Nanomedicine: Application Areas and Development Prospects
Abstract
:1. Introduction
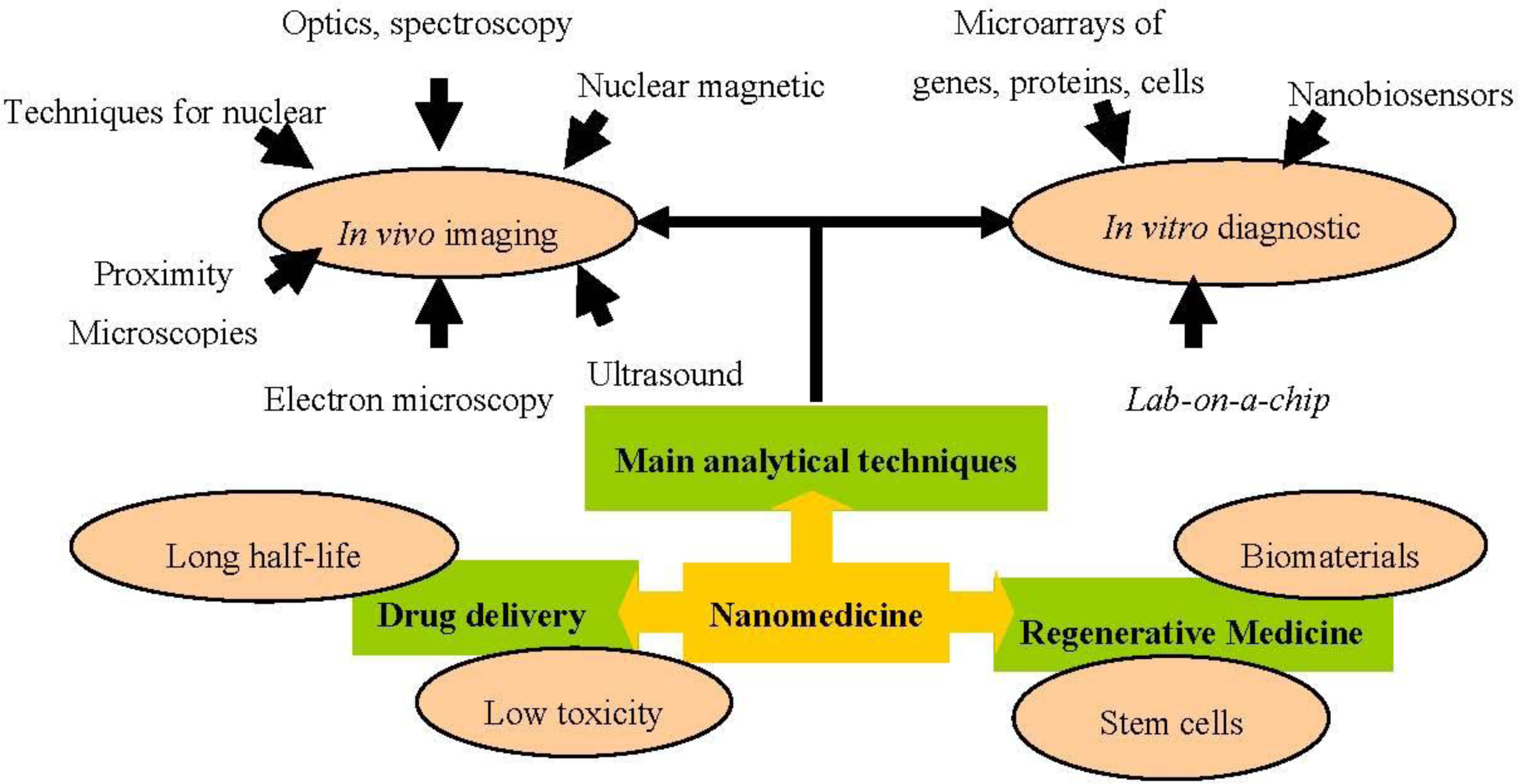
2. Nanomedicine Applications
2.1. Analytical and Diagnostic Tools
2.1.1. In Vitro Diagnostic Devices
2.1.1.1. Nanobiosensor
2.1.1.2. Microarrays
- - Gene expression analysis, used to determine gene expression patterns and simultaneously quantify the expression of a large number of genes, permitting comparison of their activation between healthy and diseased tissues.
- - Detection of mutations and polymorphisms, allowing the study of all possible polymorphisms and the detection of mutations in complex genes.
- - Sequentiation, used to sequence short DNA fragments (sequencing of long DNA fragments has not yet proven possible, although they can be used as quality controls).
- - Therapy follow-up, allowing evaluation of genetic features that may affect the response to a given therapy.
- - Preventive medicine, developing knowledge on the genetic features of diseases in order to treat and prevent them before symptom onset.
- - Drug screening and toxicology, analyzing changes in gene expression during the administration of a drug, as well as localizing new possible therapeutic targets and testing for associated toxicological effects.
- - Clinical diagnosis, allowing the rapid identification of pathogens by employing the appropriate genetic markers.
2.1.1.3. Lab-on-a-Chip
2.1.2. In Vivo Imaging
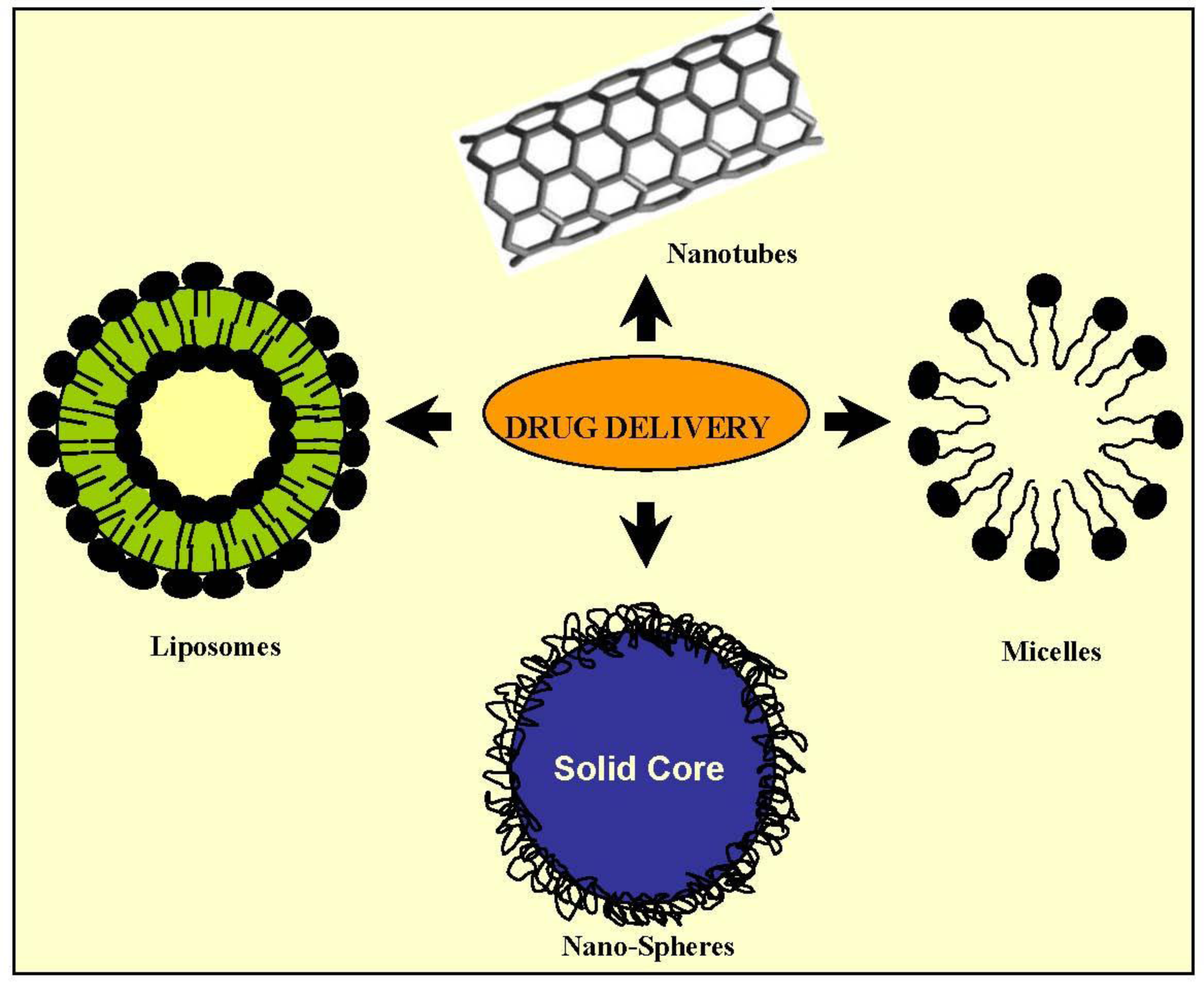
2.2. Drug Delivery
2.2.1. Micelles
2.2.2. Nanoemulsions
2.2.3. Solid Nanoparticles
2.2.4. Dendrimers
2.2.5. Liposomes
2.3. Regenerative Medicine
3. Conclusions
Acknowledgments
References
- Appenzeller, T. The man who dared to think small. Science 1991, 254, 1300–1301. [Google Scholar]
- Kang, YS; Risbud, S; Rabolt, JF; Stroeve, P. Synthesis and characterization of nanometer-size Fe3O4 and g-Fe2O3 particles. Chem. Mater 1996, 8, 2209–2211. [Google Scholar]
- Pankhurst, QA; Connoly, J; Jones, SK. Applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys 2003, 36, 167. [Google Scholar]
- Dobson, J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther 2006, 13, 283–287. [Google Scholar]
- Rudge, S; Peterson, C; Vessely, C; Koda, J; Stevens, S; Catterall, L. Adsorption and desorption of chemotherapeutic drugs from a magnetically targeted carrier (MTC). J. Control. Release 2001, 74, 335–340. [Google Scholar]
- Mody, VV; Siwale, R; Singh, A; Mody, HR. Introduction to metallic nanoparticles. J. Pharm. Bioallied. Sci 2010, 2, 282–289. [Google Scholar]
- Zhang, L; Gu, FX; Chan, JM; Wang, AZ; Langer, RS; Farokhzad, OC. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther 2008, 83, 761–769. [Google Scholar]
- Surendiran, A; Sandhiya, S; Pradhan, SC; Adithan, C. Novel applications of nanotechnology in medicine. Indian J. Med. Res 2009, 130, 689–701. [Google Scholar]
- Zarbin, MA; Montemagno, C; Leary, JF; Ritch, R. Nanotechnology in ophthalmology. Can. J. Ophthalmol 2010, 45, 457–476. [Google Scholar]
- Jain, KK. Nanotechnology in clinical laboratory diagnostics. Clin. Chim. Acta 2005, 358, 37–54. [Google Scholar]
- Jain, KK. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert Rev. Mol. Diagn 2003, 3, 153–161. [Google Scholar]
- Singh, RP; Oh, BK; Ch, JW. Application of peptide nucleic acid towards development of nanobiosensor arrays. Bioelectrochemisty 2010, 79, 153–161. [Google Scholar]
- European Technology Platform on Nanomedicine. Nanotechnology for Health. Vision Paper and Basis for a Strategic Research Agenda for Nanomedicine. Eur. Comm 2005, 1, 1–39. [Google Scholar]
- Debouck, C; Goodfellow, PN. DNA microarrays in drug discovery and development. Nat. Genet 1999, 21, 48–50. [Google Scholar]
- Cole, KA; Krizman, DB; Emmert-Buck, MR; Chuaqui, RF; Bonner, RF; Best, CJ. The genetics of cancer: A 3D model. Nat Genet 1999, 21, 38–41. [Google Scholar]
- Gerhold, DL; Jensen, RV; Gullans, SR. Better therapeutics through microarrays. Nat. Genet 2002, 32, 547–551. [Google Scholar]
- Heller, RA; Schena, M; Chai, A; Shalon, D; Bedilion, T; Gilmore, J; Woolley, DE; Davis, RW. Discovery and analysis of inflammatory disease-related genes using cDNA microarrays. Proc. Natl. Acad. Sci. USA 1997, 94, 2150–2155. [Google Scholar]
- McCaffrey, TA; Fu, C; Du, B; Eksinar, S; Kent, KC; Bush, H, Jr; Kreiger, K; Rosengart, T; Cybulsky, MI; Silverman, ES; Collins, T. High-level expression of Egr-1 and Egr-1-inducible genes in mouse and human atherosclerosis. J. Clin. Invest 2000, 105, 653–662. [Google Scholar]
- Perou, CM; Jeffrey, SS; Van de Rijn, M; Rees, CA; Eisen, MB; Ross, DT; Pergamenschikov, A; Williams, CF; Zhu, SX; Lee, JC; Lashkari, D; Shalon, D; Brown, PO; Botstein, D. Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl. Acad. Sci. USA 1999, 96, 9212–9217. [Google Scholar]
- Perou, CM; Sørlie, T; Eisen, MB; Rijn, M; Jeffrey, SS; Rees, CA; Pollack, JR; Ross, DT; Johnsen, H; Akslen, LA; Fluge, O; Pergamenschikov, A; Williams, C; Zhu, SX; Lønning, PE; Børresen-Dale, AL; Brown, PO; Botstein, D. Molecular portraits of breast tumours. Nature 2000, 406, 747–752. [Google Scholar]
- Alon, U; Barkai, N; Notterman, DA; Gish, K; Ybarra, S; Mack, D; Levine, AJ. Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 1999, 96, 6745–6750. [Google Scholar]
- Kaminski, N; Allard, JD; Pittet, JF; Zuo, F; Griffiths, MJ; Morris, D; Huang, X; Sheppard, D; Heller, RA. Global analysis of gene expression in pulmonary fibrosis reveals distinct programs regulating lung inflammation and fibrosis. Proc. Natl. Acad. Sci. USA 2000, 97, 1778–1783. [Google Scholar]
- Stoughton, RB. Applications of DNA microarrays in biology. Annu. Rev. Biochem 2005, 74, 53–82. [Google Scholar]
- Plomin, R; Schalkwyk, LC. Microarrays. Dev. Sci 2007, 10, 19–23. [Google Scholar]
- Schulze, A; Downward, J. Navigating gene expression using microarrays—a technology review. Nat. Cell Biol 2001, 3, 190–195. [Google Scholar]
- Kennedy, GC; Matsuzaki, H; Dong, S; Liu, WM; Huang, J; Liu, G; Su, X; Cao, M; Chen, W; Zhang, J; Liu, W; Yang, G; Di, X; Ryder, T; He, Z; Surti, U; Phillips, MS; Boyce-Jacino, MT; Fodor, SP; Jones, KW. Large-scale genotyping of complex DNA. Nat. Biotechnol 2003, 21, 1233–1237. [Google Scholar]
- Ulger, C; Toruner, GA; Alkan, M; Mohammed, M; Damani, S; Kang, J; Galante, A; Aviv, H; Soteropoulos, P; Tolias, PP; Schwalb, MN; Dermody, JJ. Comprehensive genome-wide comparison of DNA and RNA level scan using microarray technology for identification of candidate cancer-related genes in the HL-60 cell line. Cancer Genet. Cytogenet 2003, 147, 28–35. [Google Scholar]
- MacBeath, G. Protein microarrays and proteomics. Nat. Genet 2002, 32, 526–532. [Google Scholar]
- Braunschweig, T; Chung, JY; Hewitt, SM. Tissue microarrays: Bridging the gap between research and the clinic. Expert Rev. Proteomics 2005, 2, 325–336. [Google Scholar]
- Clarke, PA; Poele, R; Wooster, R; Workman, P. Gene expression microarray analysis in cancer biology, pharmacology, and drug development: Progress and potential. Biochem. Pharmacol 2001, 62, 1311–1336. [Google Scholar]
- Jayapal, M; Melendez, JA. DNA microarray technology for target identification and validation. Clin. Exp. Pharmacol. Physiol 2006, 33, 496–503. [Google Scholar]
- Ghallab, YH; Badawy, W. Lab-on-a-Chip: Techniques, Circuits, and Biomedical Applications; Artech House: Norwood, MA, USA, 2010; pp. 1–220. [Google Scholar]
- Kim, J; Byun, D; Mauk, MG; Bau, HH. A disposable, self-contained PCR chip. Lab Chip 2009, 9, 606–612. [Google Scholar]
- Lab-on-a-Chip: Miniaturized Systems for (Bio)Chemical Analysis and Synthesis, 2nd ed; Oosterbroek, E; Van den Berg, A (Eds.) Elsevier Science: Amsterdam, The Netherlands, 2003; pp. 1–402.
- Stybayeva, G; Mudanyali, O; Seo, S; Silangcruz, J; Macal, M; Ramanculov, E; Dandekar, S; Erlinger, A; Ozcan, A; Revzin, A. Lensfree holographic imaging of antibody microarrays for high-throughput detection of Leukocyte numbers and function. Anal. Chem 2010, 82, 3736–3744. [Google Scholar]
- Weissleder, R; Pittet, MJ. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar]
- Wang, W; Eddy, R; Condeelis, J. The cofilin pathway in breast cancer invasion and metastasis. Nat. Rev. Cancer 2007, 7, 429–440. [Google Scholar]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar]
- Jaffer, FA; Weissleder, R. Molecular imaging in the clinical arena. JAMA 2005, 293, 855–862. [Google Scholar]
- Love, Z; Wang, F; Dennis, J; Awadallah, A; Salem, N; Lin, Y; Weisenberger, A; Majewski, S; Gerson, S; Lee, Z. Imaging of mesenchymal stem cell transplant by bioluminescence and PET. J. Nucl. Med 2007, 48, 2011–2020. [Google Scholar]
- MacLaren, DC; Toyokuni, T; Cherry, SR; Barrio, JR; Phelps, ME; Herschman, HR; Gambhir, SS. PET imaging of transgene expression. Biol. Psychiatry 2000, 48, 337–348. [Google Scholar]
- Kraitchman, DL; Heldman, AW; Atalar, E; Amado, LC; Martin, BJ; Pittenger, MF; Hare, JM; Bulte, JW. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation 2003, 107, 2290–2293. [Google Scholar]
- Long, CM; Bulte, JW. In vivo tracking of cellular therapeutics using magnetic resonance imaging. Expert Opin. Biol. Ther 2009, 9, 293–306. [Google Scholar]
- Kraitchman, DL; Bulte, JW. Imaging of stem cells using MRI. Basic Res. Cardiol 2008, 103, 105–113. [Google Scholar]
- Kalish, H; Arbab, AS; Miller, BR; Lewis, BK; Zywicke, HA; Bulte, JW; Bryant, LH, Jr; Frank, JA. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: Relationship between relaxivities, electrostatic forces, and chemical composition. Magn. Reson. Med 2003, 50, 275–282. [Google Scholar]
- Muja, N; Bulte, J. Magnetic resonance imaging of cells in experimental models. Prog. Nucl. Reson. Spectrosc 2009, 55, 61–77. [Google Scholar]
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol 2004, 2, 3–9. [Google Scholar]
- Bruchez, MJ; Moronne, M; Gin, P; Weiss, S; Alivisatos, AP. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar]
- Vizirianakis, IS. Nanomedicine and personalized medicine toward the application of pharmacotyping in clinical practice to improve drug-delivery outcomes. Nanomedicine 2011, 7, 11–17. [Google Scholar]
- Kawasaki, ES; Player, A. Nanotechnology, nanomedicine, and the development of new, effective therapies for cancer. Nanomedicine 2005, 1, 101–109. [Google Scholar]
- Veiseh, O; Kievit, F; Ellenbogen, RG; Zhang, M. Cancer Cell Invasion: Treatment and Monitoring Opportunities in Nanomedicine. Adv Drug Deliv Rev 2011, in press.. [Google Scholar]
- Maeda, H; Wu, J; Sawa, T; Matsumura, Y; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar]
- Farokhzad, OC; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar]
- Danhier, F; Feron, O; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar]
- Tamilvanan, S. Formulation of multifunctional oil-in-water nanosized emulsions for active and passive targeting of drugs to otherwise inaccessible internal organs of the human body. Int. J. Pharm 2009, 381, 62–76. [Google Scholar]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev 2011, in press.. [Google Scholar]
- Arias, JL; Gallardo, V; Ruiz, MA; Delgado, AV. Magnetite/poly(alkylcyanoacrylate) (core/shell) nanoparticles as 5-Fluorouracil delivery systems for active targeting. Eur. J. Pharm. Biopharm 2008, 69, 54–63. [Google Scholar]
- Murphy, CG; Modi, S. HER2 breast cancer therapies: A review. Biologics Targets Ther 2009, 3, 289–301. [Google Scholar]
- Cirstoiu-Hapca, A; Bossy-Nobs, L; Buchegger, F; Gurny, R; Delie, F. Differential tumor cell targeting of anti-HER2 (Herceptin®) and anti-CD20 (Mabthera®) coupled nanoparticles. Int. J. Pharm 2007, 331, 190–196. [Google Scholar]
- Li, D; Yu, H; Huang, H; Shen, F; Wu, X; Li, J; Wang, J; Cao, X; Wang, Q; Tang, G. FGF receptor-mediated gene delivery using ligands coupled to polyethylenimine. J. Biomater. Appl 2007, 22, 163–180. [Google Scholar]
- Song, S; Liu, D; Peng, J; Sun, Y; Li, Z; Gu, J; Xu, Y. Peptide ligand-mediated liposome distribution and targeting to EGFR expressing tumor in vivo. Int. J. Pharm 2008, 363, 155–161. [Google Scholar]
- Serda, RE; Godin, B; Blanco, E; Chiappini, C; Ferrari, M. Multi-stage delivery nano-particle systems for therapeutic applications. Biochim. Biophys. Acta 2011, 1810, 317–329. [Google Scholar]
- Rangel-Yagui, CO; Pessoa, A; Tavares, LC. Micellar solubilization of drugs. J. Pharm. Pharm. Sci 2005, 8, 147–163. [Google Scholar]
- Sarker, DK. Engineering of nanoemulsions for drug delivery. Curr. Drug Deliv 2005, 2, 297–310. [Google Scholar]
- Husseini, GA; PittMicelles, WG. Nanoparticles for ultrasonic drug and gene delivery. Adv. Drug. Deliv. Rev 2008, 60, 1137–1152. [Google Scholar]
- Haley, B; Frenkel, E. Nanoparticles for drug delivery in cancer treatment. Urol Oncol 2008, 26, 57–64. [Google Scholar]
- Hughes, GA. Nanostructure-mediated drug delivery. Nanomedicine 2005, 1, 22–30. [Google Scholar]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug. Deliv. Rev 2011, 63, 161–169. [Google Scholar]
- Obata, Y; Tajima, S; Takeoka, S. Evaluation of pH-responsive liposomes containing amino acid-based zwitterionic lipids for improving intracellular drug delivery in vitro and in vivo. J. Control. Release 2010, 142, 267–276. [Google Scholar]
- Huang, S. Liposomes in ultrasonic drug and gene delivery. Adv. Drug. Deliv. Rev 2008, 60, 1167–1176. [Google Scholar]
- Koide, H; Asai, T; Hatanaka, K; Akai, S; Ishii, T; Kenjo, E; Ishida, T; Kiwada, H; Tsukada, H; Oku, N. T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int. J. Pharm 2010, 392, 218–223. [Google Scholar]
- Sakamoto, JH; Van de Ven, AL; Godin, B; Blanco, E; Serda, RE; Grattoni, A; Ziemys, A; Bouamrani, A; Hu, T; Ranganathan, SI; De Rosa, E; Martinez, JO; Smid, CA; Buchanan, RM; Lee, S; Srinivasan, S; Landry, M; Meyn, A; Tasciotti, E; Liu, X; Decuzzi, P; Ferrari, M. Enabling individualized therapy through nanotechnology. Pharmacol. Res 2010, 62, 57–89. [Google Scholar]
- Shekaran, A; Garcia, AJ. Nanoscale engineering of extracellular matrix-mimetic bioadhesive surfaces and implants for tissue engineering. Biochim. Biophys. Acta 2011, 1810, 350–360. [Google Scholar]
- Barnes, CP; Sell, SA; Boland, ED; Simpson, DG; Bowlin, GL. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug. Deliv. Rev 2007, 59, 1413–1433. [Google Scholar]
- Atala, A; Lanza, R; Nerem, R; Thomson, JA. Principles of Regenerative Medicine, 2nd ed; Academic Press: London, UK, 2011; pp. 733–1105. [Google Scholar]
- Prakash, S; Khan, A; Paul, A. Nanoscaffold based stem cell regeneration therapy: Recent advancement and future potential. Expert Opin. Biol. Ther 2010, 10, 1649–1661. [Google Scholar]
| Feature | QDs | Cantilevers | Gold nanoparticles |
|---|---|---|---|
| Structure | Semiconductor nanocrystals typically composed of a semiconductor core encapsulated by another semiconductor shell with a larger spectral band-gap; a third silica shell can be added for water solubility | Nano-machined silicon or a piezoelectric material such as quartz similar to those used in atomic force microscopy | Gold particles in the nanometre size domain; gold nanoshells consist of concentric sphere nanoparticles with a dielectric core (typically gold sulfide or silica) surrounded by a thin gold shell |
| Size | 2–10 nm | Nanoscale | 2–150 nm (changes in optical properties as a function of size) |
| Diagnostic applications | - In vitro diagnosis: immunohistochemistry, infectious agent detection, fluoroimmunoassays, immunoassays, intracellular imaging and tissue imaging. - In vivo imaging | DNA and protein (various biomarkers) detection and quantification. | Detection of DNA and proteins (including antibodies) |
| Method for detecting | Fluorometry and several types of microscopy, such as fluorescence, confocal, total internal reflection, wide-field epifluorescence, atomic force, and multiphoton microscopy | Operate either statically, by measuring absolute cantilever deflection, or dynamically, by measuring resonance frequency shifts | Surface plasmon resonance microscopy. Gold particles coated with silver have strong light-scattering properties and can easily be detected by standard dark-field microscopy with white light illumination |
| Advantage | - Their optical tunability, resistance to photobleaching, excitation of various QDs by a single wavelength of light (for multiplexing), narrow emission band, and exceptional stability of optical properties after conjugation to a biomolecule. - They do not need lasers for excitation. - The instrumentation needed for detection is simple. | - Their sensitivity, compatibility with silicon technology, and capacity for microfluidic integration. - Good potential for high throughput protein screening | Their optical properties, useful for imaging and photothermal therapy. Their surfaces, functionalized using various well-characterized chemical moieties (thiols, disulfides, amines) |
| Toxicity | Risk of leakage of toxic core semiconductor materials into host system or into the environment on disposal | No particular toxicity concerns | No particular toxicity concerns |
| Compounds | Nanocarrier | Application |
|---|---|---|
| CPX-1 irinotecan | Liposome | Systemic |
| DNA (gene therapy) | Solid lipid nanoparticles | Systemic |
| Cancer vaccine | Immunostimulatory acid-degradable microparticles | Subcutaneously |
| Camptothecin | Polymeric nanoparticles | Systemic |
| Tamoxifen citrate | Solid lipid nanoparticles | Systemic |
| Pilocarpine hydrochloride | Polymeric nanoparticles | Systemic |
| Clotrimazole | Solid lipid nanoparticles and nanostructured lipid carriers | Topical |
| Clozapine | Solid lipid nanoparticles | Systemic |
| Coenzyme Q 10 | Solid lipid nanoparticles | Topical |
| Titanium dioxide | Solid lipid nanoparticles | Topical |
| 5-Fluorouracil | Nanostructured lipid carriers | Systemic |
| Ibuprofen | Solid lipid nanoparticles | Topical |
| Insulin | Solid lipid nanoparticles | Systemic |
| Isotretinoin | Solid lipid nanoparticles | Systemic |
| Ketoconazole | Solid lipid nanoparticles | Topical |
| Mifepristone | Solid lipid nanoparticles | Systemic |
| N,N-Diethyltoluamide (DEET) | Solid lipid nanoparticles | Topical |
| N-dodecyl-ferulate | Solid lipid nanoparticles | Systemic |
| Oxybenzone | Solid lipid nanoparticles | Topical |
| Clobetasol propionate | Nanostructured lipid carriers | Systemic |
| Retinoids | Solid lipid nanoparticles | Topical |
| Triptolide | Solid lipid nanoparticles | Systemic |
| Vitamin A | Solid lipid nanoparticles | Topical |
| MCC465 doxorubicin | mAb-liposome | Systemic |
| NC-6004 cisplatin | Micelle | Systemic |
| NK105 paclitaxel | Micelle | Systemic |
| NK911 doxorubicin | Micelle | Systemic |
| PK1 doxorubicin | HPMA copolymer | |
| SP1049C doxorubicin | Micelle | Systemic |
| Etoposide | Nanostructured lipid carriers | Systemic |
| Docetaxel | Nanostructured lipid carriers | Systemic |
| Paclitaxel | Nanostructured lipid carriers | Orally |
| Paclitaxel | Polymeric nanoparticles | Subcutaneously |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application Areas and Development Prospects. Int. J. Mol. Sci. 2011, 12, 3303-3321. https://doi.org/10.3390/ijms12053303
Boulaiz H, Alvarez PJ, Ramirez A, Marchal JA, Prados J, Rodríguez-Serrano F, Perán M, Melguizo C, Aranega A. Nanomedicine: Application Areas and Development Prospects. International Journal of Molecular Sciences. 2011; 12(5):3303-3321. https://doi.org/10.3390/ijms12053303
Chicago/Turabian StyleBoulaiz, Houria, Pablo J. Alvarez, Alberto Ramirez, Juan A. Marchal, Jose Prados, Fernando Rodríguez-Serrano, Macarena Perán, Consolación Melguizo, and Antonia Aranega. 2011. "Nanomedicine: Application Areas and Development Prospects" International Journal of Molecular Sciences 12, no. 5: 3303-3321. https://doi.org/10.3390/ijms12053303







