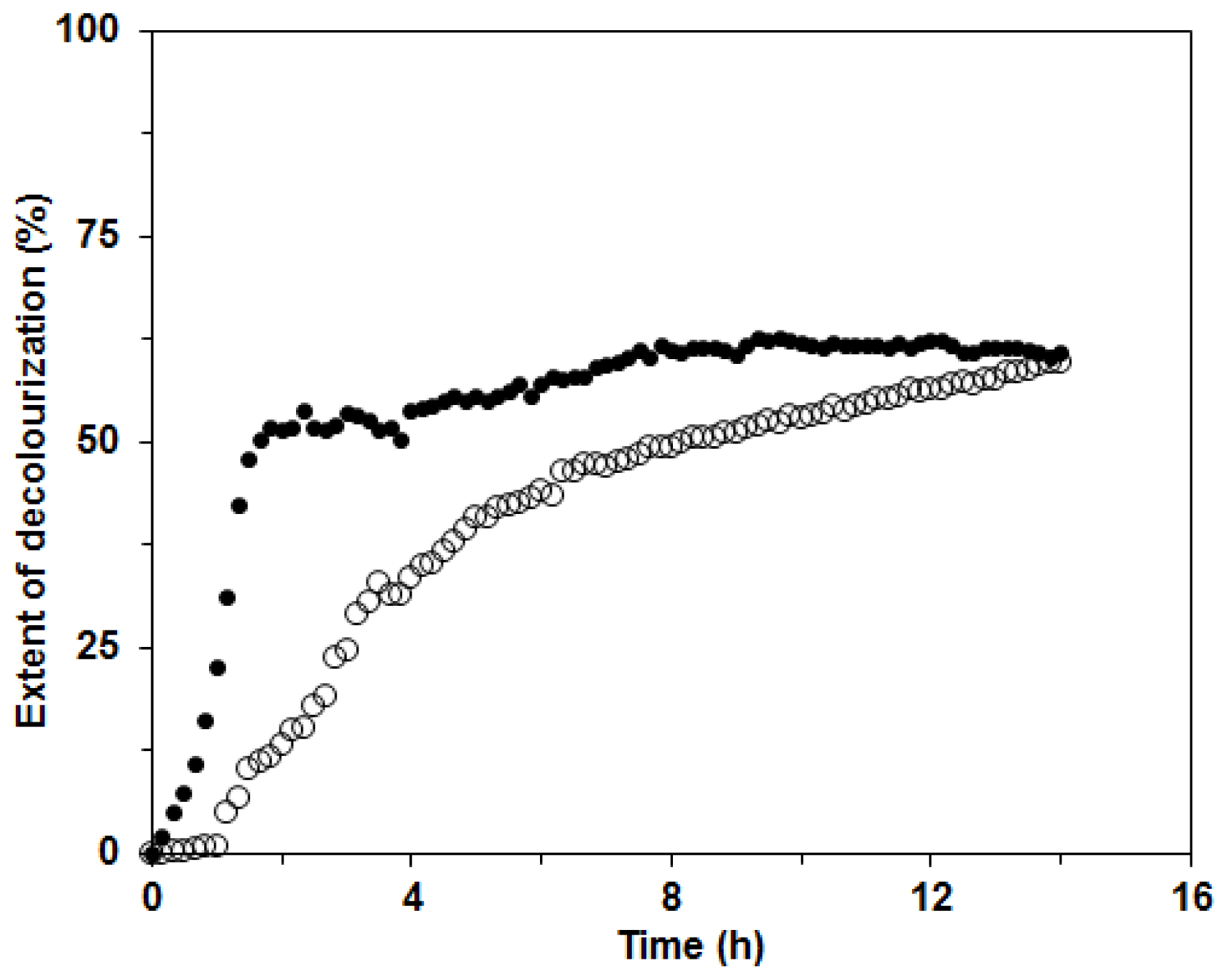
Further, the mixture of ligninolytic enzymes produced by the white rot fungus
C. subvermispora could be used in environmental applications, in the paper industry and also for the decolourization of synthetic dyes [
1,
4,
15,
17]. In our investigation, we tested the possible applicability of the ligninolytic enzyme mixture produced in BWCM and OLM for decolourization of the two structurally different dyes Remazol Brilliant Blue (RBBR) and Copper (II) phthalocyanine (CuP). The decolorization of RBBR was faster and more efficient than that of CuP with the enzyme mixture from BWCM (80% and 60% decolorization in approximately 8 and 12 h, respectively). We assumed that Lac played a crucial role in the decolorization of this dye (
Figure 8). Our results show that CuP is more difficult to degrade with Lac than RBBR. This effect was already documented during a study of the fungus
Dichomitus squalens [
20]. A similar result, that decolorization of anthraquinonic dyes is easier with laccases produced by white rot fungi, was also reported in the work by Liu
et al. [
28]. The decolorization of CuP was initially faster than that of RBBR, probably due to higher MnP activities in the OLM. When filtrates with lower Lac activities and higher MnP were used for decolorization studies, such as the filtrate from OLM in this study, the compensation and cooperation of these two enzymes in the decolorization process could be implied. These findings were suggested during dye degradation studies with Lac and MnP from
D. squalens [
29]. The extent of decolorization after 14 h was 60% for both enzyme mixtures in BWCM and OLM (
Figure 9). The results of our decolorization study with the
C. subvermispora ligninolytic enzyme system show the potential use of this fungus for environmental protection and bioremediation of pollutants. However, in the future during the enzymatic degradation studies, more attention should be paid to the analysis of the products appearing during enzyme degradation.