Chemical Composition, Starch Digestibility and Antioxidant Capacity of Tortilla Made with a Blend of Quality Protein Maize and Black Bean
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
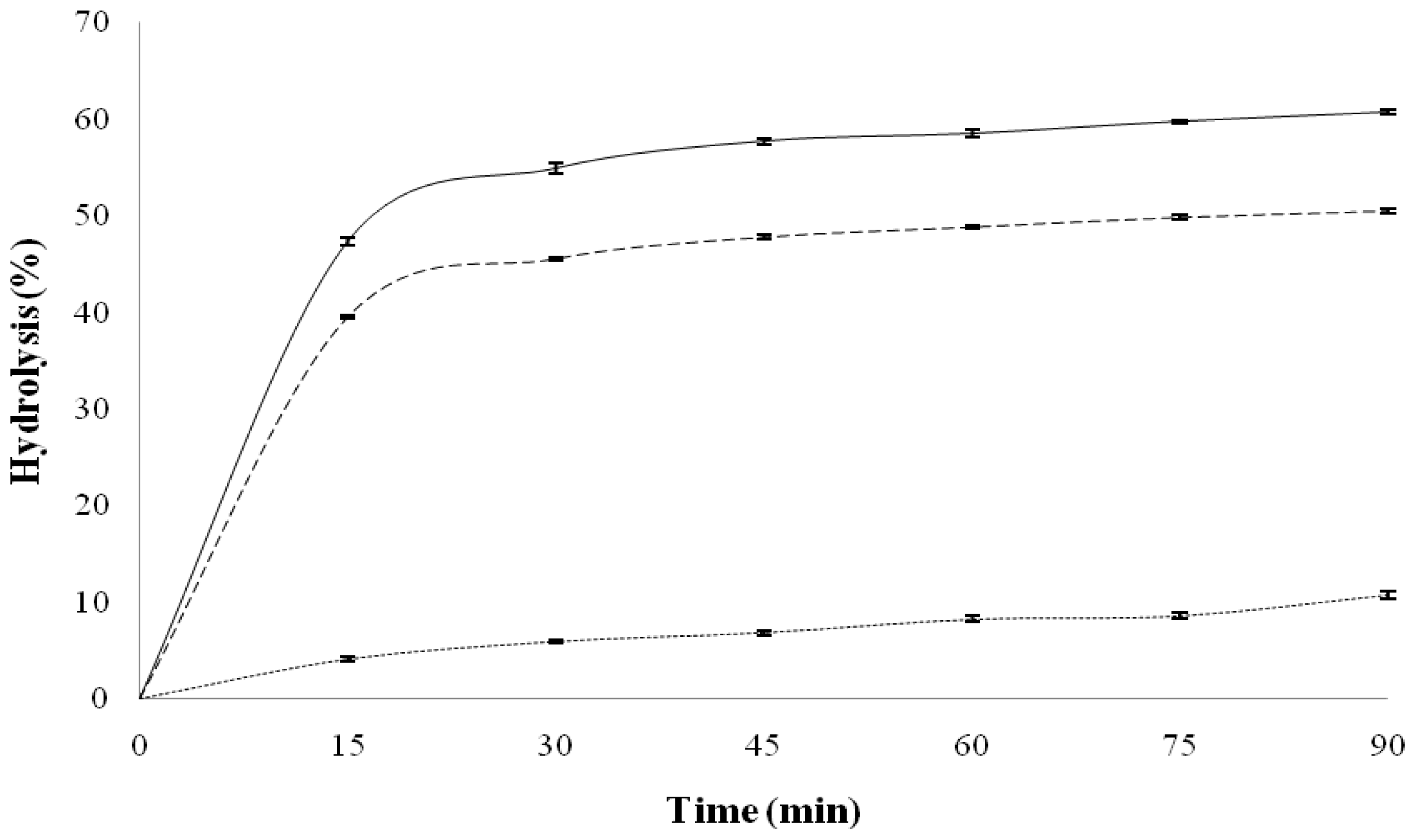
2.2. Starch Digestibility
2.3. In Vitro Kinetic of Starch Digestion
2.4. Polyphenols Content
2.5. Antioxidant Capacity
3. Experimental Section
3.1. Sample Preparation
3.2. Chemical Analysis
3.3. Total Starch
3.4. Resistant Starch
3.5. In Vitro Kinetic of Starch Digestion
3.6. Determination of Polyphenols Content
3.6.1. Extractable Polyphenols
3.6.2. Non Extractable Polyphenols
3.6.2.1. Proanthocyanidins
3.6.2.2. Hydrolysable Polyphenols
3.7. Free Radical-Scavenging Assay (ABTS)
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Paredes-López, O.; Saharopulos-Paredes, M.E. A review of tortilla production technology. Bakers Dig 1983, 13, 16–25. [Google Scholar]
- Robles, R.R.; Murray, E.D.; Paredes-López, O. Physicochemical changes of maize starch during the lime-heat treatment for tortilla making. Int. J. Food Sci. Technol 1988, 23, 91–98. [Google Scholar]
- Amaya-Guerra, C.A.; Alanis-Guzman, M.G.; Serna-Saldívar, S.O. Effects of soybean fortification on protein quality of tortilla-based diets produced from regular and quality protein maize. Plant Foods Hum. Nutr 2004, 59, 45–50. [Google Scholar]
- Serna-Saldívar, S.O.; Rooney, L.W. Quality protein maize processing and perspectives for industrial utilization. Proceedings of the International Symposium on Quality Protein Maize, Sete Lagoas, MG, Brazil, 1–3 December 1994.
- Sathe, S.R.; Rangnekar, P.D.; Deshpande, S.S.; Salunkhe, D.K. Isolation and partial characterization of black gram (Phaseolus mungo L.) starch. J. Food Sci 1982, 47, 1524–1538. [Google Scholar]
- Bourges, R.H. Las leguminosas en la alimentación. II Parte. Cuad. Nutr 1987, 10, 22–30. [Google Scholar]
- Reyes-Moreno, C.; Paredes-López, O. Hard to cook phenomenon in common beans—A review. Crit. Rev. Food Sci. Nutr 1993, 33, 227–286. [Google Scholar]
- Paredes-López, O.; Guzmán-Maldonado, S.H.; Serna-Saldívar, S.O. Los alimentos Mágicos de las Culturas Indígenas de México-El Caso de la Tortilla; Colegio de Sinaloa: Culiacán, Sinaloa, México, 2000. [Google Scholar]
- Rosado, J.L.; López, P.; Morales, M.; Muñoz, E.; Allen, L.H. Bioavailability of energy, nitrogen, fat, zinc, iron and calcium from rural and urban Mexican diets. Br. J. Nutr 1992, 68, 45–58. [Google Scholar]
- Rehman, Z.; Salariya, A.M.; Zafar, S.I. Effect of processing on available carbohydrate content and starch digestibility of kidney beans (Phaseolus vulgaris L.). Food Chem 2001, 73, 351–355. [Google Scholar]
- Foster-Powell, K.; Brand-Miller, J. International tables of glycemic index. Am. J. Clin. Nutr 1995, 62, 871S–893S. [Google Scholar]
- Hernández-Salazar, M.; Osorio-Diaz, P.; Loarca-Piña, G.; Reynoso-Camacho, R.; Tovar, J.; Bello-Pérez, L.A. In vitro fermentability and antioxidant capacity of the indigestible fraction of cooked black beans (Phaseolus vulgaris L.), lentils (Lens culinaris L.) and chickpeas (Cicer arietinum L.). J. Sci. Food Agric 2010, 90, 1417–1422. [Google Scholar]
- Sáyago-Ayerdi, S.G.; Tovar, J.; Osorio-Diaz, P.; Paredes-López, O.; Bello-Pérez, L.A. In vitro starch digestibility and predicted glycemic index of corn tortilla, black beans, and tortilla-bean mixture: Effect of cold storage. J. Agric. Food Chem 2005, 53, 1281–1285. [Google Scholar]
- Tovar, J.; Sáyago-Ayerdi, S.G.; Peñalver, C.; Paredes-López, O.; Bello-Pérez, L.A. In vitro starch hydrolysis index and predicted glycemic index of corn tortilla, black beans (Phaseolus vulgaris L.), and Mexican “taco”. Cereal Chem 2003, 80, 533–535. [Google Scholar]
- Mora-Avilés, A.; Lemus-Flores, B.; Miranda-López, R.; Hernández-López, D.; Pons-Hernández, J.L.; Acosta-Gallegos, J.A.; Guzmán-Maldonado, S.H. Effects of common bean enrichment on nutritional quality of tortillas produced from nixtamalized regular and quality protein maize flours. J. Sci. Food Agric 2007, 87, 880–886. [Google Scholar]
- Hernández-Salazar, M.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Tovar, J.; Bello-Pérez, L.A. Chemical composition and starch digestibility of tortillas prepared with non-conventional comercial nixtamalized maize flours. Int. J. Food Sci. Nutr 2006, 57, 143–150. [Google Scholar]
- Vargas-Torres, A.; Osorio-Díaz, P.; Tovar, J.; Paredes-López, O.; Ruales, J.; Bello-Pérez, L.A. Chemical composition, starch bioavailability and indigestible fraction of common beans (Phaseolus vulgaris L.). Starch Stärke 2004, 56, 74–78. [Google Scholar]
- Méndez-Montealvo, G.; Solorza-Feria, J.; Velázquez, V.M.; Gómez-Montiel, N.; Paredes-López, O.; Bello-Pérez, L.A. Composición química y caracterización calorimétrica de híbridos y variedades de maíz cultivadas en México. Agrociencia 2005, 39, 267–274. [Google Scholar]
- Rendón-Villalobos, R.; Bello-Pérez, L.A.; Osorio-Díaz, P.; Tovar, J.; Paredes-López, O. Effect of storage time on in vitro digestibility and resistant starch content of nixtamal, masa, and tortilla. Cereal Chem 2002, 79, 340–344. [Google Scholar]
- Graham, G.G. Quality-protein maize with a high fat content as a weaning food. J. Pediatr. Gastroenterol. Nutr 1993, 17, 139–144. [Google Scholar]
- Agama-Acevedo, E.; Rendón-Villalobos, R.; Tovar, J.; Trejo-Estrada, S.R.; Bello-Pérez, L.A. Effect of storage time on in vitro digestion rate and resistant starch content of tortillas elaborated from commercial corn masas. Arch. Latinoam. Nutr 2005, 55, 86–92. [Google Scholar]
- Silva-Cristobal, L.; Osorio-Díaz, P.; Tovar, J.; Bello-Pérez, L.A. Chemical composition, carbohydrate digestibility, and antioxidant capacity of cooked black bean, chickpea, and lentil Mexican varieties. CyTA J. Food 2010, 8, 7–14. [Google Scholar]
- Bednar, G.E.; Patil, A.R.; Murray, S.M.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C., Jr. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentabilityin vitroin a canine model. J. Nutr 2001, 131, 276–286. [Google Scholar]
- Reynoso-Camacho, R.; Ríos-Ugalde, M.C.; Torres-Pacheco, I.; Acosta-Gallegos, J.A.; Palomino-Salinas, A.C.; Ramos-Gómez, M.; González-Jasso, E.; Guzmán-Maldonado, S.H. El consumo de frijol común (Phaseolus vulgaris L.) y su efecto sobre el cáncer de colon en ratas Sprague-Dawley. Agric. Téc. Méx 2007, 33, 43–52. [Google Scholar]
- Agama-Acevedo, E.; Rendón-Villalobos, R.; Tovar, J.; Paredes-López, O.; Islas-Hernández, J.J.; Bello-Pérez, L.A. In vitro starch digestibility changes during storage of maize flour tortillas. Nahrung 2004, 48, 38–42. [Google Scholar]
- Vargas-Torres, A.; Osorio-Díaz, P.; Islas-Hernández, J.J.; Tovar, J.; Paredes-López, O.; Bello-Pérez, L.A. Starch digestibility of five cooked black bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal 2004, 17, 605–612. [Google Scholar]
- Osorio-Díaz, P.; Méndez-Montealvo, G.; Agama-Acevedo, E.; Islas-Hernández, J.J.; Sánchez-Muñoz, J.; Bello-Pérez, L.A. Biodisponibilidad del almidón en dos variedades comerciales de frijol (Phaseolus vulgaris L.) y en frijoles industrializados. Agrociencia 2003, 37, 565–573. [Google Scholar]
- Björck, I.; Granfeldt, Y.; Liljeberg, H.; Tovar, J.; Asp, N.G. Food properties affecting the digestion and absortion of carbohydrates. Am. J. Clin. Nutr 1994, 59, 699S–705S. [Google Scholar]
- Fredriksson, H.; Björck, I.; Andersson, R.; Liljeberg, H.; Silverio, J.; Eliasson, A.C.; Aman, P. Studies on α-amylase degradation of retrograded starch gels from waxy maize and high-amylopectin potato. Carbohydr. Polym 2000, 43, 81–87. [Google Scholar]
- Landa-Habana, L.; Piña-Hernández, A.; Agama-Acevedo, E.; Tovar, J.; Bello-Pérez, L.A. Effect of cooking procedures and storage on starch bioavailability in common beans (Phaseolus vulgaris L.). Plant Foods Hum. Nutr 2004, 59, 133–136. [Google Scholar]
- Osorio-Díaz, P.; Tovar, J.; Paredes-López, O.; Acosta-Gallegos, J.A.; Bello-Pérez, L.A. Chemical composition and in vitro starch bioavailability of Phaseolus vulgaris (L) cv Mayocoba. J. Sci. Food Agric 2005, 85, 499–504. [Google Scholar]
- Hendrich, S. Battling obesity with resistant starch. Food Technol 2010, 64, 22–30. [Google Scholar]
- Tovar, J.; Björck, I.M.; Asp, N.G. Starch content and α-amylolysis rate in precooked legume flours. J. Agric. Food Chem 1990, 38, 1818–1823. [Google Scholar]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res 1997, 17, 427–437. [Google Scholar]
- Bello-Pérez, L.A.; Rendón-Villalobos, J.R.; Agama-Acevedo, E.; Islas-Hernández, J.J. In vitro starch digestibility of tortillas elaborated by different masa preparation procedures. Cereal Chem 2006, 83, 188–193. [Google Scholar]
- Hernández-Uribe, J.P.; Agama-Acevedo, E.; Islas-Hernández, J.J.; Tovar, J.; Bello-Pérez, L.A. Chemical composition and in vitro starch digestibility of pigmented corn tortilla. J. Sci. Food Agric 2007, 87, 2482–2487. [Google Scholar]
- Xu, B.J.; Yuan, S.H.; Chang, S.K.C. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci 2007, 72, S167–S177. [Google Scholar]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Lee, C.-H.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT Food Sci. Technol 2009, 42, 1187–1192. [Google Scholar]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem 2007, 101, 492–501. [Google Scholar]
- De Mejia, E.G.; Guzmán-Maldonado, S.H.; Acosta-Gallegos, J.A.; Reynoso-Camacho, R.; Ramírez-Rodríguez, E.; Pons-Hernández, J.L.; González-Chavira, M.M.; Castellanos, J.Z.; Kelly, J.D. Effect of cultivar and growing location on the trypsin inhibitors, tannins, and lectins of common beans (Phaseolus vulgaris L.) grown in the semiarid highlands of Mexico. J. Agric. Food Chem 2003, 51, 5962–5966. [Google Scholar]
- Guzmán-Maldonado, H.S.; Castellanos, J.; González, M.E. Relationship between theoretical and experimentally detected tannin content of common beans (Phaseolus vulgaris L.). Food Chem 1996, 55, 333–335. [Google Scholar]
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem 2005, 53, 5036–5040. [Google Scholar]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem 1995, 43, 1789–1793. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem 2005, 93, 713–718. [Google Scholar]
- Saura-Calixto, F.; Goñi, I. Antioxidant capacity of the spanish mediterranean diet. Food Chem 2006, 94, 442–447. [Google Scholar]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr 2003, 133, 2812–2819. [Google Scholar]
- Dueñas, M.; Sun, B.; Hernández, T.; Estrella, I.; Spranger, M.I. Proanthocyanidin composition in the seed coat of lentils (Lens culinaris L.). J. Agric. Food Chem 2003, 51, 7999–8004. [Google Scholar]
- Plumb, G.W.; Prince, K.R.; Williamson, G. Antioxidant properties of flavonol glucosides from tea. Redox Report 1999, 4, 13–16. [Google Scholar]
- Yang, Y.-C.; Lu, F.-H.; Wu, J.-S.; Wu, C.-H.; Chang, C.-J. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med 2004, 164, 1534–1540. [Google Scholar]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. BioFactors 2000, 13, 49–54. [Google Scholar]
- Jankun, J.; Selman, S.H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar]
- Seeram, N.P.; Adams, L.S.; Hardy, M.L.; Heber, D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem 2004, 52, 2512–2517. [Google Scholar]
- Aparicio-Fernández, X.; Manzo-Bonilla, L.; Loarca-Piña, G.F. Comparison of antimutagenic activity of phenolic compounds in newly harvested and stored common beans phaseolus vulgaris against aflatoxin B1. J. Food Sci 2005, 70, S73–S78. [Google Scholar]
- AACC International Methods, Approved Methods of the American Association of Cereal Chemists, 10th ed; International Press: St. Paul, MN, USA, 2000.
- Official Methods of Analysis, 16th ed; AOAC International: Gaithersburg, MD, USA, 1997.
- Goñi, I.; García-Diz, L.; Mañas, E.; Saura-Calixto, F. Analysis of resistant starch: A method for foods and food products. Food Chem 1996, 56, 445–449. [Google Scholar]
- Holm, J.; Björck, I.; Asp, N.G.; Sjoberg, L.B.; Lundquist, I. Starch availability in vitro and in vivo after flaking, steam-cooking and popping of wheat. J. Cereal Sci 1985, 3, 193–200. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol 1999, 299, 152–178. [Google Scholar]
- Reed, J.D.; Mcdowell, R.E.; van Soest, P.J.; Horvath, P.J. Condensed tannins: A factor limiting the use of cassava forage. J. Sci. Food Agric 1982, 33, 213–220. [Google Scholar]
- Hartzfeld, P.W.; Forkner, R.; Hunter, M.D.; Hagerman, A.E. Determination of hydrolizable tannins (gallotannins and ellagitanins) after reaction with potassium iodate. J. Agric. Food Chem 2002, 50, 1785–1790. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med 1999, 26, 1231–1237. [Google Scholar]
- Pulido, R.; Hernández-García, M.; Saura-Calixto, F. Contribution of beverages to the intake of lipophilic and hydrophilic antioxidants in the Spanish diet. Eur. J. Clin. Nutr 2003, 57, 1275–1282. [Google Scholar]
| Sample | Protein * | Fat | Ash | TDF |
|---|---|---|---|---|
| Black bean flour | 21.45 ± 0.36 a | 1.24 ± 0.03 a | 4.66 ± 0.02 a | 36.24 ± 0.70 a |
| QPM masa | 7.89 ± 0.11 b | 5.03 ± 0.22 b | 1.61 ± 0.02 b | 8.55 ± 0.41 b |
| QPM tortilla | 8.78 ± 0.06 b | 4.37 ± 0.23 bc | 1.67 ± 0.00 b | 9.36 ± 0.27 b |
| QPM-black bean tortilla | 12.04 ± 0.43 c | 3.89 ± 0.25 c | 2.58 ± 0.01 c | 14.72 ± 0.30 c |
| Sample | Total starch | Digestible starch* | Resistant starch |
|---|---|---|---|
| Black bean flour | 44.36 ± 0.52 a | 37.92 ± 0.55 a | 6.44 ± 0.07 a |
| QPM masa | 77.68 ± 0.20 b | 74.50 ± 0.20 b | 3.17 ± 0.01 b |
| QPM tortilla | 76.69 ± 0.82 b | 71.65 ± 0.80 c | 5.04 ± 0.05 c |
| QPM-black bean tortilla | 66.75 ± 0.47 c | 60.45 ± 0.48 d | 6.30 ± 0.08 a |
| Sample | Hydrolysis at 90 min (%) | pGI * |
|---|---|---|
| Black bean flour | 10.77 ± 0.35 a | 47.92 ± 0.27 a |
| QPM tortilla | 60.72 ± 0.27 b | 87.97 ± 0.22 b |
| QPM-black bean tortilla | 50.50 ± 0.23 c | 79.76 ± 0.18 c |
| Sample | Polyphenols content (mg/g) | ||
|---|---|---|---|
| EP | PA | HP | |
| Black bean flour | 5.55 ± 0.08a | 22.94 ± 2.12a | 12.13 ± 0.23a |
| QPM tortilla | 0.96 ± 0.05b | N.d. | 5.70 ± 0.13b |
| QPM-black bean tortilla | 1.99 ± 0.08c | 8.68 ± 1.28b | 6.17 ± 0.18b |
| Sample | Antioxidant capacity (ABTS method) (μmol Trolox eq/g) | ||
|---|---|---|---|
| EP | PA | HP | |
| Black bean flour | 57.58 ± 0.18 a | 11.27 ± 0.65 a | 14.54 ± 0.24 a |
| QPM tortilla | 6.95 ± 0.49 b | N.d. | 0.87 ± 0.46 b |
| QPM-black bean tortilla | 12.55 ± 0.42 c | 3.62 ± 0.23 b | 1.44 ± 0.35 b |
© 2012 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Grajales-García, E.M.; Osorio-Díaz, P.; Goñi, I.; Hervert-Hernández, D.; Guzmán-Maldonado, S.H.; Bello-Pérez, L.A. Chemical Composition, Starch Digestibility and Antioxidant Capacity of Tortilla Made with a Blend of Quality Protein Maize and Black Bean. Int. J. Mol. Sci. 2012, 13, 286-301. https://doi.org/10.3390/ijms13010286
Grajales-García EM, Osorio-Díaz P, Goñi I, Hervert-Hernández D, Guzmán-Maldonado SH, Bello-Pérez LA. Chemical Composition, Starch Digestibility and Antioxidant Capacity of Tortilla Made with a Blend of Quality Protein Maize and Black Bean. International Journal of Molecular Sciences. 2012; 13(1):286-301. https://doi.org/10.3390/ijms13010286
Chicago/Turabian StyleGrajales-García, Eva M., Perla Osorio-Díaz, Isabel Goñi, Deisy Hervert-Hernández, Salvador H. Guzmán-Maldonado, and Luis A. Bello-Pérez. 2012. "Chemical Composition, Starch Digestibility and Antioxidant Capacity of Tortilla Made with a Blend of Quality Protein Maize and Black Bean" International Journal of Molecular Sciences 13, no. 1: 286-301. https://doi.org/10.3390/ijms13010286




