An Inverse Relationship Links Temperature and Substrate Apparent Affinity in the Ion-Coupled Cotransporters rGAT1 and KAAT1
Abstract
:1. Introduction
2. Results and Discussion
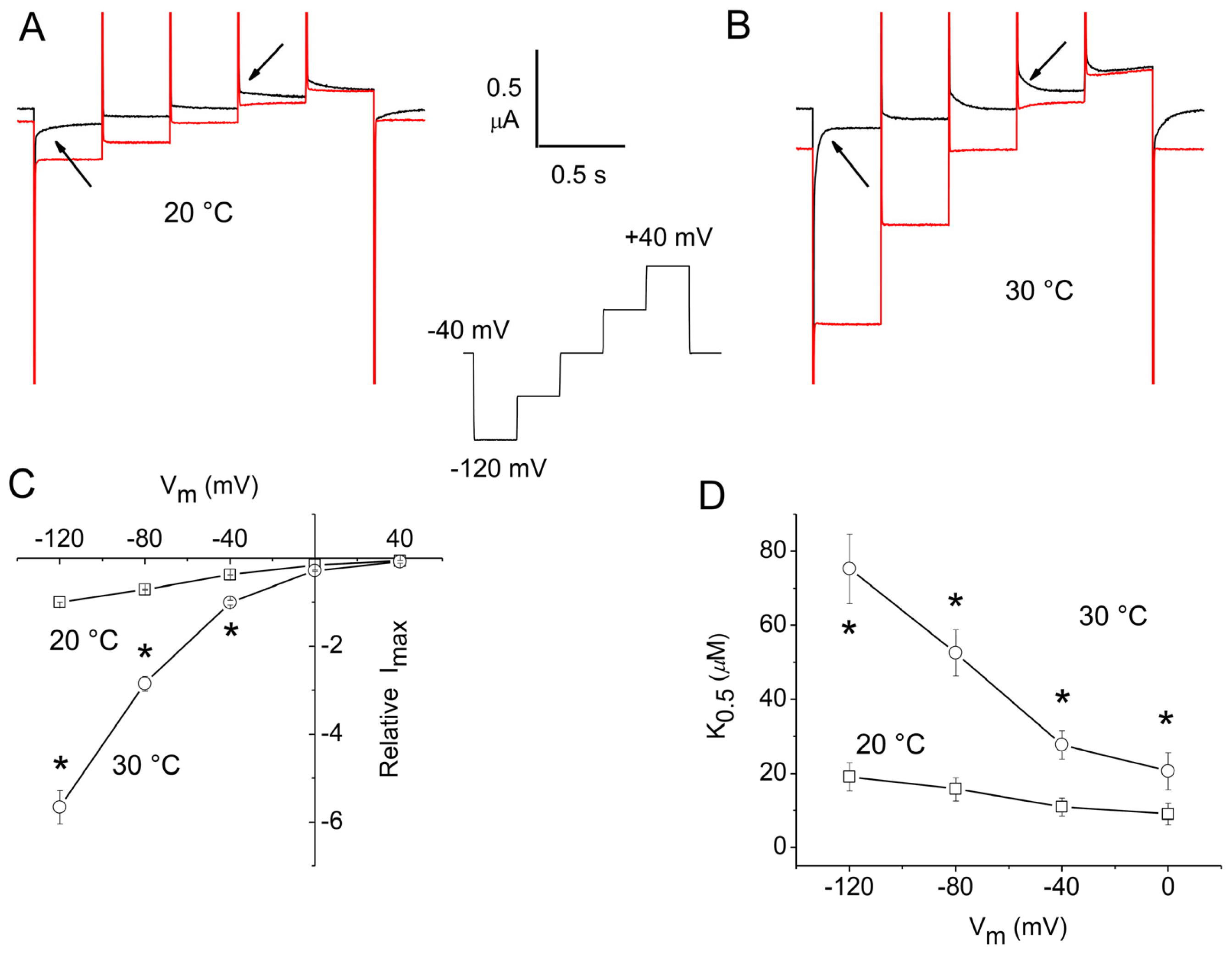
2.1. Apparent Affinity Changes Induced by Temperature in rGAT1
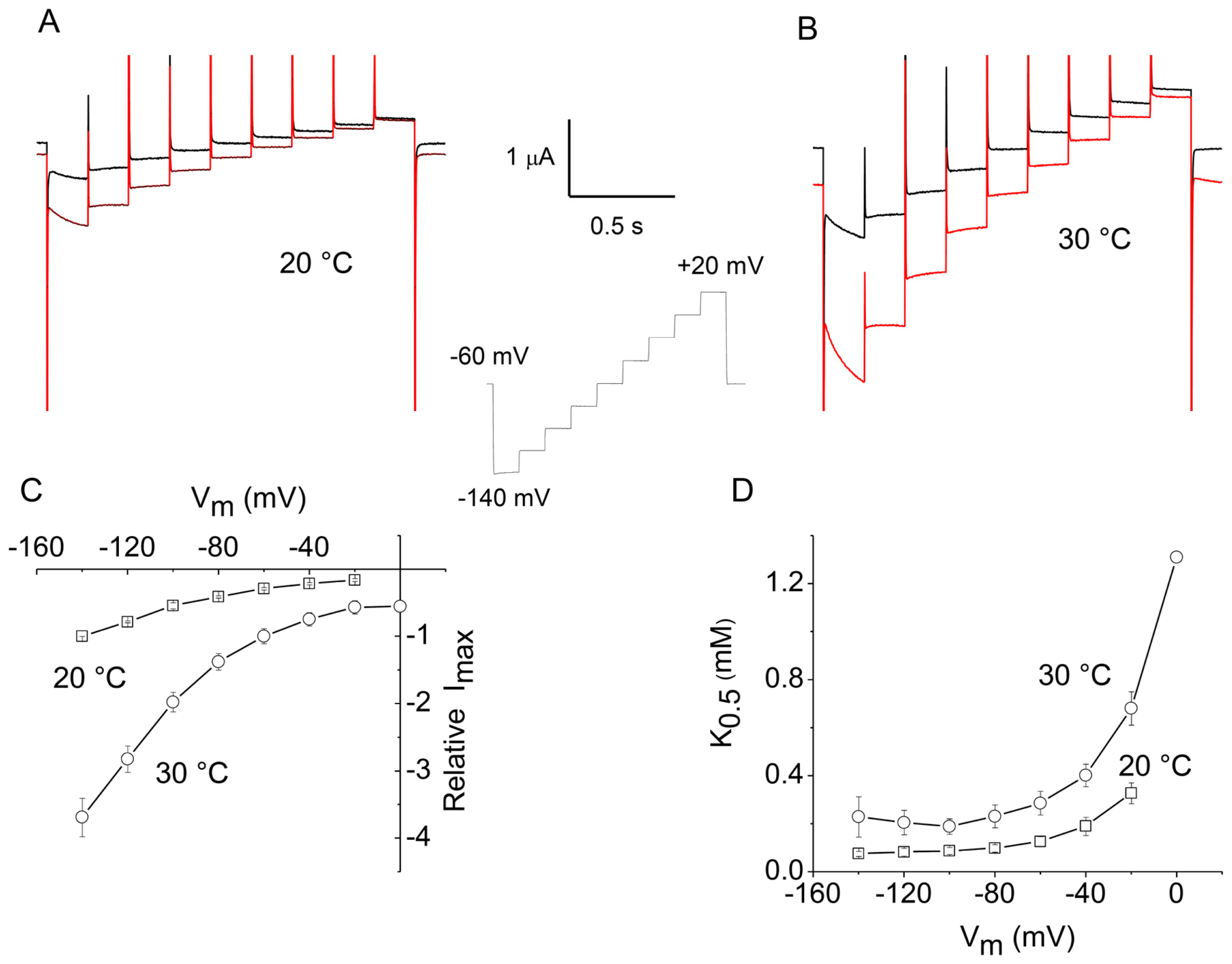
2.2. Apparent Affinity Changes Induced by Temperature in KAAT1
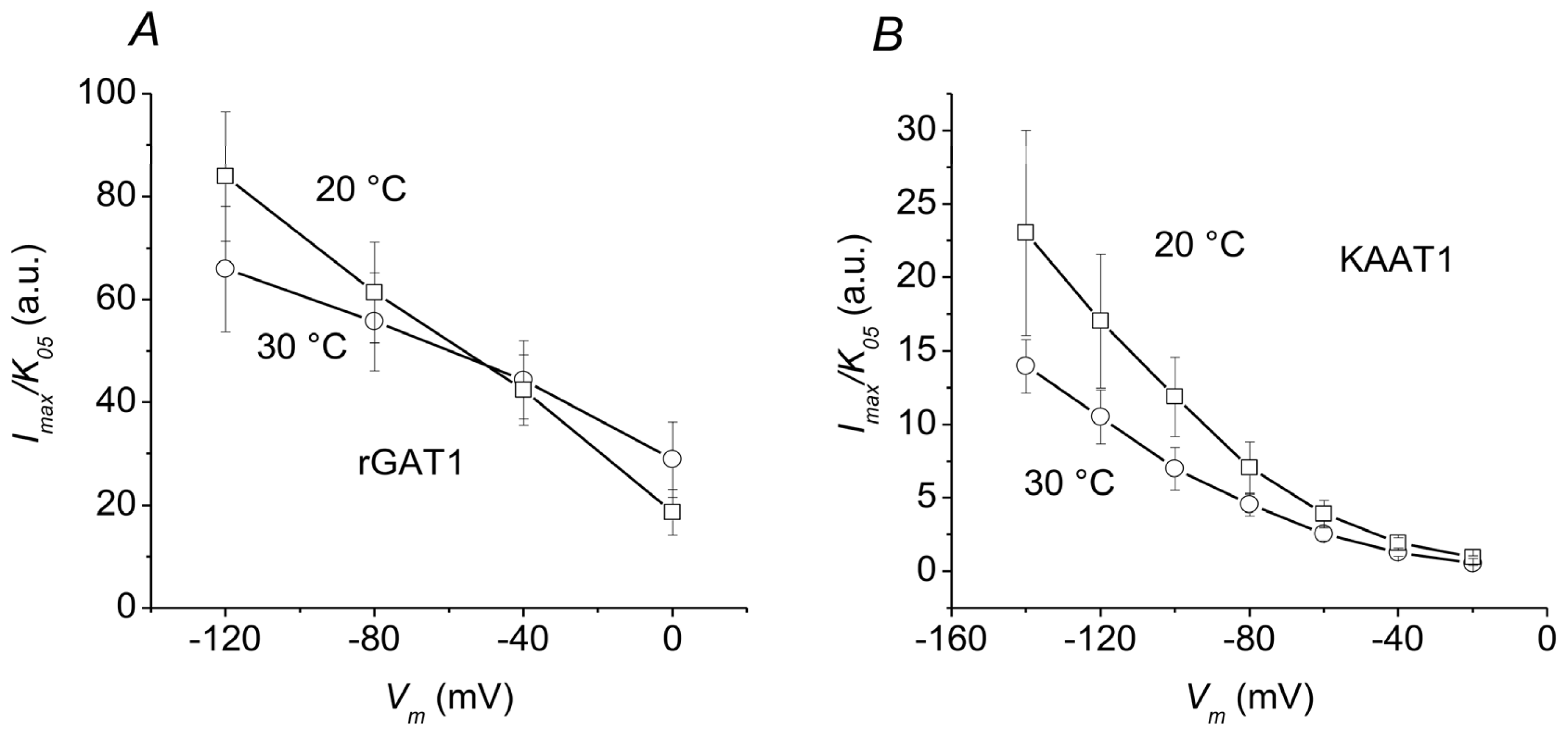
2.3. Overall Efficiency
3. Experimental Section
3.1. Oocyte Expression
3.2. Electrophysiology and Data Analysis
3.3. Temperature Control
3.4. Solutions
4. Conclusions
Acknowledgments
References
- Parent, L.; Supplisson, S.; Loo, D.D.F.; Wright, E.M. Electrogenic properties of the cloned Na+/glucose transporter: I. Voltage-clamp studies. J. Membr. Biol 1992, 125, 49–62. [Google Scholar]
- Forster, I.C.; Virkki, L.; Bossi, E.; Murer, H.; Biber, J. Electrogenic kinetics of a mammalian intestinal type IIb Na+/Pi cotransporter. J. Membr. Biol 2006, 212, 177–190. [Google Scholar]
- Pérez-Siles, G.; Núñez, E.; Morreale, A.; Jiménez, E.; Leo-Macías, A.; Pita, G.; Cherubino, F.; Sangaletti, R.; Bossi, E.; Ortìz, Á.; et al. An aspartate residue in the external vestibule of glycine transporter 2 (GLYT2) controls cation access and transport coupling. Biochem. J. 2012, 442, 323–334. [Google Scholar]
- Mager, S.; Kleinberger-Doron, N.; Keshet, G.I.; Davidson, N.; Kanner, B.I.; Lester, H.A. Ion binding and permeation at the GABA transporter GAT1. J. Neurosci 1996, 16, 5405–5414. [Google Scholar]
- Hazama, A.; Loo, D.D.F.; Wright, E.M. Presteady-state currents of the rabbit Na+/glucose cotransporter (SGLT1). J. Membr. Biol 1997, 155, 175–186. [Google Scholar]
- Binda, F.; Bossi, E.; Giovannardi, S.; Forlani, G.; Peres, A. Temperature effects on the presteady-state and transport-associated currents of GABA cotransporter rGAT1. FEBS Lett 2002, 512, 303–307. [Google Scholar]
- Hilgemann, D.W.; Lu, C.-C. GAT1 (GABA:Na+:Cl−) cotransport function. Database reconstruction with an alternating access model. J. Gen. Physiol 1999, 114, 459–475. [Google Scholar]
- Beckman, M.L.; Quick, M.W. Substrates and temperature differentiate ion flux from serotonin flux in a serotonin transporter. Neuropharmacology 2001, 40, 526–535. [Google Scholar]
- Bacconi, A.; Ravera, S.; Virkki, L.V.; Murer, H.; Forster, I.C. Temperature dependence of steady-state and presteady-state kinetics of a type IIb Na+/Pi cotransporter. J. Membr. Biol 2007, 215, 81–92. [Google Scholar]
- Mager, S.; Cao, Y.; Lester, H.A. Measurement of transient currents from neurotransmitter transporters expressed in Xenopus oocytes. Methods Enzymol 1998, 296, 551–566. [Google Scholar]
- Bossi, E.; Centinaio, E.; Castagna, M.; Giovannardi, S.; Vincenti, S.; Sacchi, V.F.; Peres, A. Ion binding and permeation through the lepidopteran amino acid transporter KAAT1 expressed in Xenopus oocytes. J. Physiol 1999, 515, 729–742. [Google Scholar]
- Bossi, E.; Cherubino, F.; Margheritis, E.; Oyadeyi, A.S.; Vollero, A.; Peres, A. Temperature effects on the kinetic properties of the rabbit intestinal oligopeptide cotransporter PepT1. Pflugers Arch 2012, 464, 183–191. [Google Scholar]
- Fesce, R.; Giovannardi, S.; Binda, F.; Bossi, E.; Peres, A. The relation between charge movement and transport-associated currents in the GABA cotransporter rGAT1. J. Physiol 2002, 545, 739–750. [Google Scholar]
- Peres, A.; Giovannardi, S.; Bossi, E.; Fesce, R. Electrophysiological insights on the mechanism of ion-coupled cotransporters. News Physiol. Sci 2004, 19, 80–84. [Google Scholar]
- Giovannardi, S.; Fesce, R.; Bossi, E.; Binda, F.; Peres, A. Cl− effects on the function of the GABA cotransporter rGAT1 preserve the mutual relation between transient and transport currents. CMLS 2003, 60, 550–556. [Google Scholar]
- Soragna, A.; Bossi, E.; Giovannardi, S.; Pisani, R.; Peres, A. Relations between substrate affinities and charge equilibration rates in the GABA cotransporter rGAT1. J. Physiol 2005, 562, 333–345. [Google Scholar]
- Castagna, M.; Shayakul, C.; Trotti, D.; Sacchi, V.F.; Harvey, W.R.; Hediger, M.A. Cloning and characterization of a potassium-coupled amino acid transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 5395–5400. [Google Scholar]
- Castagna, M.; Bossi, E.; Sacchi, V.F. Molecular physiology of the insect K-activated amino acid transporter 1 (KAAT1) and cation-anion activated amino acid transporter/channel 1 (CAATCH1) in the light of the structure of the homologous protein LeuT. Ins. Mol. Biol 2009, 18, 265–279. [Google Scholar]
- Mager, S.; Naeve, J.; Quick, M.; Labarca, C.; Davidson, N.; Lester, H.A. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron 1993, 10, 177–188. [Google Scholar]
- Forlani, G.; Bossi, E.; Ghirardelli, R.; Giovannardi, S.; Binda, F.; Bonadiman, L.; Ielmini, L.; Peres, A. Mutation K448E in the external loop 5 of rGAT1 transporter induces pH sensitivity and altered substrates interactions. J. Physiol 2001, 536, 479–494. [Google Scholar]
- Boudko, D.Y.; Kohn, A.B.; Meleshkevitch, E.A.; Dasher, M.K.; Seron, T.J.; Stevens, B.R.; Harvey, W.R. Ancestry and progeny of nutrient amino acid transporters. Proc. Natl. Acad. Sci. USA 2005, 102, 1360–1365. [Google Scholar]
- Camargo, S.M.R.; Makrides, V.; Virkki, L.V.; Forster, I.C.; Verrey, F. Steady-state kinetic characterization of the mouse B0AT1 sodium-dependent neutral amino acid transporter. Pflugers Arch 2005, 451, 338–348. [Google Scholar]
- Soragna, A.; Mari, S.; Peres, A.; Pisani, R.; Castagna, M.; Sacchi, V.F.; Bossi, E. Structural domains involved in substrate selectivity in two neutral amino acid transporters. Am. J. Physiol. Cell Physiol 2004, 287, C754–C761. [Google Scholar]
- Miszner, A.; Peres, A.; Castagna, M.; Betté, S.; Giovannardi, S.; Cherubino, F.; Bossi, E. Structural and functional basis of amino acid specificity in the invertebrate cotransporter KAAT1. J. Physiol 2007, 581, 899–913. [Google Scholar]
- Gonzales, A.L.; Lee, W.; Spencer, S.R.; Oropeza, R.A.; Chapman, J.V.; Ku, J.Y.; Eskandari, S. Turnover rate of the gamma-aminobutyric acid transporter GAT1. J. Membr. Biol 2007, 220, 33–51. [Google Scholar]
- Stryer, L. Biochemistry, 3rd. Ed ed; W.H. Freeman and Company: New York, NY, USA; p. 1988.
- Dow, J.A.T.; Peacock, J.M. Microelectrode evidence for the electrical isolation of goblet cell cavities in Manduca sexta middle midgut. J. Exp. Biol 1989, 143, 101–114. [Google Scholar]
- Bossi, E.; Fabbrini, M.S.; Ceriotti, A. Exogenous protein expression in Xenopus Laevis oocyte, basic procedure. Methods Mol. Biol 2007, 375, 107–131. [Google Scholar]
- Zeuthen, T.; MacAulay, N. Cotransport of water by Na+-K+-2Cl− cotransporters expressed in Xenopus oocytes: NKCC1 versus NKCC2. J. Physiol 2012, 590, 1139–1154. [Google Scholar]
- De Oliveira, A.M.; Shoemaker, H.; Segonzac, A.; Langer, S.Z. Differences in the temperature dependence of drug interaction with the noradrenaline and serotonin transporters. Neuropharmacology 1989, 28, 823–828. [Google Scholar]
- Sala-Rabanal, M.; Loo, D.D.F.; Hirayama, B.A.; Turk, E.; Wright, E.M. Molecular interactions between dipeptides, drugs and the human intestinal H+/oligopeptide cotransporter, hPEPT1. J. Physiol 2006, 574, 149–166. [Google Scholar]
- Renna, M.D.; Sangaletti, R.; Bossi, E.; Cherubino, F.; Kottra, G.; Peres, A. Unified modeling of the mammalian and fish proton-dependent oligopeptide transporter PepT1. Channels 2011, 5, 89–99. [Google Scholar]
- Nussberger, S.; Steel, A.; Trotti, D.; Romero, M.F.; Boron, W.F.; Hediger, M.A. Symmetry of H+ binding to the intra- and extracellular side of the H+-coupled oligopeptide cotransporter PepT1. J. Biol. Chem 1997, 272, 7777–7785. [Google Scholar]
- Richerson, G.B.; Wu, Y. Dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J. Neurophysiol 2003, 90, 1363–1374. [Google Scholar]
- Richerson, G.B.; Wu, Y. Role of the GABA transporter in epilepsy. Adv. Exp. Med. Biol 2004, 548, 76–91. [Google Scholar]
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peres, A.; Vollero, A.; Margheritis, E.; D'Antoni, F.; Bossi, E. An Inverse Relationship Links Temperature and Substrate Apparent Affinity in the Ion-Coupled Cotransporters rGAT1 and KAAT1. Int. J. Mol. Sci. 2012, 13, 15565-15574. https://doi.org/10.3390/ijms131215565
Peres A, Vollero A, Margheritis E, D'Antoni F, Bossi E. An Inverse Relationship Links Temperature and Substrate Apparent Affinity in the Ion-Coupled Cotransporters rGAT1 and KAAT1. International Journal of Molecular Sciences. 2012; 13(12):15565-15574. https://doi.org/10.3390/ijms131215565
Chicago/Turabian StylePeres, Antonio, Alessandra Vollero, Eleonora Margheritis, Francesca D'Antoni, and Elena Bossi. 2012. "An Inverse Relationship Links Temperature and Substrate Apparent Affinity in the Ion-Coupled Cotransporters rGAT1 and KAAT1" International Journal of Molecular Sciences 13, no. 12: 15565-15574. https://doi.org/10.3390/ijms131215565



