Phenolic Compounds from Halimodendron halodendron (Pall.) Voss and Their Antimicrobial and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
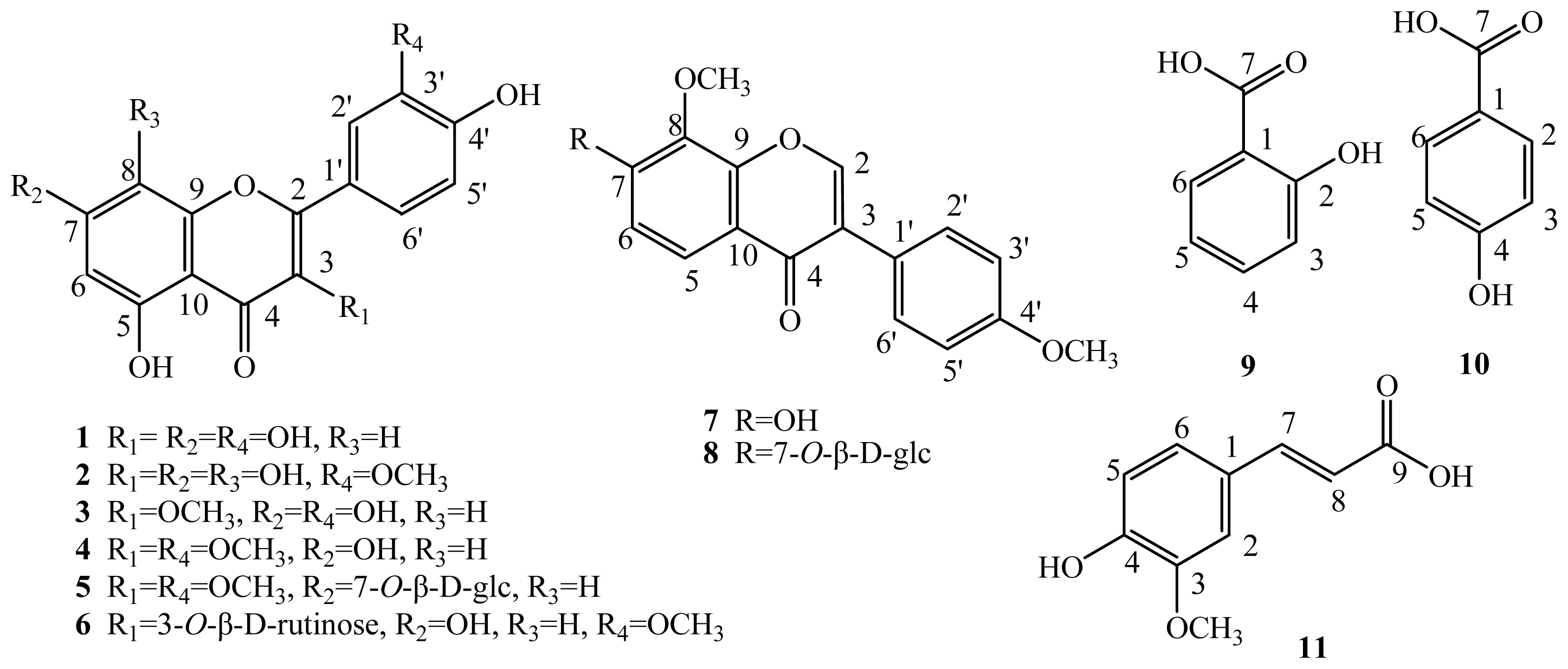
2.1. Elucidation of the Purified Phenolic Compounds
2.2. Antibacterial Activity
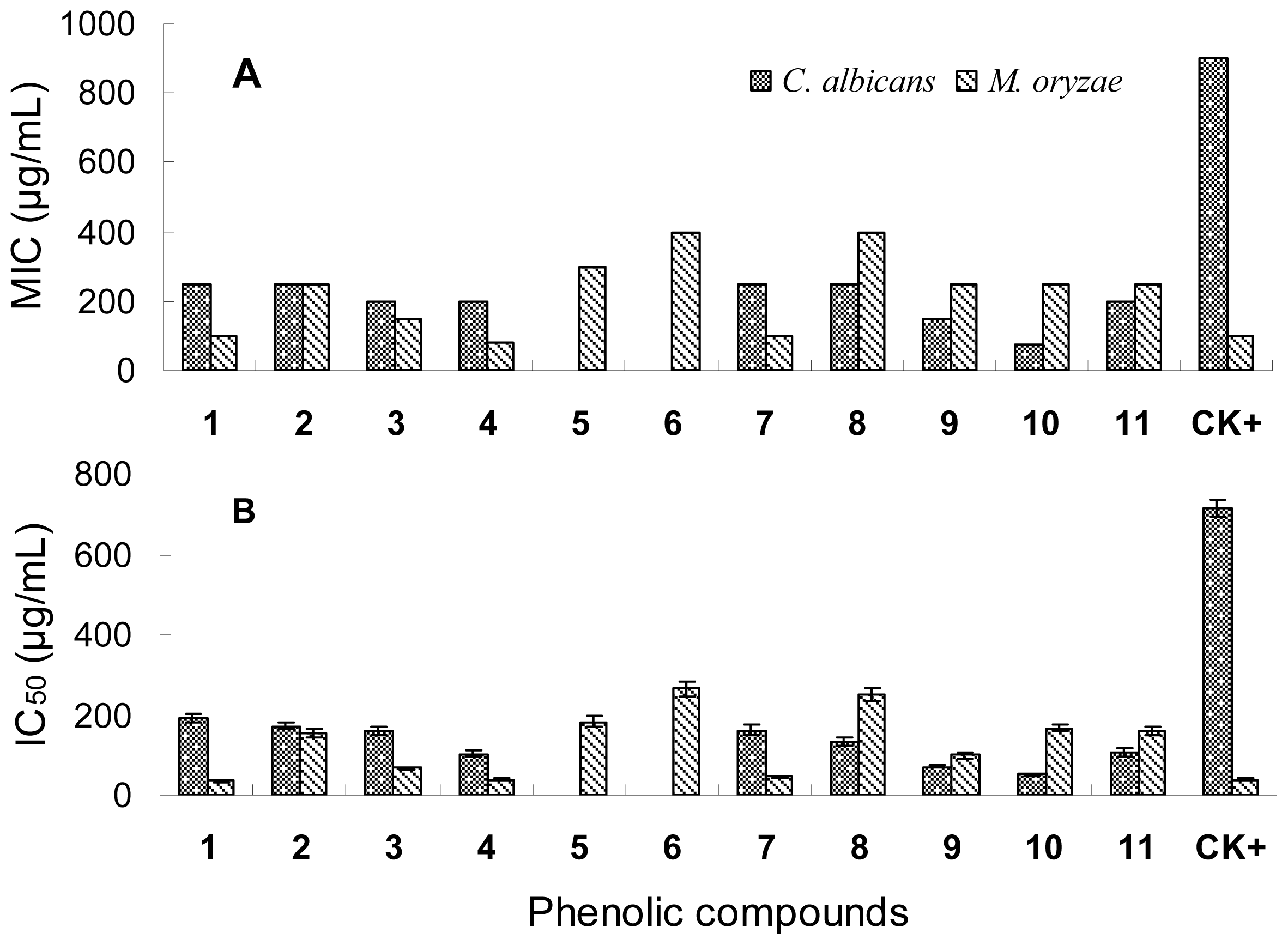
2.3. Antifungal Activity
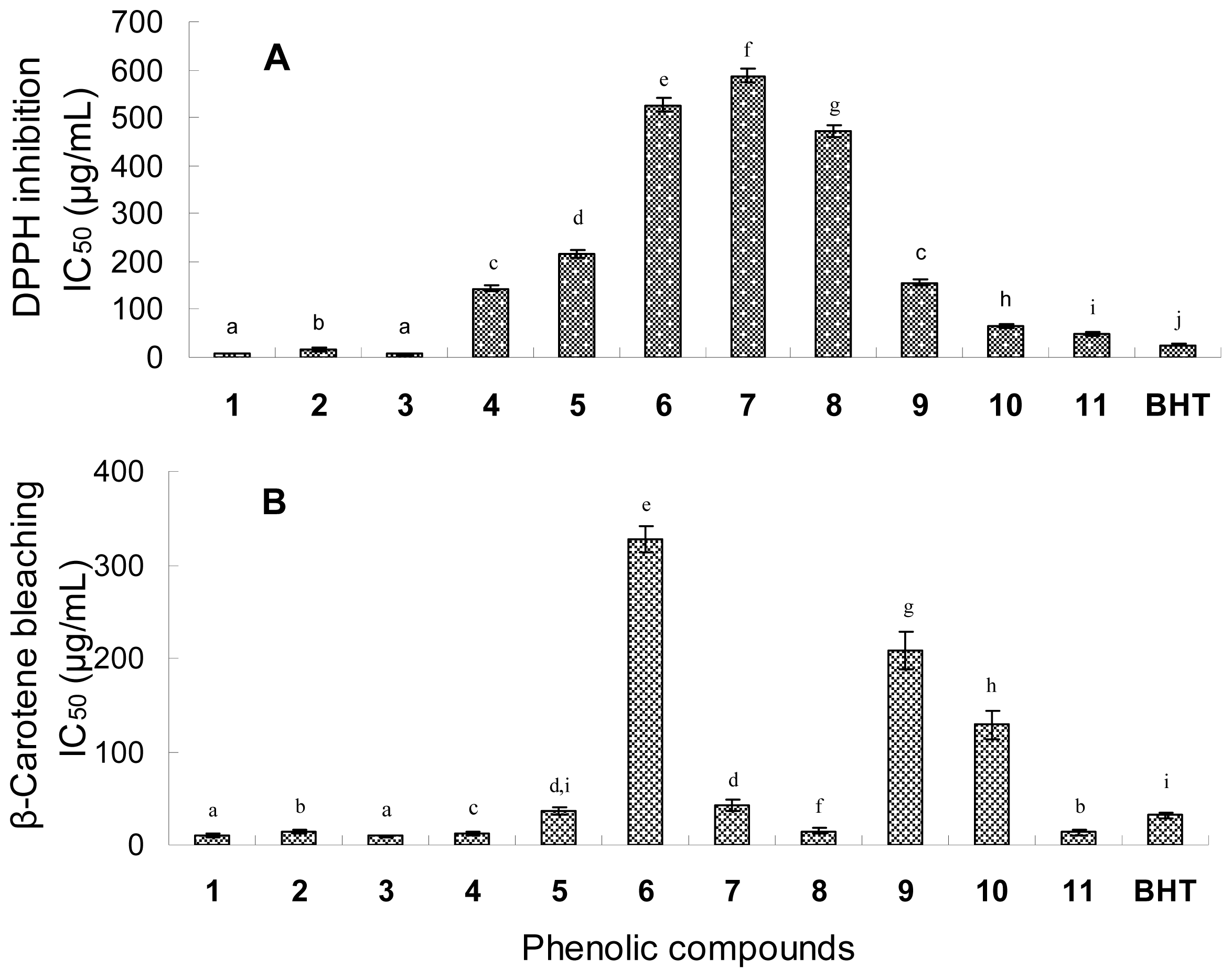
2.4. Antioxidant Activity
3. Experimental Section
3.1. Plant Materials
3.2. Solvents and Chemicals
3.3. General Analytical Methods
3.4. Extraction and Fractionation of the Compounds from Plant Materials
3.5. Physicochemical and Spectrometric Data of the Compounds
3.6. Antibacterial Activity Assay
3.7. Antifungal Activity Assay
3.8. Antioxidant Activity Assays
3.9. Statistical Analysis
4. Conclusions
Acknowledgements
References
- Moure, A.; Cruz, J.M.; Franco, D.; Dominguez, M.; Sineiro, J.; Dominguez, H.; Nunez, M.J.; Parajo, J.C. Natural antioxidants from residual sources. Food Chem 2001, 72, 145–171. [Google Scholar]
- Ames, B.N. Dietary carcinogens and anticarcinogens: Oxygen radical and degenerative diseases. Science 1983, 221, 1256–1264. [Google Scholar]
- Ito, N.; Fukushima, S.; Hagiwara, A. Carcinogenicity of butylated hydroxyanisole in F344 rats. J. Natl. Canc. Inst 1983, 70, 343–352. [Google Scholar]
- Ebrahimabadi, A.H.; Ebrahimabadi, E.H.; Djafari-Bidgoli, Z.; Kashi, F.J.; Mazoochi, A.; Batooli, H. Composition and antioxidant and antimicrobial activity of the essential oil and extracts of Stachys inflata Benth from Iran. Food Chem 2010, 119, 452–458. [Google Scholar]
- Ammar, R.B.; Bhouri, W.; Sghaier, M.B.; Boubaker, J.; Skandrani, I.; Neffati, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.-M.; Chekir-Ghedira, L. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternaus L. (Rhamnaceae): A structure-activity relationship study. Food Chem 2009, 116, 258–264. [Google Scholar]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar]
- Bailey, G.S.; Williams, D.E. Potential mechanisms for food related carcinogens and anti-carcinogens. Food Technol 1993, 47, 105–118. [Google Scholar]
- Zhou, L.; Li, D.; Wang, J.; Liu, Y.; Wu, J. Antibacterial phenolic compounds from the spines of Gleditsia sinensis Lam. Nat. Prod. Res 2007, 21, 283–291. [Google Scholar]
- Du, H.; Wang, Y.; Hao, X.; Li, C.; Peng, Y.; Wang, J.; Liu, H.; Zhou, L. Antimicrobial phenolic compounds from Anabasis aphylla. Nat. Prod. Commun 2009, 4, 385–388. [Google Scholar]
- Wang, J.; Liu, H.; Zhao, J.; Gao, H.; Zhou, L.; Liu, Z.; Chen, Y.; Sui, P. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxy benzaldehyde. Molecules 2010, 15, 5807–5817. [Google Scholar]
- Liverio, L.; Puglisi, P.P.; Morazzoni, P.; Bombardelli, E. Antimutagenic activity of procyanidins from Vitis vinifera. J. Stud. Med. Plants 1994, 65, 203–209. [Google Scholar]
- Ueda, H.; Yamazaki, C.; Yamazaki, M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol. Pharm. Bull 2002, 25, 1197–1202. [Google Scholar]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar]
- Zhang, J.; Shen, Q.; Lu, J.C.; Li, J.Y.; Liu, W.Y.; Yang, J.J.; Li, J.; Xiao, K. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem 2010, 119, 1491–1496. [Google Scholar]
- Fu, K.J. Flora Reipublicae Popularis Sinicae, Tomus; Volume 42, 1, Science Press: Beijing, China, 1993. [Google Scholar]
- Zhang, Q.; Chen, Y.; Li, J.; He, S.; Lin, Y. The assessment and development of wild ligneous feed plants in Xinjiang. Pratacult. Sci 1996, 13, 1–4. [Google Scholar]
- Wang, J.; Gao, H.; Zhao, J.; Wang, Q.; Zhou, L.; Han, J.; Yu, Z.; Yang, F. Preparative separation of phenolic compounds from Halimodendron halodendron by high-speed counter-current chromatography. Molecules 2010, 15, 5998–6007. [Google Scholar]
- Wang, J.; Liu, H.; Gao, H.; Zhao, J.; Zhou, L.; Han, J.; Yu, Z.; Yang, F. Antimicrobial and antioxidant activities of the flower essential oil of Halimodendron halodendron. Nat. Prod. Commun 2011, 6, 1749–1753. [Google Scholar]
- Barat, I. Chromosome karyotype analysis of Halimondendron holodendron. Chin. J. Grassland 1996, 18, 23–25. [Google Scholar]
- Zhang, M.; Fritsch, P.W.; Cruz, B.C. Phylogeny of Caragana (Fabaceae) based on DNA sequence data from rbcL, trnS-trnG, and ITS. Mol. Phylogenet. Evol 2009, 50, 547–559. [Google Scholar]
- Zhu, Z.J.; Pan, R.; Si, J.Y.; Fu, Y.; Huang, Q.Q. Study on the chemical constituents of Bupleurum bicaule Helm. Nat. Prod. Res. Dev 2008, 20, 833–835. [Google Scholar]
- Shi, J.; Chen, B.; Sun, Z.H.; Hu, C.Q. Studies on flavonoid constituents of Caragana intermedia. Acta Pharm. Sin 2003, 38, 599–602. [Google Scholar]
- Li, L.; Henry, G.E.; Seeram, N.P. Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. J. Agric. Food Chem 2009, 57, 7282–7287. [Google Scholar]
- Al-Dabbas, M.M.; Kitahara, K.; Suganuma, T.; Hashimoto, F.; Tadera, K. Antioxidant and α-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci. Biotechnol. Biochem 2006, 70, 2178–2184. [Google Scholar]
- Brandao, M.G.; Nery, C.G.; Mamao, M.A.; Krettli, A.U. Two methoxylated flavone glycosides from Bidens pilosa. Phytochemistry 1998, 48, 397–399. [Google Scholar]
- Tang, Y.P.; Wang, Y.; Long, F.C.; Li, Y.F.; Wang, J.H. Flavonoid glycosides from leaves of Ginkgo biloba. Acta Pharm. Sin 2000, 35, 363–366. [Google Scholar]
- Shirataki, Y.; Matsuoka, S.; Komatsu, M.; Ohyama, M.; Tanaka, T.; Iinuma, M. Studies on the constituents of Sophora species. Phytochemistry 1999, 50, 695–701. [Google Scholar]
- Rukachaisirikul, V.; Sukpondma, Y.; Jansakul, C.; Taylor, W.C. Isoflavone glycosides from Derris scandens. Phytochemistry 2002, 60, 827–834. [Google Scholar]
- Niu, X.M.; Li, S.H.; Na, Z.; Mei, S.X.; Zhao, Q.S.; Sun, H.D. Studies on chemical constituents of Isodon eriocalyx var. laxiflora. Chin. Tradit. Herb. Drugs 2003, 34, 300–303. [Google Scholar]
- Chung, H.S.; Shin, J.C. Characterization of antioxidant alkaloids and phenolic acids from anthocyanin-pigmented rice (Oryza sativa cv. Heugjinjubyeo). Food Chem 2007, 104, 1670–1677. [Google Scholar]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol 1996, 50, 27–34. [Google Scholar]
- Kaul, T.N.; Middleton, E.J.; Ogra, P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol 1985, 15, 71–79. [Google Scholar]
- Osawa, K.; Yasuda, H.; Maruyama, T.; Morita, H.; Takeya, K.; Itokawa, H. Isoflavanones from the heartwood of Swartzia polyphylla and their antibacterial activity against cariogenic bacteria. Chem. Pharm. Bull 1992, 40, 2970–2974. [Google Scholar]
- Huang, D.J.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem 2005, 53, 1841–1856. [Google Scholar]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radical Res 2002, 36, 1219–1227. [Google Scholar]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem 2007, 105, 940–949. [Google Scholar]
- Uriarte-Pueyo, I.; Calvo, M.I. Structure-activity relationships of acetylated flavone glycosides from Galeopsis ladanum L. (Lamiaceae). Food Chem 2010, 120, 679–683. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem 2008, 56, 8404–8411. [Google Scholar]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Radical chemistry of flavonoid antioxidants. Adv. Exp. Med. Biol 1990, 264, 165–170. [Google Scholar]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Method. Enzymol 1990, 186, 343–355. [Google Scholar]
- Benavente-Garcia, O.; Castillo, J.; Marin, F.R.; Ortuno, A.; Del Rio, J.A. Uses and properties of citrus flavonoids. J. Agric. Food Chem 1997, 45, 4505–4515. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod 2000, 63, 1035–1042. [Google Scholar]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoid is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar]
- Langfied, R.D.; Scarano, F.J.; Heitzman, M.E.; Kondo, M.; Hammond, G.B.; Neto, C.C. Use of a modified microplate bioassay method to investigate antibacterial activity in the Peruvian medicinal plant Peperomia galioides. J. Ethnopharmacol 2004, 94, 279–281. [Google Scholar]
- Abe, K.; Matsuki, N. Measurement of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci. Res 2000, 38, 325–329. [Google Scholar]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool 1998, 33, 339–347. [Google Scholar]
- Liu, H.; Wang, J.; Zhao, J.; Lu, S.; Wang, J.; Jiang, W.; Ma, Z.; Zhou, L. Isoquinoline alkaloids from Macleaya cordata active against plant microbial pathogens. Nat. Prod. Commun 2009, 4, 1557–1560. [Google Scholar]
- Ono, M.; Oda, E.; Tanaka, T.; Iida, Y.; Yamasaki, T.; Masuoka, C.; Ikeda, T.; Nohara, T. DPPH radical-scavenging effect on some constituents from the aerial parts of Lippia triphylla. J. Nat. Med 2008, 62, 101–106. [Google Scholar]

© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Lou, J.; Luo, C.; Zhou, L.; Wang, M.; Wang, L. Phenolic Compounds from Halimodendron halodendron (Pall.) Voss and Their Antimicrobial and Antioxidant Activities. Int. J. Mol. Sci. 2012, 13, 11349-11364. https://doi.org/10.3390/ijms130911349
Wang J, Lou J, Luo C, Zhou L, Wang M, Wang L. Phenolic Compounds from Halimodendron halodendron (Pall.) Voss and Their Antimicrobial and Antioxidant Activities. International Journal of Molecular Sciences. 2012; 13(9):11349-11364. https://doi.org/10.3390/ijms130911349
Chicago/Turabian StyleWang, Jihua, Jingfeng Lou, Chao Luo, Ligang Zhou, Mingan Wang, and Lan Wang. 2012. "Phenolic Compounds from Halimodendron halodendron (Pall.) Voss and Their Antimicrobial and Antioxidant Activities" International Journal of Molecular Sciences 13, no. 9: 11349-11364. https://doi.org/10.3390/ijms130911349
APA StyleWang, J., Lou, J., Luo, C., Zhou, L., Wang, M., & Wang, L. (2012). Phenolic Compounds from Halimodendron halodendron (Pall.) Voss and Their Antimicrobial and Antioxidant Activities. International Journal of Molecular Sciences, 13(9), 11349-11364. https://doi.org/10.3390/ijms130911349





